The Regulations of Essential WalRK Two-Component System on Enterococcus faecalis
Abstract
:1. Introduction
2. Material and Methods
3. WalKR Two-Component Signal Transduction System and Resistance of Enterococcus Faecalis
3.1. Regulation of E. faecalis on Biofilm Formation
3.2. Antibiotic Resistance of Enterococcus faecalis
3.3. Regulatory Roles of the WalRK TCS in Response to Host Immunity
4. Chemical Compounds Act on WalRK to Inhibit the Pathogenicity of Bacteria
4.1. Antibiotics-Based Agents on WalRK
4.2. Novel Screened Antibacterial Substances on WalRK
4.3. Nucleic-Acid-Based Interfering walRK for Treatment
4.4. Bacteriophage Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Facklam, R. What happened to the streptococci: Overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 2002, 15, 613–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rams, T.E.; Feik, D.; Young, V.; Hammond, B.F.; Slots, J. Enterococci in human periodontitis. Oral Microbiol. Immunol. 1992, 7, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Distel, J.W.; Hatton, J.F.; Gillespie, M.J. Biofilm formation in medicated root canals. J. Endod. 2002, 28, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.A.; Huang, D.B. Biofilm formation by enterococci. J. Med. Microbiol. 2007, 56 Pt 2, 1581–1588. [Google Scholar] [CrossRef] [Green Version]
- Figdor, D.; Davies, J.K.; Sundqvist, G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol. Immunol. 2003, 18, 234–239. [Google Scholar] [CrossRef]
- McHugh, C.P.; Zhang, P.; Michalek, S.; Eleazer, P.D. pH required to kill Enterococcus faecalis in vitro. J. Endod. 2004, 30, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Sedgley, C.M.; Lennan, S.L.; Appelbe, O.K. Survival of Enterococcus faecalis in root canals ex vivo. Int. Endod. J. 2005, 38, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Stevens, R.H.; Grossman, L.I. Evaluation of the antimicrobial potential of calcium hydroxide as an intracanal medicament. J. Endod. 1983, 9, 372–374. [Google Scholar] [CrossRef]
- Gilmore, M.S.; Ferretti, J.J. Microbiology. The thin line between gut commensal and pathogen. Science 2003, 299, 1999–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, C.P. The emergence of enterococci as a cause of nosocomial infection. Br. J. Biomed. Sci. 1998, 55, 149–156. [Google Scholar]
- Jett, B.D.; Huycke, M.M.; Gilmore, M.S. Virulence of enterococci. Clin. Microbiol. Rev. 1994, 7, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Moellering, R.C., Jr. Emergence of Enterococcus as a significant pathogen. Clin. infect. Dis. 1992, 14, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N. Glycopeptide-resistant enterococci: A decade of experience. J. Med. Microbiol. 1998, 47, 849–862. [Google Scholar] [CrossRef]
- Ali, L.; Goraya, M.U.; Arafat, Y.; Ajmal, M.; Chen, J.L.; Yu, D. Molecular Mechanism of Quorum-Sensing in Enterococcus faecalis: Its Role in Virulence and Therapeutic Approaches. Int. J. Mol. Sci. 2017, 18, 960. [Google Scholar] [CrossRef] [Green Version]
- Marino, A.; Munafò, A.; Zagami, A.; Ceccarelli, M.; Di Mauro, R.; Cantarella, G.; Bernardini, R.; Nunnari, G.; Cacopardo, B. Ampicillin Plus Ceftriaxone Regimen against Enterococcus faecalis Endocarditis: A Literature Review. J. Clin. Med. 2021, 10, 4594. [Google Scholar] [CrossRef] [PubMed]
- Romay, E.; Pericàs, J.M.; García-País, M.J.; Hernández-Meneses, M.; Ayuso, B.; García-González, J.; Garcés-Durán, R.V.; Rabuñal, R.; Alonso-García, P.; García-Garrote, F.; et al. On Behalf Of Lucus Augusti And Hospital Clinic Endocarditis Teams. Relationship among Streptococcus gallolyticus Subsp. gallolyticus, Enterococcus faecalis and Colorectal Neoplasms in Recurrent Endocarditis: A Historical Case Series. J. Clin. Med. 2022, 11, 2181. [Google Scholar] [CrossRef] [PubMed]
- Comenge, Y.; Quintiliani, R., Jr.; Li, L.; Dubost, L.; Brouard, J.P.; Hugonnet, J.E.; Arthur, M. The CroRS two-component regulatory system is required for intrinsic beta-lactam resistance in Enterococcus faecalis. J. Bacteriol. 2003, 185, 7184–7192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frère, J.M.; Joris, B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit. Rev. Microbiol. 1985, 11, 299–396. [Google Scholar] [CrossRef]
- Tinoco, J.M.; Buttaro, B.; Zhang, H.; Liss, N.; Sassone, L.; Stevens, R. Effect of a genetically engineered bacteriophage on Enterococcus faecalis biofilms. Arch. Oral Biol. 2016, 71, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Lindenstrauss, A.G.; Behr, J.; Ehrmann, M.A.; Haller, D.; Vogel, R.F. Identification of fitness determinants in Enterococcus faecalis by differential proteomics. Arch. Microbiol. 2013, 195, 121–130. [Google Scholar] [CrossRef]
- Centeno, J.A.; Menendez, S.; Hermida, M.; Rodríguez-Otero, J.L. Effects of the addition of Enterococcus faecalis in Cebreiro cheese manufacture. Int. J. Food Microbiol. 1999, 48, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Arribas, P.; Seseña, S.; Poveda, J.M.; Chicón, R.; Cabezas, L.; Palop, L. Enterococcus populations in artisanal Manchego cheese: Biodiversity, technological and safety aspects. Food Microbiol. 2011, 28, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Metaxopoulos, J.; Samelis, J.; Papadelli, M. Technological and Microbiological Evaluation of Traditional Processes as Modified for the Industrial Manufacturing of Dry Fermented Sausage in Greece. Ital. J. Food Sci. 2001, 13, 3–18. [Google Scholar]
- Marchesini, B.; Bruttin, A.; Romailler, N.; Moreton, R.S.; Stucchi, C.; Sozzi, T. Microbiological events during commercial meat fermentations. J. Appl. Bacteriol. 1992, 73, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, P.F.; Braun, L.; Fumagalli, I.; D’Apuzzo, V.; Heim, F.; Karly, M.; Lodi, R.; Politta, G.; Vonbank, F.; Zeltner, L. Double-blind report on the efficacy of lactic acid-producing Enterococcus SF68 in the prevention of antibiotic-associated diarrhoea and in the treatment of acute diarrhoea. J. Int. Med. Res. 1989, 17, 333–338. [Google Scholar] [CrossRef]
- Hancock, L.E.; Perego, M. Systematic inactivation and phenotypic characterization of two-component signal transduction systems of Enterococcus faecalis V583. J. Bacteriol. 2004, 186, 7951–7958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.; Yuille, H.M.; Blessie, V.; Göhring, N.; Iglói, Z.; Nishiguchi, K.; Nakayama, J.; Henderson, P.J.; Phillips-Jones, M.K. Expression, purification and activities of the entire family of intact membrane sensor kinases from Enterococcus faecalis. Mol. Membr. Biol. 2008, 25, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Laguri, C.; Phillips-Jones, M.K.; Williamson, M.P. Solution structure and DNA binding of the effector domain from the global regulator PrrA (RegA) from Rhodobacter sphaeroides: Insights into DNA binding specificity. Nucleic Acids Res. 2003, 31, 6778–6787. [Google Scholar] [CrossRef] [Green Version]
- Hoch, J.A.; Silhavy, T.J. Two-Component Signal Transduction; ASM Press: Washington, DC, USA, 1995; Volume xvi, p. 488. [Google Scholar]
- Tierney, A.R.; Rather, P.N. Roles of two-component regulatory systems in antibiotic resistance. Future Microbiol. 2019, 14, 533–552. [Google Scholar] [CrossRef]
- Hancock, L.E.; Perego, M. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J. Bacteriol. 2004, 186, 5629–5639. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Singh, K.V.; Weinstock, G.M.; Murray, B.E. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. infect. Immun. 2000, 68, 2579–2586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sifri, C.D.; Mylonakis, E.; Singh, K.V.; Qin, X.; Garsin, D.A.; Murray, B.E.; Ausubel, F.M.; Calderwood, S.B. Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. infect. Immun. 2002, 70, 5647–5650. [Google Scholar] [CrossRef] [PubMed]
- Goh, H.M.S.; Yong, M.H.A.; Chong, K.K.L.; Kline, K.A. Model systems for the study of Enterococcal colonization and infection. Virulence 2017, 8, 1525–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurlow, L.R.; Thomas, V.C.; Narayanan, S.; Olson, S.; Fleming, S.D.; Hancock, L.E. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. infect. Immun. 2010, 78, 4936–4943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Kim, K.M.; Lee, J.H.; Seo, S.J.; Lee, I.H. Extracellular gelatinase of Enterococcus faecalis destroys a defense system in insect hemolymph and human serum. infect. Immun. 2007, 75, 1861–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evers, S.; Courvalin, P. Regulation of VanB-type vancomycin resistance gene expression by the VanS(B)-VanR (B) two-component regulatory system in Enterococcus faecalis V583. J. Bacteriol. 1996, 178, 1302–1309. [Google Scholar] [CrossRef] [Green Version]
- Hancock, L.; Perego, M. Two-component signal transduction in Enterococcus faecalis. J. Bacteriol. 2002, 184, 5819–5825. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, M.; Kuwahara-Arai, K.; Hiramatsu, K. Identification of the up- and down-regulated genes in vancomycin-resistant Staphylococcus aureus strains Mu3 and Mu50 by cDNA differential hybridization method. Biochem. Biophys. Res. Commun. 2000, 269, 485–490. [Google Scholar] [CrossRef]
- West, A.H.; Stock, A.M. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 2001, 26, 369–376. [Google Scholar] [CrossRef]
- Fabret, C.; Hoch, J.A. A two-component signal transduction system essential for growth of Bacillus subtilis: Implications for anti-infective therapy. J. Bacteriol. 1998, 180, 6375–6383. [Google Scholar] [CrossRef]
- Inouye, M.; Dutta, R. Histidine Kinases in Signal Transduction; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2003; Volume xviii, 520p. [Google Scholar] [CrossRef]
- Mascher, T.; Helmann, J.D.; Unden, G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 2006, 70, 910–938. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.E.; Hoch, J.A. Essentiality, bypass, and targeting of the YycFG (VicRK) two-component regulatory system in gram-positive bacteria. J. Bacteriol. 2008, 190, 2645–2648. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Mack, T.R.; Stock, A.M. Bacterial response regulators: Versatile regulatory strategies from common domains. Trends Biochem. Sci. 2007, 32, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-component signal transduction. Annu. Rev Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubrac, S.; Bisicchia, P.; Devine, K.M.; Msadek, T. A matter of life and death: Cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol. Microbiol. 2008, 70, 1307–1322. [Google Scholar] [CrossRef]
- Tran, T.T.; Panesso, D.; Gao, H.; Roh, J.H.; Munita, J.M.; Reyes, J.; Diaz, L.; Lobos, E.A.; Shamoo, Y.; Mishra, N.N.; et al. Whole-genome analysis of a daptomycin-susceptible enterococcus faecium strain and its daptomycin-resistant variant arising during therapy. Antimicrob. Agents Chemother. 2013, 57, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.; Saizieu, A.d.; Schönfeld, H.J.; Kamber, M.; Lange, R.; Thompson, C.J.; Page, M.G. Genetic analysis and functional characterization of the Streptococcus pneumoniae vic operon. infect. Immun. 2002, 70, 6121–6128. [Google Scholar] [CrossRef] [Green Version]
- Teng, F.; Singh, K.V.; Bourgogne, A.; Zeng, J.; Murray, B.E. Further characterization of the epa gene cluster and Epa polysaccharides of Enterococcus faecalis. infect. Immun. 2009, 77, 3759–3767. [Google Scholar] [CrossRef] [Green Version]
- Rice, K.C.; Mann, E.E.; Endres, J.L.; Weiss, E.C.; Cassat, J.E.; Smeltzer, M.S.; Bayles, K.W. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2007, 104, 8113–8118. [Google Scholar] [CrossRef] [Green Version]
- Mesnage, S.; Chau, F.; Dubost, L.; Arthur, M. Role of N-acetylglucosaminidase and N-acetylmuramidase activities in Enterococcus faecalis peptidoglycan metabolism. J. Biol. Chem. 2008, 283, 19845–19853. [Google Scholar] [CrossRef] [Green Version]
- Guiton, P.S.; Hung, C.S.; Kline, K.A.; Roth, R.; Kau, A.L.; Hayes, E.; Heuser, J.; Dodson, K.W.; Caparon, M.G.; Hultgren, S.J. Contribution of autolysin and Sortase a during Enterococcus faecalis DNA-dependent biofilm development. infect. Immun. 2009, 77, 3626–3638. [Google Scholar] [CrossRef] [PubMed]
- Rohde, H.; Burdelski, C.; Bartscht, K.; Hussain, M.; Buck, F.; Horstkotte, M.A.; Knobloch, J.K.; Heilmann, C.; Herrmann, M.; Mack, D. Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol. Microbiol. 2005, 55, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, S.; Rodríguez-Martínez, S.; Cancino-Diaz, M.E.; Cancino-Diaz, J.C. Extracellular proteases of Staphylococcus epidermidis: Roles as virulence factors and their participation in biofilm. APMIS 2018, 126, 177–185. [Google Scholar] [CrossRef]
- Nešuta, O.; Budešínský, M.; Hadravová, R.; Monincová, L.; Humpolicková, J.; Cerovský, V. How proteases from Enterococcus faecalis contribute to its resistance to short α-helical antimicrobial peptides. Pathog. Dis. 2017, 29, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arciola, C.R.; Baldassarri, L.; Campoccia, D.; Creti, R.; Pirini, V.; Huebner, J.; Montanaro, L. Strong biofilm production, antibiotic multi-resistance and high gelE expression in epidemic clones of Enterococcus faecalis from orthopaedic implant infections. Biomaterials 2008, 29, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, F.L.; Willems, R.J.; Leavis, H.L. Optimizing future treatment of enterococcal infections: Attacking the biofilm? Trends Microbiol. 2012, 20, 40–49. [Google Scholar] [CrossRef]
- Shankar, N.; Lockatell, C.V.; Baghdayan, A.S.; Drachenberg, C.; Gilmore, M.S.; Johnson, D.E. Role of Enterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection. infect. Immun. 2001, 69, 4366–4372. [Google Scholar] [CrossRef] [Green Version]
- Coupri, D.; Budin-Verneuil, A.; Hartke, A.; Benachour, A.; Léger, L.; Lequeux, T.; Pfund, E.; Verneuil, N. Genetic and pharmacological inactivation of d-alanylation of teichoic acids sensitizes pathogenic enterococci to β-lactams. J. Antimicrob. Chemother. 2019, 74, 3162–3169. [Google Scholar] [CrossRef]
- Chuang-Smith, O.N.; Wells, C.L.; Henry-Stanley, M.J.; Dunny, G.M. Acceleration of Enterococcus faecalis biofilm formation by aggregation substance expression in an ex vivo model of cardiac valve colonization. PLoS ONE 2010, 5, e15798. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.R.; Clabots, C.; Hirt, H.; Waters, C.; Dunny, G. Enterococcal aggregation substance and binding substance are not major contributors to urinary tract colonization by Enterococcus faecalis in a mouse model of ascending unobstructed urinary tract infection. infect. Immun. 2004, 72, 2445–2448. [Google Scholar] [CrossRef] [Green Version]
- Dunny, G.M. Enterococcal sex pheromones: Signaling, social behavior, and evolution. Annu. Rev. Genet. 2013, 47, 457–482. [Google Scholar] [CrossRef]
- Telford, J.L.; Barocchi, M.A.; Margarit, I.; Rappuoli, R.; Grandi, G. Pili in gram-positive pathogens. Nat. Rev. Microbiol. 2006, 4, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, S.B.; Kao, S.M.; van Putte, L.J.; Gallo, J.C.; Dunny, G.M. Role of the pheromone-inducible surface protein Asc10 in mating aggregate formation and conjugal transfer of the Enterococcus faecalis plasmid pCF10. J. Bacteriol. 1991, 173, 7665–7672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, C.M.; Hirt, H.; McCormick, J.K.; Schlievert, P.M.; Wells, C.L.; Dunny, G.M. An amino-terminal domain of Enterococcus faecalis aggregation substance is required for aggregation, bacterial internalization by epithelial cells and binding to lipoteichoic acid. Mol. Microbiol. 2004, 52, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Sava, I.G.; Heikens, E.; Huebner, J. Pathogenesis and immunity in enterococcal infections. Clin. Microbiol. Infect. 2010, 16, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Jiang, K.; Camacho, M.I.; Jonna, V.R.; Hofer, A.; Westerlund, F.; Christie, P.J.; Berntsson, R.P. PrgB promotes aggregation, biofilm formation, and conjugation through DNA binding and compaction. Mol. Microbiol. 2018, 109, 291–305. [Google Scholar] [CrossRef] [Green Version]
- Dunny, G.M.; Leonard, B.A.; Hedberg, P.J. Pheromone-inducible conjugation in Enterococcus faecalis: Interbacterial and host-parasite chemical communication. J. Bacteriol. 1995, 177, 871–876. [Google Scholar] [CrossRef] [Green Version]
- Leonard, B.A.; Podbielski, A.; Hedberg, P.J.; Dunny, G.M. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc. Natl. Acad. Sci. USA 1996, 93, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Chandler, J.R.; Hirt, H.; Dunny, G.M. A paracrine peptide sex pheromone also acts as an autocrine signal to induce plasmid transfer and virulence factor expression in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 15617–15622. [Google Scholar] [CrossRef] [Green Version]
- Hirt, H.; Schlievert, P.M.; Dunny, G.M. In vivo induction of virulence and antibiotic resistance transfer in Enterococcus faecalis mediated by the sex pheromone-sensing system of pCF10. infect. Immun. 2002, 70, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Galli, D.; Lottspeich, F.; Wirth, R. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol. Microbiol. 1990, 4, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Galli, D.; Friesenegger, A.; Wirth, R. Transcriptional control of sex-pheromone-inducible genes on plasmid pAD1 of Enterococcus faecalis and sequence analysis of a third structural gene for (pPD1-encoded) aggregation substance. Mol. Microbiol. 1992, 6, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- French, G.L. Enterococci and vancomycin resistance. Clin. Infect. Dis. 1998, 27 (Suppl. 1), S75–S83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Liu, Y.; Zhang, H.; Lei, L. The Susceptibility to Calcium Hydroxide Modulated by the Essential walR Gene Reveals the Role for Enterococcus faecalis Biofilm Aggregation. J. Endod. 2019, 45, 295–301.e2. [Google Scholar] [CrossRef]
- Chen, Z.; Song, K.; Shang, Y.; Xiong, Y.; Lyu, Z.; Chen, J.; Zheng, J.; Li, P.; Wu, Y.; Gu, C.; et al. Selection and Identification of Novel Antibacterial Agents against Planktonic Growth and Biofilm Formation of Enterococcus faecalis. J. Med. Chem. 2021, 64, 15037–15052. [Google Scholar] [CrossRef]
- Rice, L.B. Enterococcal Physiology and Antimicrobial Resistance: The Streetlight Just Got a Little Brighter. mBio 2021, 12, e03511–e03520. [Google Scholar] [CrossRef]
- Van Harten, R.M.; Willems, R.J.L.; Martin, N.I.; Hendrickx, A.P.A. Multidrug-Resistant Enterococcal Infections: New Compounds, Novel Antimicrobial Therapies? Trends Microbiol. 2017, 25, 467–479. [Google Scholar] [CrossRef]
- Jahansepas, A.; Aghazadeh, M.; Rezaee, M.A.; Hasani, A.; Sharifi, Y.; Aghazadeh, T.; Mardaneh, J. Occurrence of Enterococcus faecalis and Enterococcus faecium in Various Clinical Infections: Detection of Their Drug Resistance and Virulence Determinants. Microb. Drug Resist. 2018, 24, 76–82. [Google Scholar] [CrossRef]
- Arthur, M.; Quintiliani, R., Jr. Regulation of VanA- and VanB-type glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 2001, 45, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Hegstad, K.; Mikalsen, T.; Coque, T.M.; Werner, G.; Sundsfjord, A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin. Microbiol. Infect. 2010, 16, 541–554. [Google Scholar] [CrossRef]
- Kellogg, S.L.; Kristich, C.J. Functional Dissection of the CroRS Two-Component System Required for Resistance to Cell Wall Stressors in Enterococcus faecalis. J. Bacteriol. 2016, 198, 1326–1336. [Google Scholar] [CrossRef]
- Depardieu, F.; Mejean, V.; Courvalin, P. Competition between VanU(G) repressor and VanR(G) activator leads to rheostatic control of vanG vancomycin resistance operon expression. PLoS Genet. 2015, 11, e1005170. [Google Scholar] [CrossRef] [PubMed]
- Bloem, A.; Bax, H.I.; Yusuf, E.; Verkaik, N.J. New-Generation Antibiotics for Treatment of Gram-Positive Infections: A Review with Focus on Endocarditis and Osteomyelitis. J. Clin. Med. 2021, 10, 1743. [Google Scholar] [CrossRef]
- Smith, J.R.; Barber, K.E.; Raut, A.; Rybak, M.J. β-Lactams enhance daptomycin activity against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium in in vitro pharmacokinetic/pharmacodynamic models. Antimicrob. Agents Chemother. 2015, 59, 2842–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, C.A.; Panesso, D.; McGrath, D.M.; Qin, X.; Mojica, M.F.; Miller, C.; Diaz, L.; Tran, T.T.; Rincon, S.; Barbu, E.M.; et al. Genetic basis for in vivo daptomycin resistance in enterococci. N. Engl. J. Med. 2011, 365, 892–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stipp, R.N.; Boisvert, H.; Smith, D.J.; Höfling, J.F.; Duncan, M.J.; Mattos-Graner, R.O. CovR and VicRK regulate cell surface biogenesis genes required for biofilm formation in Streptococcus mutans. PLoS ONE 2013, 8, e58271. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, J.; Peng, Q.; Liu, Y.; Lei, L.; Zhang, H. The Role of Staphylococcus aureus YycFG in Gene Regulation, Biofilm Organization and Drug Resistance. Antibiotics 2021, 10, 1555. [Google Scholar] [CrossRef]
- Voyich, J.M.; Vuong, C.; DeWald, M.; Nygaard, T.K.; Kocianova, S.; Griffith, S.; Jones, J.; Iverson, C.; Sturdevant, D.E.; Braughton, K.R.; et al. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J. Infect. Dis. 2009, 199, 1698–1706. [Google Scholar] [CrossRef] [Green Version]
- Karauzum, H.; Datta, S.K. Adaptive Immunity gainst Staphylococcus aureus. Curr. Top Microbiol. Immunol. 2017, 409, 419–439. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, D.; Xia, X.; Zhang, K.; Aadil, R.M.; Batool, Z.; Wang, J. Five major two components systems of Staphylococcus aureus for adaptation in diverse hostile environment. Microb. Pathog. 2021, 159, 105119. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Lei, L.; Zhang, H. Endogenous antisense walR RNA modulates biofilm organization and pathogenicity of Enterococcus faecalis. Exp. Ther. Med. 2021, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Fakhruzzaman, M.; Inukai, Y.; Yanagida, Y.; Kino, H.; Igarashi, M.; Eguchi, Y.; Utsumi, R. Study on in vivo effects of bacterial histidine kinase inhibitor, Waldiomycin, in Bacillus subtilis and Staphylococcus aureus. J. Gen. Appl. Microbiol. 2015, 61, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotoh, Y.; Doi, A.; Furuta, E.; Dubrac, S.; Ishizaki, Y.; Okada, M.; Igarashi, M.; Misawa, N.; Yoshikawa, H.; Okajima, T.; et al. Novel antibacterial compounds specifically targeting the essential WalR response regulator. J. Antibiot. 2010, 63, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, M.; Watanabe, T.; Hashida, T.; Umekita, M.; Hatano, M.; Yanagida, Y.; Kino, H.; Kimura, T.; Kinoshita, N.; Inoue, K.; et al. Waldiomycin, a novel WalK-histidine kinase inhibitor from Streptomyces sp. MK844-mF10. J. Antibiot. 2013, 66, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Igarashi, M.; Okajima, T.; Ishii, E.; Kino, H.; Hatano, M.; Sawa, R.; Umekita, M.; Kimura, T.; Okamoto, S.; et al. Isolation and characterization of signermycin B, an antibiotic that targets the dimerization domain of histidine kinase WalK. Antimicrob. Agents. Chemother. 2012, 56, 3657–3663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrela, C.; Estrela, C.R.; Hollanda, A.C.; Decurcio Dde, A.; Pécora, J.D. Influence of iodoform on antimicrobial potential of calcium hydroxide. J. Appl. Oral Sci. 2006, 14, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motiwala, M.A.; Habib, S.; Ghafoor, R.; Irfan, S. Comparison of antimicrobial efficacy of Calcipex and Metapex in endodontic treatment of chronic apical periodontitis: A randomised controlled trial study protocol. BMJ Open 2021, 11, e048947. [Google Scholar] [CrossRef]
- Babalska, Z.Ł.; Korbecka-Paczkowska, M.; Karpiński, T.M. Wound Antiseptics and European Guidelines for Antiseptic Application in Wound Treatment. Pharmaceuticals 2021, 14, 1253. [Google Scholar] [CrossRef]
- Okada, A.; Igarashi, M.; Okajima, T.; Kinoshita, N.; Umekita, M.; Sawa, R.; Inoue, K.; Watanabe, T.; Doi, A.; Martin, A.; et al. Walkmycin B targets WalK (YycG), a histidine kinase essential for bacterial cell growth. J. Antibiot. 2010, 63, 89–94. [Google Scholar] [CrossRef]
- Kato, A.; Ueda, S.; Oshima, T.; Inukai, Y.; Okajima, T.; Igarashi, M.; Eguchi, Y.; Utsumi, R. Characterization of H-box region mutants of WalK inert to the action of waldiomycin in Bacillus subtilis. J. Gen. Appl. Microbiol. 2017, 63, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Kitayama, T.; Iwabuchi, R.; Minagawa, S.; Sawada, S.; Okumura, R.; Hoshino, K.; Cappiello, J.; Utsumi, R. Synthesis of a novel inhibitor against MRSA and VRE: Preparation from zerumbone ring opening material showing histidine-kinase inhibition. Bioorg. Med. Chem. Lett. 2007, 17, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hashimoto, Y.; Yamamoto, K.; Hirao, K.; Ishihama, A.; Hino, M.; Utsumi, R. Isolation and characterization of inhibitors of the essential histidine kinase, YycG in Bacillus subtilis and Staphylococcus aureus. J. Antibiot. 2003, 56, 1045–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Wu, C.Y.; Huang, H.T.; Lu, M.K.; Hu, W.S.; Lee, K.T. Bacillus subtilis natto Derivatives Inhibit Enterococcal Biofilm Formation via Restructuring of the Cell Envelope. Front Microbiol. 2021, 12, 785351. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhang, J.; Xu, B.; Chen, L.; Wu, Y.; Yang, X.; Shen, X.; Molin, S.; Danchin, A.; Jiang, H.; et al. Structure-based discovery of inhibitors of the YycG histidine kinase: New chemical leads to combat Staphylococcus epidermidis infections. BMC Microbiol. 2006, 6, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhao, Y.; Zhao, D.; Gong, T.; Wu, Y.; Han, H.; Xu, T.; Peschel, A.; Han, S.; Qu, D. Antibacterial and anti-biofilm activities of thiazolidione derivatives against clinical staphylococcus strains. Emerg. Microbes Infect. 2015, 4, e1. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, Y.; Tang, Y.; Zhao, Y.; Chen, J.; Zheng, J.; Wu, Y.; Deng, Q.; Qu, D.; Yu, Z. In vitro activities of thiazolidione derivatives combined with daptomycin against clinical Enterococcus faecium strains. BMC Microbiol. 2022, 22, 16. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, C.; Chen, J.; Shi, Y.; Ma, X.; Wang, Y.; Wang, Z.; Yu, Z.; Zheng, J.; Chen, Z. Antibacterial and anti-biofilm activities of histidine kinase YycG inhibitors against Streptococcus agalactiae. J. Antibiot. 2021, 74, 874–883. [Google Scholar] [CrossRef]
- Furuta, E.; Yamamoto, K.; Tatebe, D.; Watabe, K.; Kitayama, T.; Utsumi, R. Targeting protein homodimerization: A novel drug discovery system. FEBS Lett. 2005, 579, 2065–2070. [Google Scholar] [CrossRef] [Green Version]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Lei, L.; Zhang, H. Nanographene oxides carrying antisense walR RNA regulates the Enterococcus faecalis biofilm formation and its susceptibility to chlorhexidine. Lett. Appl. Microbiol. 2020, 71, 451–458. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Zhang, H.; Lei, L. Nano-graphene oxide with antisense walR RNA inhibits the pathogenicity of Enterococcus faecalis in periapical periodontitis. J. Dent. Sci. 2020, 15, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Moryl, M.; Palatyńska-Ulatowska, A.; Maszewska, A.; Grzejdziak, I.; Dias de Oliveira, S.; Pradebon, M.C.; Steier, L.; Różalski, A.; Poli de Figueiredo, J.A. Benefits and Challenges of the Use of Two Novel vB_Efa29212_2e and vB_Efa29212_3e Bacteriophages in Biocontrol of the Root Canal Enterococcus faecalis Infections. J. Clin. Med. 2022, 11, 6494. [Google Scholar] [CrossRef] [PubMed]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Merril, C.R.; Biswas, B.; Carlton, R.; Jensen, N.C.; Creed, G.J.; Zullo, S.; Adhya, S. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. USA 1996, 93, 3188–3192. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.P.; Cha, J.D.; Jang, E.H.; Klumpp, J.; Hagens, S.; Hardt, W.D.; Lee, K.Y.; Loessner, M.J. PEGylation of bacteriophages increases blood circulation time and reduces T-helper type 1 immune response. Microb. Biotechnol. 2008, 1, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.K.; Collins, J.J. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. USA 2007, 104, 11197–11202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaber, J.W.; Hochhut, B.; Waldor, M.K. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 2004, 427, 72–74. [Google Scholar] [CrossRef]
- Chatterjee, A.; Johnson, C.N.; Luong, P.; Hullahalli, K.; McBride, S.W.; Schubert, A.M.; Palmer, K.L.; Carlson, P.E., Jr.; Duerkop, B.A. Bacteriophage Resistance Alters Antibiotic-Mediated Intestinal Expansion of Enterococci. infect. Immun. 2019, 87, e00085-19. [Google Scholar] [CrossRef] [Green Version]
- Duerkop, B.A.; Huo, W.; Bhardwaj, P.; Palmer, K.L.; Hooper, L.V. Molecular Basis for Lytic Bacteriophage Resistance in Enterococci. mBio 2016, 7, e01304-16. [Google Scholar] [CrossRef]

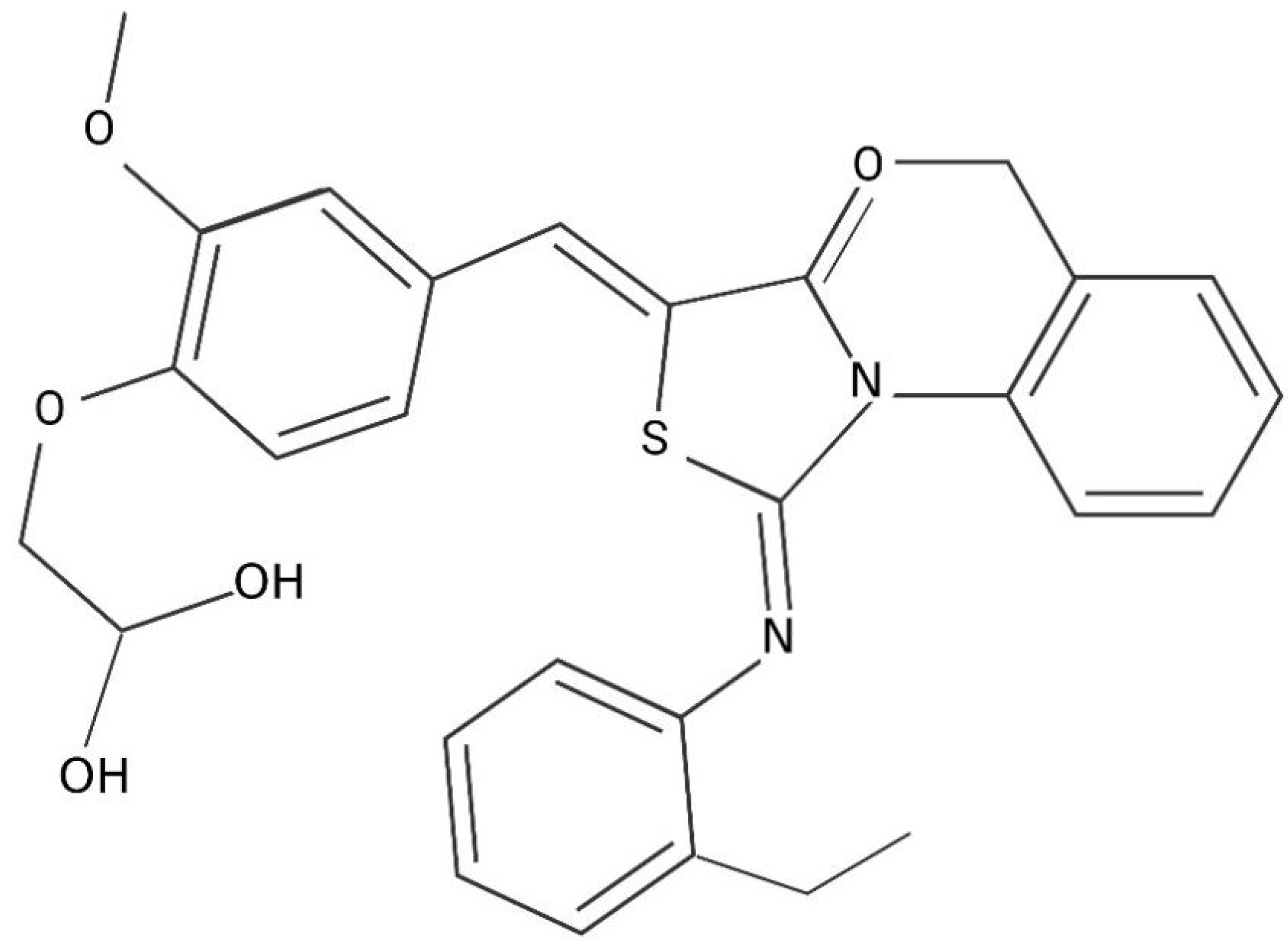
| Thiazolidinone Analogues Compound 2 Derivatives | |
|---|---|
| Name | Chemical Formula |
| H2-38 | 3-{5-{{3-(4-chlorophenyl)-2-[(4-chlorophenyl)imino]-4-oxothiazolidin-5-ylidene}methyl}furan-2-yl}benzoic acid |
| H2-39 | 4-{5-{{3-(4-chlorophenyl)-2-[(4-chlorophenyl)imino]-4-oxothiazolidin-5-ylidene}methyl}furan-2-yl}benzoic acid |
| H2-74 | 2-{4-{{3-(4-chlorophenyl)-2-[(4-chlorophenyl)imino]-4-oxothiazolidin-5-ylidene}methyl}phenoxy}acetic acid |
| H2-81 | 4-{5-{{3-(4-fluorophenyl)-2-[(4-phenyl)imino]-4-oxothiazolidin-5-ylidene}methyl}thiophene-2-yl} benzoic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Fang, R.; Peng, Q.; Wu, S.; Lei, L. The Regulations of Essential WalRK Two-Component System on Enterococcus faecalis. J. Clin. Med. 2023, 12, 767. https://doi.org/10.3390/jcm12030767
Zhang J, Fang R, Peng Q, Wu S, Lei L. The Regulations of Essential WalRK Two-Component System on Enterococcus faecalis. Journal of Clinical Medicine. 2023; 12(3):767. https://doi.org/10.3390/jcm12030767
Chicago/Turabian StyleZhang, Junqi, Rong Fang, Qi Peng, Shizhou Wu, and Lei Lei. 2023. "The Regulations of Essential WalRK Two-Component System on Enterococcus faecalis" Journal of Clinical Medicine 12, no. 3: 767. https://doi.org/10.3390/jcm12030767






