Gastrointestinal Ultrasound in Emergency Setting
Abstract
1. Introduction
2. Ultrasonographic Anatomy of Gastrointestinal Tract
3. Flares and Complication of Inflammatory Bowel Diseases (IBD)
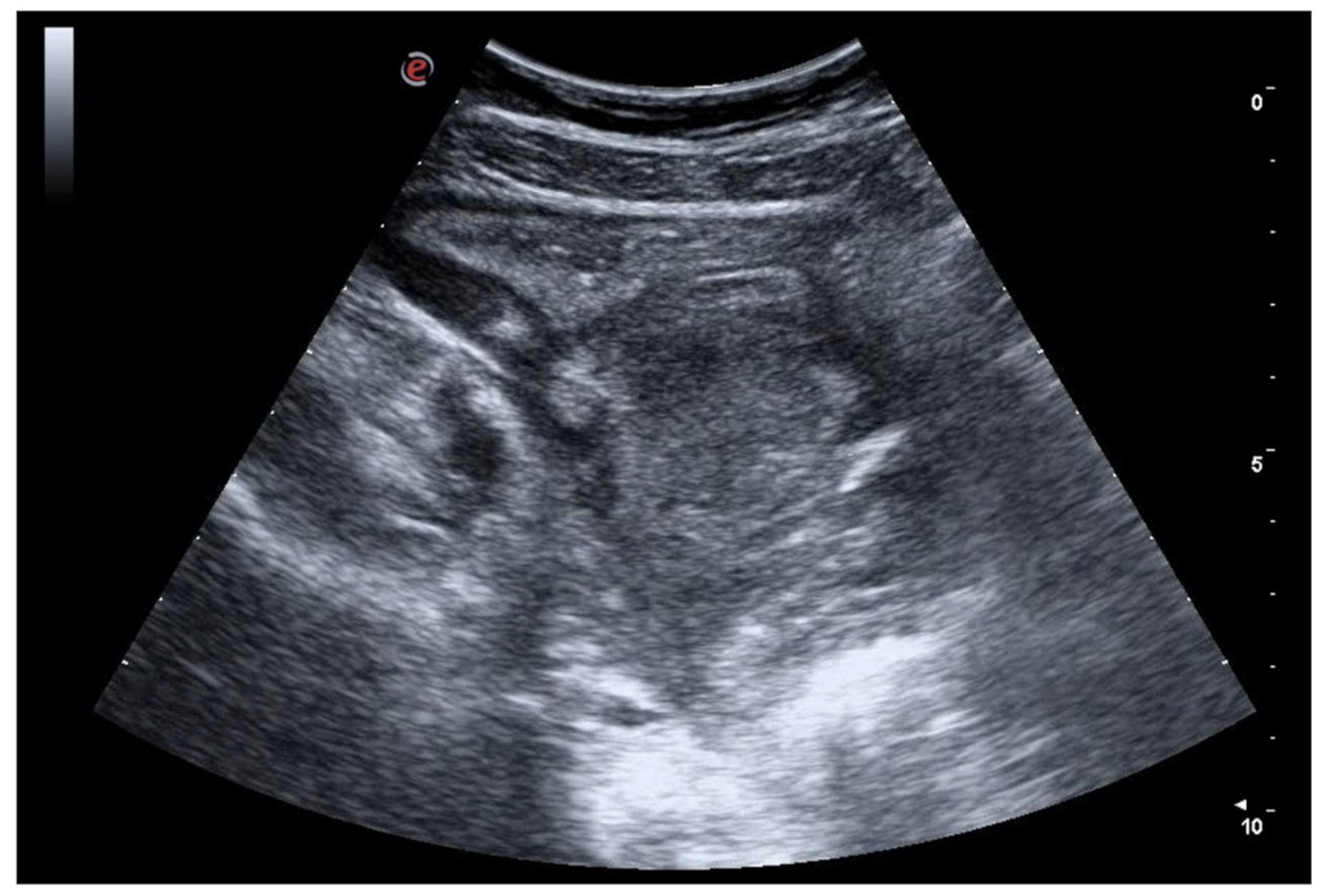
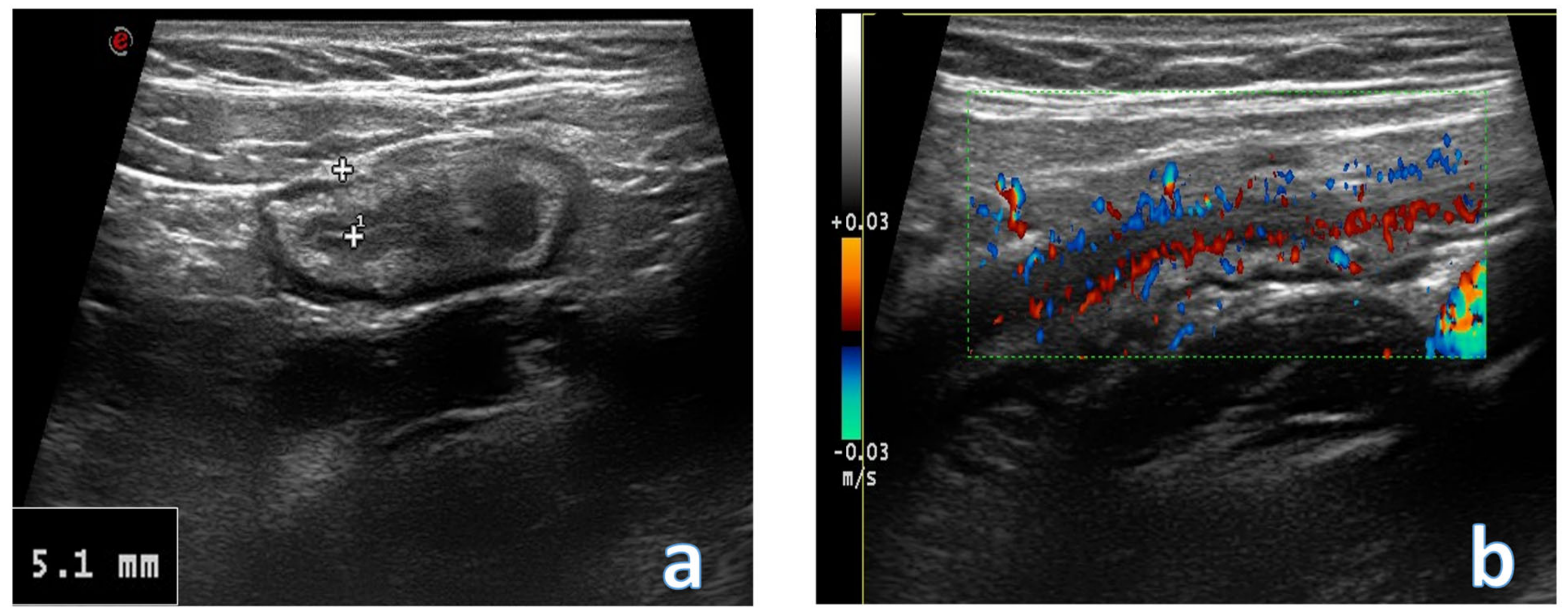
4. Acute Appendicitis
5. Acute Diverticulitis
6. Bowel Obstruction
6.1. GIUS Signs of SBO
6.2. GIUS Signs of LBO
7. Gastrointestinal Perforation
8. Ischemic Bowel Disease
8.1. Non-Occlusive Mesenteric Ischemia
8.2. Acute Venous Mesenteric Ischemia
8.3. Ischemic Colitis
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Hollerweger, A.; Maconi, G.; Ripolles, T.; Nylund, K.; Higginson, A.; Serra, C.; Dietrich, C.F.; Dirks, K.; Gilja, O.H. Gastrointestinal Ultrasound (GIUS) in Intestinal Emergencies—An EFSUMB Position Paper. Ultraschall Med. 2020, 41, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Dirks, K.; Calabrese, E.; Dietrich, C.F.; Gilja, O.H.; Hausken, T.; Higginson, A.; Hollerweger, A.; Maconi, G.; Maaser, C.; Nuernberg, D.; et al. EFSUMB Position Paper: Recommendations for Gastrointestinal Ultrasound (GIUS) in Acute Appendicitis and Diverticulitis. Ultraschall Med. 2019, 40, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Di Serafino, M.; Mercogliano, C.; Ferrara, D.; Vezzali, N.; Di Nardo, G.; Martemucci, L.; Vallone, G.; Zeccolini, M. The pediatric gastrointestinal tract: Ultrasound findings in acute diseases. J. Ultrasound. 2019, 22, 409–422. [Google Scholar] [CrossRef]
- Cocco, G.; Basilico, R.; Delli Pizzi, A.; Cocco, N.; Boccatonda, A.; D’Ardes, D.; Fabiani, S.; Anzoletti, N.; D’Alessandro, P.; Vallone, G.; et al. Gallbladder polyps ultrasound: What the sonographer needs to know. J. Ultrasound. 2021, 24, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; D’Ardes, D.; Cocco, G.; Cipollone, F.; Schiavone, C. Ultrasound and hepatic abscess: A successful alliance for the internist. Eur. J. Intern. Med. 2019, 68, e19–e21. [Google Scholar] [CrossRef]
- Atkinson, N.S.; Bryant, R.V.; Dong, Y.; Maaser, C.; Kucharzik, T.; Maconi, G.; Asthana, A.K.; Blaivas, M.; Goudie, A.; Gilja, O.H.; et al. WFUMB Position Paper. Learning Gastrointestinal Ultrasound: Theory and Practice. Ultrasound Med. Biol. 2016, 42, 2732–2742. [Google Scholar] [CrossRef]
- Long, B.; Robertson, J.; Koyfman, A. Emergency Medicine Evaluation and Management of Small Bowel Obstruction: Evidence-Based Recommendations. J. Emerg. Med. 2019, 56, 166–176. [Google Scholar] [CrossRef]
- Choe, J.; Wortman, J.R.; Michaels, A.; Sarma, A.; Fulwadhva, U.P.; Sodickson, A.D. Beyond appendicitis: Ultrasound findings of acute bowel pathology. Emerg. Radiol. 2019, 26, 307–317. [Google Scholar] [CrossRef]
- Maturen, K.E.; Wasnik, A.P.; Kamaya, A.; Dillman, J.R.; Kaza, R.K.; Pandya, A.; Maheshwary, R.K. Ultrasound imaging of bowel pathology: Technique and keys to diagnosis in the acute abdomen. AJR Am. J. Roentgenol. 2011, 197, W1067–W1075. [Google Scholar] [CrossRef]
- Nylund, K.; Maconi, G.; Hollerweger, A.; Ripolles, T.; Pallotta, N.; Higginson, A.; Serra, C.; Dietrich, C.F.; Sporea, I.; Saftoiu, A.; et al. EFSUMB Recommendations and Guidelines for Gastrointestinal Ultrasound. Ultraschall Med. 2017, 38, e1–e15. [Google Scholar]
- Zenobii, M.F.; Accogli, E.; Domanico, A.; Arienti, V. Update on ultrasound in bowel obstruction. Intern. Emerg. Med. 2016, 11, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, N.; Baccini, F.; Corazziari, E. Small intestine contrast ultrasonography. J. Ultrasound Med. 2000, 19, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hollerweger, A. Colonic diseases: The value of US examination. Eur. J. Radiol. 2007, 64, 239–249. [Google Scholar] [CrossRef]
- Yabunaka, K.; Katsuda, T.; Sanada, S.; Fukutomi, T. Sonographic appearance of the normal appendix in adults. J. Ultrasound Med. 2007, 26, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Rettenbacher, T.; Hollerweger, A.; Macheiner, P.; Rettenbacher, L.; Tomaselli, F.; Schneider, B.; Gritzmann, N. Outer diameter of the vermiform appendix as a sign of acute appendicitis: Evaluation at, U.S. Radiology 2001, 218, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Bonvicini, U.; Pisani, M. [Ultrasonography of normal vermiform appendix]. Chir. Ital. 2001, 53, 231–238. [Google Scholar]
- Kadasne, R.; Sabih, D.E.; Puri, G.; Sabih, Q. Sonographic diagnosis of appendicitis: A pictorial essay and a new diagnostic maneuver. J. Clin. Ultrasound. 2021, 49, 847–859. [Google Scholar] [CrossRef]
- Maconi, G.; Nylund, K.; Ripolles, T.; Calabrese, E.; Dirks, K.; Dietrich, C.F.; Hollerweger, A.; Sporea, I.; Saftoiu, A.; Maaser, C.; et al. EFSUMB Recommendations and Clinical Guidelines for Intestinal Ultrasound (GIUS) in Inflammatory Bowel Diseases. Ultraschall Med. 2018, 39, 304–317. [Google Scholar] [CrossRef]
- Goodsall, T.M.; Nguyen, T.M.; Parker, C.E.; Ma, C.; Andrews, J.M.; Jairath, V.; Bryant, R.V. Systematic Review: Gastrointestinal Ultrasound Scoring Indices for Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 125–142. [Google Scholar] [CrossRef]
- Smith, R.L.; Taylor, K.M.; Friedman, A.B.; Gibson, R.N.; Gibson, P.R. Systematic Review: Clinical Utility of Gastrointestinal Ultrasound in the Diagnosis, Assessment and Management of Patients With Ulcerative Colitis. J. Crohns Colitis 2020, 14, 465–479. [Google Scholar] [CrossRef]
- Calabrese, E.; Maaser, C.; Zorzi, F.; Kannengiesser, K.; Hanauer, S.B.; Bruining, D.H.; Iacucci, M.; Maconi, G.; Novak, K.L.; Panaccione, R.; et al. Bowel Ultrasonography in the Management of Crohn’s Disease. A Review with Recommendations of an International Panel of Experts. Inflamm. Bowel Dis. 2016, 22, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- Fraquelli, M.; Castiglione, F.; Calabrese, E.; Maconi, G. Impact of intestinal ultrasound on the management of patients with inflammatory bowel disease: How to apply scientific evidence to clinical practice. Dig. Liver Dis. 2020, 52, 9–18. [Google Scholar] [CrossRef]
- Horsthuis, K.; Bipat, S.; Bennink, R.J.; Stoker, J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: Meta-analysis of prospective studies. Radiology 2008, 247, 64–79. [Google Scholar] [CrossRef]
- Arienti, V.; Zamboni, L.; Gionchetti, P.; Rizzello, F.; Campieri, M. Ultrasonographic findings in Crohn’s disease. Gut 2000, 46, 293. [Google Scholar] [CrossRef] [PubMed]
- Parente, F.; Greco, S.; Molteni, M.; Cucino, C.; Maconi, G.; Sampietro, G.M.; Danelli, P.G.; Cristaldi, M.; Bianco, R.; Gallus, S.; et al. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment. Pharmacol. Ther. 2003, 18, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, H.; Zhao, J.; Zhu, W.; Zhang, L.; Gong, J.; Li, Y.; Gu, L.; Li, J. Ultrasound as a diagnostic tool in detecting active Crohn’s disease: A meta-analysis of prospective studies. Eur. Radiol. 2014, 24, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ellrichmann, M.; Wietzke-Braun, P.; Dhar, S.; Nikolaus, S.; Arlt, A.; Bethge, J.; Kuehbacher, T.; Wintermeyer, L.; Balschun, K.; Klapper, W.; et al. Endoscopic ultrasound of the colon for the differentiation of Crohn’s disease and ulcerative colitis in comparison with healthy controls. Aliment. Pharmacol. Ther. 2014, 39, 823–833. [Google Scholar] [CrossRef]
- Roushan, N.; Ebrahimi Daryani, N.; Azizi, Z.; Pournaghshband, H.; Niksirat, A. Differentiation of Crohn’s disease and ulcerative colitis using intestinal wall thickness of the colon: A Diagnostic accuracy study of endoscopic ultrasonography. Med. J. Islam. Repub. Iran 2019, 33, 57. [Google Scholar] [CrossRef]
- Kunihiro, K.; Hata, J.; Haruma, K.; Manabe, N.; Tanaka, S.; Chayama, K. Sonographic detection of longitudinal ulcers in Crohn disease. Scand. J. Gastroenterol. 2004, 39, 322–326. [Google Scholar] [CrossRef]
- Kucharzik, T.; Wittig, B.M.; Helwig, U.; Börner, N.; Rössler, A.; Rath, S.; Maaser, C. Use of Intestinal Ultrasound to Monitor Crohn’s Disease Activity. Clin. Gastroenterol. Hepatol. 2017, 15, 535–542.e2. [Google Scholar] [CrossRef]
- Buckius, M.T.; McGrath, B.; Monk, J.; Grim, R.; Bell, T.; Ahuja, V. Changing epidemiology of acute appendicitis in the United States: Study period 1993-2008. J. Surg. Res. 2012, 175, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, A. Evaluation of appendicitis risk prediction models in adults with suspected appendicitis. Br. J. Surg. 2020, 107, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, B.A.; Wilson, S.R. Appendicitis at the millennium. Radiology 2000, 215, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Bom, W.J.; Scheijmans, J.C.G.; Salminen, P.; Boermeester, M.A. Diagnosis of Uncomplicated and Complicated Appendicitis in Adults. Scand. J. Surg. 2021, 110, 170–179. [Google Scholar] [CrossRef]
- Podda, M.; Pisanu, A.; Sartelli, M.; Coccolini, F.; Damaskos, D.; Augustin, G.; Khan, M.; Pata, F.; De Simone, B.; Ansaloni, L.; et al. Diagnosis of acute appendicitis based on clinical scores: Is it a myth or reality? Acta Biomed. 2021, 92, e2021231. [Google Scholar]
- Gorter, R.R.; Eker, H.H.; Gorter-Stam, M.A.; Abis, G.S.; Acharya, A.; Ankersmit, M.; Antoniou, S.A.; Arolfo, S.; Babic, B.; Boni, L.; et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg. Endosc. 2016, 30, 4668–4690. [Google Scholar] [CrossRef]
- Shirah, B.H.; Shirah, H.A.; Alhaidari, W.A.; Elraghi, M.A.; Chughtai, M.A. The role of preoperative graded compression ultrasound in detecting acute appendicitis and influencing the negative appendectomy rate. Abdom. Radiol. (NY) 2017, 42, 109–114. [Google Scholar] [CrossRef]
- Lahaye, M.J.; Lambregts, D.M.; Mutsaers, E.; Essers, B.A.; Breukink, S.; Cappendijk, V.C.; Beets, G.L.; Beets-Tan, R.G. Mandatory imaging cuts costs and reduces the rate of unnecessary surgeries in the diagnostic work-up of patients suspected of having appendicitis. Eur. Radiol. 2015, 25, 1464–1470. [Google Scholar] [CrossRef]
- van Rossem, C.C.; Bolmers, M.D.; Schreinemacher, M.H.; van Geloven, A.A.; Bemelman, W.A. Prospective nationwide outcome audit of surgery for suspected acute appendicitis. Br. J. Surg. 2016, 103, 144–151. [Google Scholar] [CrossRef]
- Cundy, T.P.; Gent, R.; Frauenfelder, C.; Lukic, L.; Linke, R.J.; Goh, D.W. Benchmarking the value of ultrasound for acute appendicitis in children. J. Pediatr. Surg. 2016, 51, 1939–1943. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, M.; Chen, J.; Zhu, D.; Byanju, S. Ultrasound, computed tomography or magnetic resonance imaging—Which is preferred for acute appendicitis in children? A Meta-analysis. Pediatr. Radiol. 2017, 47, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.L.; Orth, R.C.; Zhang, W.; Lopez, M.E.; Mangona, K.L.; Guillerman, R.P. Diagnostic Performance of US for Differentiating Perforated from Nonperforated Pediatric Appendicitis: A Prospective Cohort Study. Radiology 2017, 282, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Puylaert, J.B. Ultrasonography of the acute abdomen: Gastrointestinal conditions. Radiol. Clin. North. Am. 2003, 41, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, R.B.; Laing, F.C., Jr.; Townsend, R.R. Acute appendicitis: Sonographic criteria based on 250 cases. Radiology 1988, 167, 327–329. [Google Scholar] [CrossRef]
- Rioux, M. Sonographic detection of the normal and abnormal appendix. AJR Am. J. Roentgenol. 1992, 158, 773–778. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, Y.K.; Hwang, J.C.; Ham, S.Y.; Yang, S.O. Graded compression sonography with adjuvant use of a posterior manual compression technique in the sonographic diagnosis of acute appendicitis. AJR Am. J. Roentgenol. 2002, 178, 863–868. [Google Scholar] [CrossRef]
- Sivit, C.J. Diagnosis of acute appendicitis in children: Spectrum of sonographic findings. AJR Am. J. Roentgenol. 1993, 161, 147–152. [Google Scholar] [CrossRef]
- Simonovský, V. Sonographic detection of normal and abnormal appendix. Clin. Radiol. 1999, 54, 533–539. [Google Scholar] [CrossRef]
- Xu, Y.; Jeffrey, R.B.; Shin, L.K.; DiMaio, M.A.; Olcott, E.W. Color Doppler Imaging of the Appendix: Criteria to Improve Specificity for Appendicitis in the Borderline-Size Appendix. J. Ultrasound Med. 2016, 35, 2129–2138. [Google Scholar] [CrossRef]
- Summa, M.; Perrone, F.; Priora, F.; Testa, S.; Quarati, R.; Spinoglio, G. Integrated clinical-ultrasonographic diagnosis in acute appendicitis. J. Ultrasound. 2007, 10, 175–178. [Google Scholar] [CrossRef]
- Quigley, A.J.; Stafrace, S. Ultrasound assessment of acute appendicitis in paediatric patients: Methodology and pictorial overview of findings seen. Insights Imaging 2013, 4, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Park, N.H.; Oh, H.E.; Park, H.J.; Park, J.Y. Ultrasonography of normal and abnormal appendix in children. World J. Radiol. 2011, 3, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Atema, J.J.; van Rossem, C.C.; Leeuwenburgh, M.M.; Stoker, J.; Boermeester, M.A. Scoring system to distinguish uncomplicated from complicated acute appendicitis. Br. J. Surg. 2015, 102, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Rawolle, T.; Reismann, M.; Minderjahn, M.I.; Bassir, C.; Hauptmann, K.; Rothe, K.; Reismann, J. Sonographic differentiation of complicated from uncomplicated appendicitis. Br. J. Radiol. 2019, 92, 20190102. [Google Scholar] [CrossRef] [PubMed]
- Mahida, J.B.; Lodwick, D.L.; Nacion, K.M.; Sulkowski, J.P.; Leonhart, K.L.; Cooper, J.N.; Ambeba, E.J.; Deans, K.J.; Minneci, P.C. High failure rate of nonoperative management of acute appendicitis with an appendicolith in children. J. Pediatr. Surg. 2016, 51, 908–911. [Google Scholar] [CrossRef]
- Maconi, G.; Carmagnola, S.; Guzowski, T. Intestinal Ultrasonography in the Diagnosis and Management of Colonic Diverticular Disease. J. Clin. Gastroenterol. 2016, 50 (Suppl. S1), S20–S22. [Google Scholar] [CrossRef]
- Loffeld, R.J. Long-term follow-up and development of diverticulitis in patients diagnosed with diverticulosis of the colon. Int. J. Color. Dis. 2016, 31, 15–17. [Google Scholar] [CrossRef]
- Cuomo, R.; Barbara, G.; Pace, F.; Annese, V.; Bassotti, G.; Binda, G.A.; Casetti, T.; Colecchia, A.; Festi, D.; Fiocca, R.; et al. Italian consensus conference for colonic diverticulosis and diverticular disease. United Eur. Gastroenterol. J. 2014, 2, 413–442. [Google Scholar] [CrossRef]
- Sartelli, M.; Catena, F.; Ansaloni, L.; Coccolini, F.; Griffiths, E.A.; Abu-Zidan, F.M.; Di Saverio, S.; Ulrych, J.; Kluger, Y.; Ben-Ishay, O.; et al. WSES Guidelines for the management of acute left sided colonic diverticulitis in the emergency setting. World J. Emerg. Surg. 2016, 11, 37. [Google Scholar] [CrossRef]
- Francis, N.K.; Sylla, P.; Abou-Khalil, M.; Arolfo, S.; Berler, D.; Curtis, N.J.; Dolejs, S.C.; Garfinkle, R.; Gorter-Stam, M.; Hashimoto, D.A.; et al. EAES and SAGES 2018 consensus conference on acute diverticulitis management: Evidence-based recommendations for clinical practice. Surg. Endosc. 2019, 33, 2726–2741. [Google Scholar] [CrossRef]
- Andeweg, C.S.; Wegdam, J.A.; Groenewoud, J.; van der Wilt, G.J.; van Goor, H.; Bleichrodt, R.P. Toward an evidence-based step-up approach in diagnosing diverticulitis. Scand. J. Gastroenterol. 2014, 49, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Puylaert, J.B. Ultrasound of colon diverticulitis. Dig. Dis. 2012, 30, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Valentino, M.; Serra, C.; Ansaloni, L.; Mantovani, G.; Pavlica, P.; Barozzi, L. Sonographic features of acute colonic diverticulitis. J. Clin. Ultrasound. 2009, 37, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Shirahama, M.; Ishibashi, H.; Onohara, S.; Dohmen, K.; Miyamoto, Y. Colour Doppler ultrasound for the evaluation of bowel wall thickening. Br. J. Radiol. 1999, 72, 1164–1169. [Google Scholar] [CrossRef]
- Ripollés, T.; Martínez-Pérez, M.J.; Paredes, J.M.; Vizuete, J.; García-Martínez, E.; Jiménez-Restrepo, D.H. Contrast-enhanced ultrasound in the differentiation between phlegmon and abscess in Crohn’s disease and other abdominal conditions. Eur. J. Radiol. 2013, 82, e525–e531. [Google Scholar] [CrossRef] [PubMed]
- Drożdż, W.; Budzyński, P. Change in mechanical bowel obstruction demographic and etiological patterns during the past century: Observations from one health care institution. Arch. Surg. 2012, 147, 175–180. [Google Scholar] [CrossRef]
- Taylor, M.R.; Lalani, N. Adult small bowel obstruction. Acad. Emerg. Med. 2013, 20, 528–544. [Google Scholar] [CrossRef]
- Cocco, G.; Delli Pizzi, A.; Basilico, R.; Fabiani, S.; Taraschi, A.L.; Pascucci, L.; Boccatonda, A.; Catalano, O.; Schiavone, C. Imaging of gallbladder metastasis. Insights Imaging 2021, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Hastings, R.S.; Powers, R.D. Abdominal pain in the ED: A 35 year retrospective. Am. J. Emerg. Med. 2011, 29, 711–716. [Google Scholar] [CrossRef]
- Hollerweger, A.; Wüstner, M.; Dirks, K. Bowel Obstruction: Sonographic Evaluation. Ultraschall Med. 2015, 36, 216–235, quiz 36-8. [Google Scholar] [CrossRef]
- Gottlieb, M.; Peksa, G.D.; Pandurangadu, A.V.; Nakitende, D.; Takhar, S.; Seethala, R.R. Utilization of ultrasound for the evaluation of small bowel obstruction: A systematic review and meta-analysis. Am. J. Emerg. Med. 2018, 36, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Yu, Y.C.; Huang, Y.T.; Wu, Y.Y.; Wang, T.C.; Huang, W.C.; Yang, M.D.; Hsu, Y.P. Diagnostic accuracy of ultrasound for small bowel obstruction: A systematic review and meta-analysis. Eur. J. Radiol. 2021, 136, 109565. [Google Scholar] [CrossRef]
- Tan, H.L.; Shankar, K.R.; Ade-Ajayi, N.; Guelfand, M.; Kiely, E.M.; Drake, D.P.; De Bruyn, R.; McHugh, K.; Smith, A.J.; Morris, L.; et al. Reduction in visceral slide is a good sign of underlying postoperative viscero-parietal adhesions in children. J. Pediatr. Surg. 2003, 38, 714–716. [Google Scholar] [CrossRef]
- Ferris, B.; Bastian-Jordan, M.; Fenwick, J.; Hislop-Jambrich, J. Vascular assessment in small bowel obstruction: Can CT predict requirement for surgical intervention? Abdom. Radiol. 2021, 46, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, S.; Serra, N.; Lugarà, M.; Mercogliano, G.; Liguori, C.; Toro, G.; Somma, F.; Mandato, Y.; Guerra, M.V.; Sarti, G.; et al. Ultrasound Signs in the Diagnosis and Staging of Small Bowel Obstruction. Diagnostics 2020, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Di Mizio, R.; Grassi, R.; Marchese, E.; Basti, M.; Di Campli, G.; Catalano, O.; Rotondo, A.; Fanucci, A. ["Uncompensated" small bowel obstruction in adults. Ultrasonographic findings of free fluid between loops and its prognostic value]. Radiol. Med. 1995, 89, 787–791. [Google Scholar]
- Shokoohi, H.; Boniface, K.S.; Loesche, M.A.; Duggan, N.M.; King, J.B. Development of a nomogram to predict small bowel obstruction using point-of-care ultrasound in the emergency department. Am. J. Emerg. Med. 2020, 38, 2356–2360. [Google Scholar] [CrossRef]
- Grassi, R.; Romano, S.; D’Amario, F.; Giorgio Rossi, A.; Romano, L.; Pinto, F.; Di Mizio, R. The relevance of free fluid between intestinal loops detected by sonography in the clinical assessment of small bowel obstruction in adults. Eur. J. Radiol. 2004, 50, 5–14. [Google Scholar] [CrossRef]
- Pourmand, A.; Dimbil, U.; Drake, A.; Shokoohi, H. The Accuracy of Point-of-Care Ultrasound in Detecting Small Bowel Obstruction in Emergency Department. Emerg. Med. Int. 2018, 2018, 3684081. [Google Scholar] [CrossRef]
- Nazerian, P.; Tozzetti, C.; Vanni, S.; Bartolucci, M.; Gualtieri, S.; Trausi, F.; Vittorini, M.; Catini, E.; Cibinel, G.A.; Grifoni, S. Accuracy of abdominal ultrasound for the diagnosis of pneumoperitoneum in patients with acute abdominal pain: A pilot study. Crit. Ultrasound J. 2015, 7, 15. [Google Scholar] [CrossRef]
- Coppolino, F.; Gatta, G.; Di Grezia, G.; Reginelli, A.; Iacobellis, F.; Vallone, G.; Giganti, M.; Genovese, E. Gastrointestinal perforation: Ultrasonographic diagnosis. Crit. Ultrasound J. 2013, 5 (Suppl. S1), S4. [Google Scholar] [CrossRef] [PubMed]
- Pattison, P.; Jeffrey, R.B.; Mindelzun, R.E., Jr.; Sommer, F.G. Sonography of intraabdominal gas collections. AJR Am. J. Roentgenol. 1997, 169, 1559–1564. [Google Scholar] [CrossRef]
- Shokoohi, H.; Boniface, K.S.; Abell, B.M.; Pourmand, A.; Salimian, M. Ultrasound and Perforated Viscus; Dirty Fluid, Dirty Shadows, and Peritoneal Enhancement. Emergency 2016, 4, 101–105. [Google Scholar] [PubMed]
- Hefny, A.F.; Abu-Zidan, F.M. Sonographic diagnosis of intraperitoneal free air. J. Emerg. Trauma Shock 2011, 4, 511–513. [Google Scholar]
- Chadha, D.; Kedar, R.P.; Malde, H.M. Sonographic detection of pneumoperitoneum: An experimental and clinical study. Australas. Radiol. 1993, 37, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Karahan, O.I.; Kurt, A.; Yikilmaz, A.; Kahriman, G. New method for the detection of intraperitoneal free air by sonography: Scissors maneuver. J. Clin. Ultrasound 2004, 32, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Braccini, G.; Lamacchia, M.; Boraschi, P.; Bertellotti, L.; Marrucci, A.; Goletti, O.; Perri, G. Ultrasound versus plain film in the detection of pneumoperitoneum. Abdom. Imaging 1996, 21, 404–412. [Google Scholar] [CrossRef]
- Chen, S.C.; Yen, Z.S.; Wang, H.P.; Lin, F.Y.; Hsu, C.Y.; Chen, W.J. Ultrasonography is superior to plain radiography in the diagnosis of pneumoperitoneum. Br. J. Surg. 2002, 89, 351–354. [Google Scholar] [CrossRef]
- Woodring, J.H.; Heiser, M.J. Detection of pneumoperitoneum on chest radiographs: Comparison of upright lateral and posteroanterior projections. AJR Am. J. Roentgenol. 1995, 165, 45–47. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Lembcke, B.; Jenssen, C.; Hocke, M.; Ignee, A.; Hollerweger, A. Intestinal ultrasound in rare gastrointestinal diseases, update, part 1. Ultraschall Med. 2014, 35, 400–421. [Google Scholar] [CrossRef]
- van den Heijkant, T.C.; Aerts, B.A.; Teijink, J.A.; Buurman, W.A.; Luyer, M.D. Challenges in diagnosing mesenteric ischemia. World J. Gastroenterol. 2013, 19, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Balletta, M.; Vicari, S.; Hoxha, A.; Simioni, P.; Campello, E. The Journey through the Pathogenesis and Treatment of Venous Thromboembolism in Inflammatory Bowel Diseases: A Narrative Review. Semin. Thromb. Hemost. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- D’Ardes, D.; Boccatonda, A.; Cocco, G.; Fabiani, S.; Rossi, I.; Bucci, M.; Guagnano, M.T.; Schiavone, C.; Cipollone, F. Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship. World J. Gastroenterol. 2022, 28, 1102–1112. [Google Scholar] [CrossRef]
- Luther, B.; Mamopoulos, A.; Lehmann, C.; Klar, E. The Ongoing Challenge of Acute Mesenteric Ischemia. Visc. Med. 2018, 34, 217–223. [Google Scholar] [CrossRef]
- Walker, T.G. Mesenteric ischemia. Semin. Interv. Radiol. 2009, 26, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Genovese, E.; Cappabianca, S.; Iacobellis, F.; Berritto, D.; Fonio, P.; Coppolino, F.; Grassi, R. Intestinal Ischemia: US-CT findings correlations. Crit. Ultrasound J. 2013, 5 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Danse, E.M.; Van Beers, B.E.; Goffette, P.; Dardenne, A.N.; Laterre, P.F.; Pringot, J. Acute intestinal ischemia due to occlusion of the superior mesenteric artery: Detection with Doppler sonography. J. Ultrasound Med. 1996, 15, 323–326. [Google Scholar] [CrossRef]
- Hamada, T.; Yamauchi, M.; Tanaka, M.; Hashimoto, Y.; Nakai, K.; Suenaga, K. Prospective evaluation of contrast-enhanced ultrasonography with advanced dynamic flow for the diagnosis of intestinal ischaemia. Br. J. Radiol. 2007, 80, 603–608. [Google Scholar] [CrossRef]
- Martinez, J.P.; Hogan, G.J. Mesenteric ischemia. Emerg. Med. Clin. North. Am. 2004, 22, 909–928. [Google Scholar] [CrossRef]
- Hata, J.; Kamada, T.; Haruma, K.; Kusunoki, H. Evaluation of bowel ischemia with contrast-enhanced US: Initial experience. Radiology 2005, 236, 712–715. [Google Scholar] [CrossRef]
- Wiesner, W.; Khurana, B.; Ji, H.; Ros, P.R. CT of acute bowel ischemia. Radiology 2003, 226, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.J.; Feuerstadt, P.; Blaszka, M.C. Anatomic patterns, patient characteristics, and clinical outcomes in ischemic colitis: A study of 313 cases supported by histology. Am. J. Gastroenterol. 2010, 105, 2245–2252, quiz 53. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Juan, M.D.R.; Ripollés, T.; Martí-Bonmatí, L.; Martínez, M.J.; Simó, L.; Gómez, D.; Revert, R. Predictors of severity in ischemic colitis: Usefulness of early ultrasonography. Eur. J. Radiol. 2017, 96, 21–26. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Ripolles, T.; Martinez, M.J.; Bartumeus, P.; Blay, J.; López, A. Positive Predictive Value of Abdominal Sonography in the Diagnosis of Ischemic Colitis. Ultrasound Int. Open 2015, 1, E41–E45. [Google Scholar] [CrossRef] [PubMed]
- Danse, E.M.; Van Beers, B.E.; Jamart, J.; Hoang, P.; Laterre, P.F.; Thys, F.C.; Kartheuser, A.; Pringot, J. Prognosis of ischemic colitis: Comparison of color doppler sonography with early clinical and laboratory findings. AJR Am. J. Roentgenol. 2000, 175, 1151–1154. [Google Scholar] [CrossRef]
- Ripollés, T.; Simó, L.; Martínez-Pérez, M.J.; Pastor, M.R.; Igual, A.; López, A. Sonographic findings in ischemic colitis in 58 patients. AJR Am. J. Roentgenol. 2005, 184, 777–785. [Google Scholar] [CrossRef]
- Kricun, B.J.; Horrow, M.M. Pneumoperitoneum. Ultrasound Q. 2012, 28, 137–138. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccatonda, A.; D’Ardes, D.; Tallarico, V.; Vicari, S.; Bartoli, E.; Vidili, G.; Guagnano, M.T.; Cocco, G.; Cipollone, F.; Schiavone, C.; et al. Gastrointestinal Ultrasound in Emergency Setting. J. Clin. Med. 2023, 12, 799. https://doi.org/10.3390/jcm12030799
Boccatonda A, D’Ardes D, Tallarico V, Vicari S, Bartoli E, Vidili G, Guagnano MT, Cocco G, Cipollone F, Schiavone C, et al. Gastrointestinal Ultrasound in Emergency Setting. Journal of Clinical Medicine. 2023; 12(3):799. https://doi.org/10.3390/jcm12030799
Chicago/Turabian StyleBoccatonda, Andrea, Damiano D’Ardes, Viola Tallarico, Susanna Vicari, Elena Bartoli, Gianpaolo Vidili, Maria Teresa Guagnano, Giulio Cocco, Francesco Cipollone, Cosima Schiavone, and et al. 2023. "Gastrointestinal Ultrasound in Emergency Setting" Journal of Clinical Medicine 12, no. 3: 799. https://doi.org/10.3390/jcm12030799
APA StyleBoccatonda, A., D’Ardes, D., Tallarico, V., Vicari, S., Bartoli, E., Vidili, G., Guagnano, M. T., Cocco, G., Cipollone, F., Schiavone, C., & Accogli, E. (2023). Gastrointestinal Ultrasound in Emergency Setting. Journal of Clinical Medicine, 12(3), 799. https://doi.org/10.3390/jcm12030799








