Wnt7b: Is It an Important Factor in the Bone Formation Process after Calvarial Damage?
Abstract
1. Background
2. Methods
2.1. Experimental Animals
2.2. Main Instruments and Reagents
2.3. Model Establishment
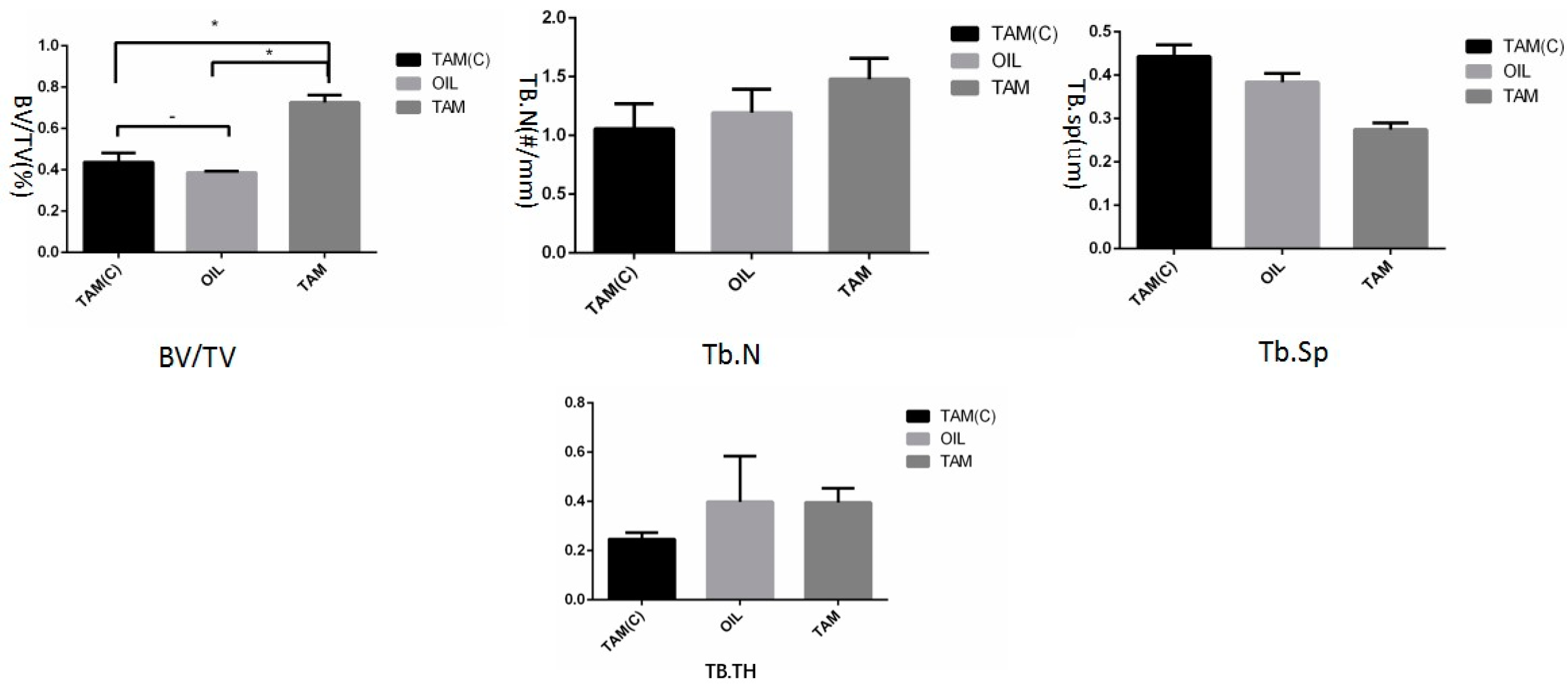
2.4. Micro-CT Scan
2.5. Tissue RNA Extraction and Quantitative PCR
2.6. Aniline Blue Staining
2.7. Statistical Analysis
3. Results
3.1. Establishment of Mouse Skull Defect Model
3.2. Micro-CT Analysis
3.3. QPCR Detection of Wnt7b Expression in Each Group
3.4. Bone Mass Index Test Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letic-Gavrilovic, A.; Piattelli, A.; Abe, K. Nerve growth factor beta(NGF beta) delivery via a collagen/hydroxyapatite (Col/HAp) composite and its effects on new bone ingrowth. J. Mater. Sci. Mater. Med. 2003, 14, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Pršo, I.B.; Kocjan, W.; Šimic, H.; Brumini, G.; Pezelj-Ribaric, S.; Borcic, J.; Ferreri, S.; Karlovic, I.M. Tumor necrosis factor-alpha and interleukin 6 in human periapical lesions. Mediat. Inflamm. 2007, 2007, 38210. [Google Scholar]
- Papakostidis, C.; Bhandari, M.; Giannoudis, P.V. Distraction osteogenesis in the treatment of long bone defects of the lower limbs: Effectiveness, complications and clinical results; a systematic review and meta-analysis. Bone Jt. J. 2013, 95, 1673–1680. [Google Scholar] [CrossRef]
- Carulli, C.; Matassi, F.; Civinini, R.; Innocenti, M. Tissue engineering applications in the management of bone loss. Clinical cases in mineral and bone metabolism. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2013, 10, 22–25. [Google Scholar]
- Masquelet, A.C.; Begue, T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. North Am. 2010, 41, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, B.; Rocco, T.; Esposito, M.T.; Cantilena, B.; Gargiulo, S.; Greco, A.; Montanaro, D.; Brunetti, A.; Pastore, L. Thymidine kinase-mediated shut down of bone morphogenetic protein-4 expression allows regulated bone production. Curr. Gene Ther. 2013, 13, 202–210. [Google Scholar] [CrossRef]
- Einhorn, T.A. Clinically applied models of bone regeneration in tissue engineering research. Clin. Orthop. Relat. Res. 1999, 367, S59–S67. [Google Scholar] [CrossRef]
- Yilgor, P.; Tuzlakoglu, K.; Reis, R.L.; Hasirci, N.; Hasirci, V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 2009, 30, 3551–3559. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Kontakis, G.; Giannoudis, P.V. Efficacy of minimally invasive techniques for enhancement of fracture healing: Evidence today. Int. Orthop. 2010, 34, 3–12. [Google Scholar] [CrossRef]
- Gong, Y.; Slee, R.B.; Fukai, N.; Rawadi, G.; Roman-Roman, S.; Reginato, A.M.; Wang, H.; Cundy, T.; Glorieux, F.H.; Lev, D.; et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 2001, 107, 513–523. [Google Scholar] [CrossRef]
- Little, R.D.; Recker, R.R.; Johnson, M.L. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 2002, 347, 943–944. [Google Scholar] [PubMed]
- Little, R.D.; Folz, C.; Manning, S.P.; Swain, P.M.; Zhao, S.-C.; Eustace, B.; Lappe, M.M.; Spitzer, L.; Zweier, S.; Braunschweiger, K.; et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 2002, 70, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Wu, W.; Li, Y.; Hoppe, D.; Stannek, P.; Glinka, A.; Niehrs, C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 2001, 411, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Semënov, M.V.; Tamai, K.; Brott, B.K.; Kühl, M.; Sokol, S.; He, X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. CB 2001, 11, 951–961. [Google Scholar] [CrossRef]
- Semënov, M.; Tamai, K.; He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005, 280, 26770–26775. [Google Scholar] [CrossRef]
- Bourhis, E.; Wang, W.; Tam, C.; Hwang, J.; Zhang, Y.; Spittler, D.; Huang, O.W.; Gong, Y.; Estevez, A.; Zilberleyb, I.; et al. Wnt antagonists bind through a short peptide to the first β-propeller domain of LRP5/6. Structure 2011, 19, 1433–1442. [Google Scholar] [CrossRef]
- Maupin, K.A.; Droscha, C.J.; Williams, B.O. A Comprehensive Overview of Skeletal Phenotypes Associated with Alterations in Wnt/β-catenin Signaling in Humans and Mice. Bone Res. 2013, 1, 27–71. [Google Scholar] [CrossRef]
- Parr, B.A.; Cornish, V.A.; Cybulsky, M.I.; McMahon, A.P. Wnt7b regulates placental development in mice. Dev. Biol. 2001, 237, 324–332. [Google Scholar] [CrossRef]
- Shu, W.; Jiang, Y.Q.; Lu, M.M.; Morrisey, E.E. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 2002, 129, 4831–4842. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Liu, W.; Maye, P.; Zhang, J.; Zhang, Y.; Hurley, M.M.; Guo, C.; Boskey, A.; Sun, L.; et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat. Genet. 2005, 37, 945–952. [Google Scholar] [CrossRef]
- Tu, X.; Joeng, K.S.; Nakayama, K.I.; Nakayama, K.; Rajagopal, J.; Carroll, T.J.; McMahon, A.P.; Long, F. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev. Cell 2007, 12, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tu, X.; Esen, E.; Joeng, K.S.; Lin, C.; Arbeit, J.M.; Rüegg, M.A.; Hall, M.N.; Ma, L.; Long, F. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. 2014, 10, e1004145. [Google Scholar] [CrossRef] [PubMed]
- Sauer, B. Inducible gene targeting in mice using the Cre/lox system. Methods 1998, 14, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Kobayashi, T.; Selig, M.K.; Torrekens, S.; Roth, S.I.; Mackem, S.; Carmeliet, G.; Kronenberg, H.M. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 2010, 19, 329–344. [Google Scholar] [CrossRef]
- Indra, A.K.; Warot, X.; Brocard, J.; Bornert, J.M.; Xiao, J.H.; Chambon, P.; Metzger, D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: Comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999, 27, 4324–4327. [Google Scholar] [CrossRef]
- Kim, J.E.; Nakashima, K.; de Crombrugghe, B. Transgenic mice expressing a ligand-inducible cre recombinase in osteoblasts and odontoblasts: A new tool to examine physiology and disease of postnatal bone and tooth. Am. J. Pathol. 2004, 165, 1875–1882. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 1995, 15, 1858–1869. [Google Scholar] [CrossRef]
- Rossert, J.; Eberspaecher, H.; de Crombrugghe, B. Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J. Cell Biol. 1995, 129, 1421–1432. [Google Scholar] [CrossRef]
- Bedore, J.; Quesnel, K.; Quinonez, D.; Séguin, C.A.; Leask, A. Targeting the annulus fibrosus of the intervertebral disc: Col1a2-Cre(ER)T mice show specific activity of Cre recombinase in the outer annulus fibrosus. J. Cell Commun. Signal. 2016, 10, 137–142. [Google Scholar] [CrossRef]
- Aalami, O.O.; Nacamuli, R.P.; Lenton, K.A.; Cowan, C.M.; Fang, T.D.; Fong, K.D.; Shi, Y.-Y.; Song, H.M.; Sahar, D.E.; Longaker, M.T. Applications of a mouse model of calvarial healing: Differences in regenerative abilities of juveniles and adults. Plast. Reconstr. Surg. 2004, 114, 713–720. [Google Scholar] [CrossRef]
- Levi, B.; James, A.W.; Nelson, E.R.; Li, S.; Peng, M.; Commons, G.W.; Lee, M.; Wu, B.; Longaker, M.T. Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine Hedgehog signaling with calvarial osteoblasts. Stem Cells Dev. 2011, 20, 243–257. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Behr, B.; Panetta, N.J.; Longaker, M.T.; Quarto, N. Different endogenous threshold levels of Fibroblast Growth Factor-ligands determine the healing potential of frontal and parietal bones. Bone 2010, 47, 281–294. [Google Scholar] [CrossRef]
- Minear, S.; Leucht, P.; Jiang, J.; Liu, B.; Zeng, A.; Fuerer, C.; Nusse, R.; Helms, J.A. Wnt proteins promote bone regeneration. Sci. Transl. Med. 2010, 2, 29ra30. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Mulliken, J.B.; Glowacki, J. Induced osteogenesis for repair and construction in the craniofacial region. Plast. Reconstr. Surg. 1980, 65, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Downes, S.; Watts, D.C. Evaluation of critical size defects of mouse calvarial bone: An organ culture study. Microsc. Res. Tech. 2010, 73, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, R.S.; Freeman, E. Use of wounds in the parietal bone of the rat for evaluating bone marrow for grafting into periodontal defects. J. Periodontal Res. 1974, 9, 39–43. [Google Scholar] [CrossRef]
- Elefteriou, F.; Yang, X. Genetic mouse models for bone studies—Strengths and limitations. Bone 2011, 49, 1242–1254. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, B.; Pei, J.; Gu, S. Wnt7b: Is It an Important Factor in the Bone Formation Process after Calvarial Damage? J. Clin. Med. 2023, 12, 800. https://doi.org/10.3390/jcm12030800
Feng B, Pei J, Gu S. Wnt7b: Is It an Important Factor in the Bone Formation Process after Calvarial Damage? Journal of Clinical Medicine. 2023; 12(3):800. https://doi.org/10.3390/jcm12030800
Chicago/Turabian StyleFeng, Bo, Jun Pei, and Shensheng Gu. 2023. "Wnt7b: Is It an Important Factor in the Bone Formation Process after Calvarial Damage?" Journal of Clinical Medicine 12, no. 3: 800. https://doi.org/10.3390/jcm12030800
APA StyleFeng, B., Pei, J., & Gu, S. (2023). Wnt7b: Is It an Important Factor in the Bone Formation Process after Calvarial Damage? Journal of Clinical Medicine, 12(3), 800. https://doi.org/10.3390/jcm12030800





