Influence of the Tibial Tunnel Angle and Posterior Tibial Slope on “Killer Turn” during Posterior Cruciate Ligament Reconstruction: A Three-Dimensional Finite Element Analysis
Abstract
1. Introduction
2. Methods
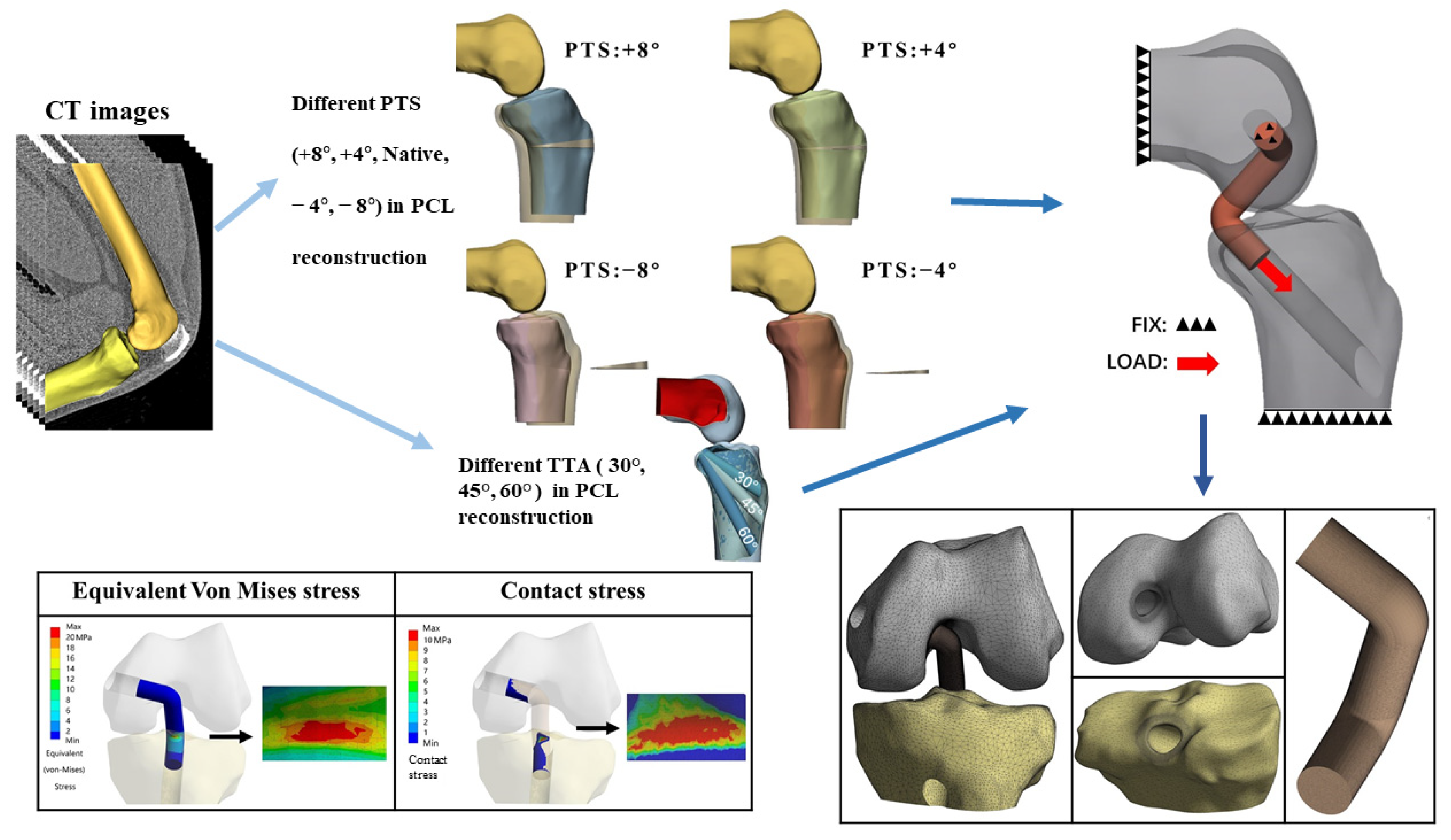
2.1. Data Acquisition
2.2. 3D Reconstruction of the Knee Joint and FEA Modeling
2.2.1. Preparation for PCL Anatomical Footprints, Bone Tunnels, and PCL Grafts
2.2.2. Preparation for aOW-HTO
2.3. Material Properties
2.4. Boundary Conditions and Loads
3. Results
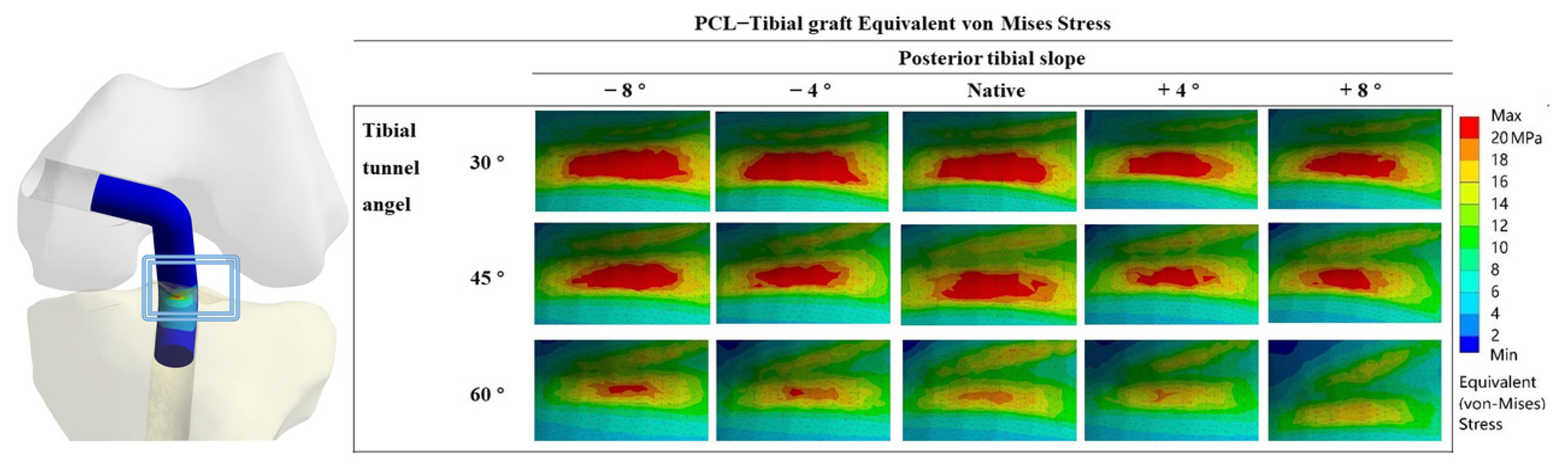
3.1. Equivalent VMS in the PCL Graft
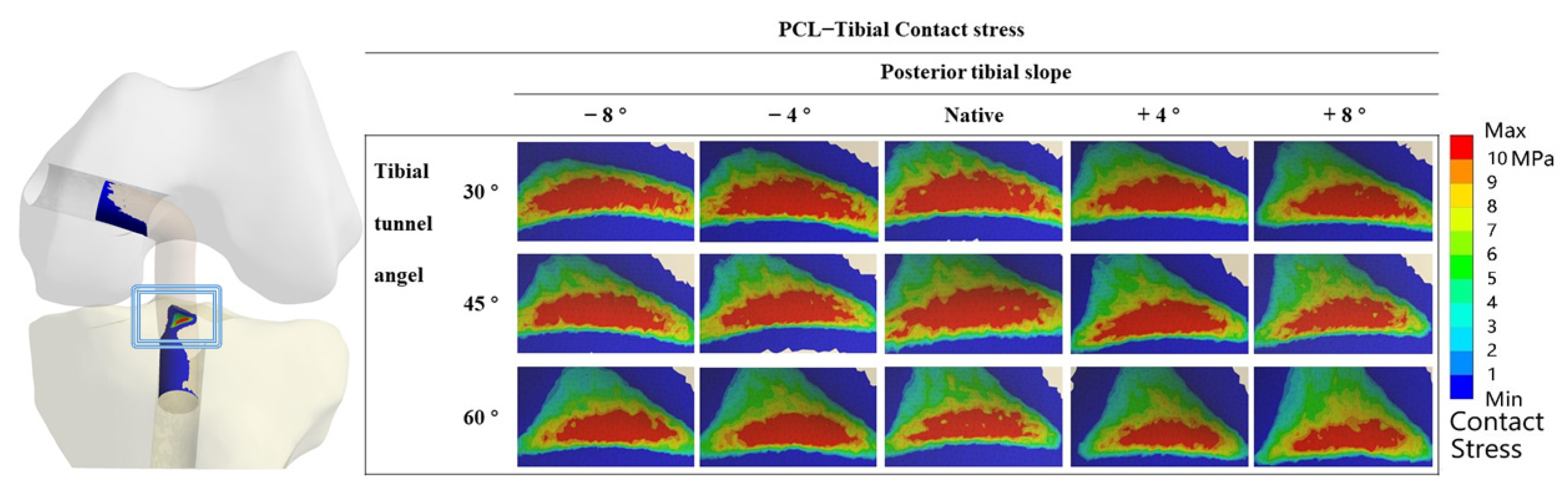
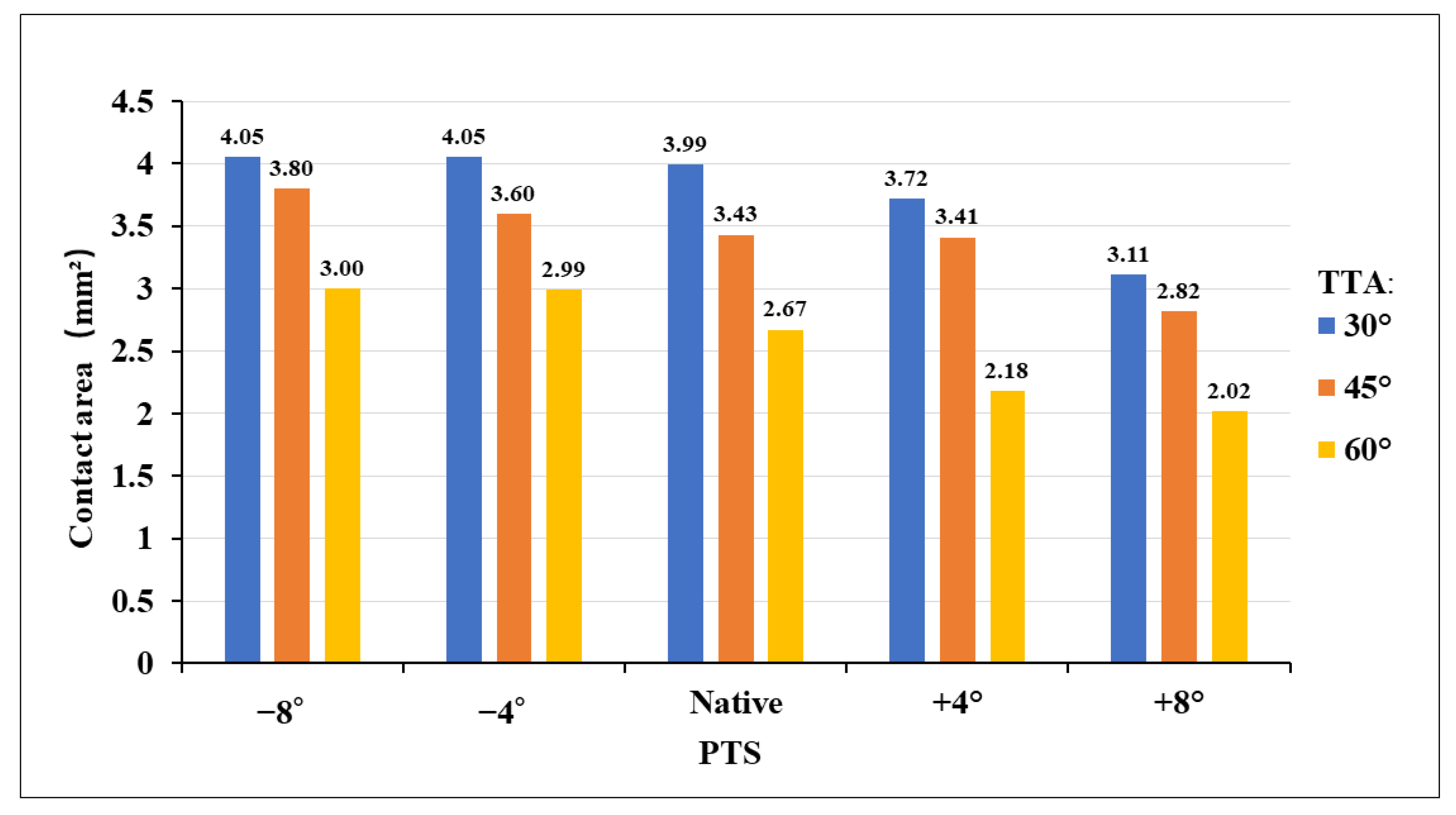
3.2. Contact Stress in “Killer Turn”
4. Discussion
4.1. Different TTAs and PTSs during PCL Reconstruction
4.2. High-Contact Stress Area
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Logterman, S.L.; Wydra, F.B.; Frank, R.M. Posterior Cruciate Ligament: Anatomy and Biomechanics. Curr. Rev. Musculoskelet. Med. 2018, 11, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Gwinner, C.; Weiler, A.; Roider, M.; Schaefer, F.M.; Jung, T.M. Tibial Slope Strongly Influences Knee Stability After Posterior Cruciate Ligament Reconstruction: A Prospective 5- to 15-Year Follow-up. Am. J. Sports Med. 2017, 45, 355–361. [Google Scholar] [CrossRef]
- Sanders, T.L.; Pareek, A.; Barrett, I.J.; Kremers, H.M.; Bryan, A.J.; Stuart, M.J.; Levy, B.A.; Krych, A.J. Incidence and long-term follow-up of isolated posterior cruciate ligament tears. Knee. Surg. Sports Traumatol. Arthrosc. 2017, 25, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Schroven, W.; Vles, G.; Verhaegen, J.; Roussot, M.; Bellemans, J.; Konan, S. Operative management of isolated posterior cruciate ligament injuries improves stability and reduces the incidence of secondary osteoarthritis: A systematic review. Knee. Surg. Sports. Traumatol. Arthrosc. 2022, 30, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Winkler, P.W.; Zsidai, B.; Wagala, N.N.; Hughes, J.D.; Horvath, A.; Senorski, E.H.; Samuelsson, K.; Musahl, V. Evolving evidence in the treatment of primary and recurrent posterior cruciate ligament injuries, part 2: Surgical techniques, outcomes and rehabilitation. Knee. Surg. Sports Traumatol. Arthrosc. 2021, 29, 682–693. [Google Scholar] [CrossRef]
- Noyes, F.R.; Barber-Westin, S.D. Posterior cruciate ligament revision reconstruction, part 1: Causes of surgical failure in 52 consecutive operations. Am. J. Sports Med. 2005, 33, 646–654. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, Y.B.; Rhee, S.M.; Lee, H.J.; Jung, H.J. Revision Posterior Cruciate Ligament Reconstruction with a Modified Tibial-Inlay Double-Bundle Technique. JBJS. Essent. Surg. Tech. 2014, 4, e1. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, C.A.; Matava, M.J. Clinical results of arthroscopic single-bundle transtibial posterior cruciate ligament reconstruction: A systematic review. Am. J. Sports Med. 2011, 39, 425–434. [Google Scholar] [CrossRef]
- Norbakhsh, S.T.; Zafarani, Z.; Najafi, A.; Aslani, H. Arthroscopic posterior cruciate ligament reconstruction by using hamstring tendon autograft and transosseous screw fixation: Minimal 3 years follow-up. Arch. Orthop. Trauma. Surg. 2014, 134, 1723–1730. [Google Scholar] [CrossRef]
- Huang, T.W.; Wang, C.J.; Weng, L.H.; Chan, Y.S. Reducing the “killer turn” in posterior cruciate ligament reconstruction. Arthroscopy 2003, 19, 712–716. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, D.H.; Kim, H.J.; Ahn, H.S.; Lee, T.H.; Hwang, S.C. Posterior Cruciate Ligament Reconstruction With Transtibial or Tibial Inlay Techniques: A Meta-analysis of Biomechanical and Clinical Outcomes. Am. J. Sports Med. 2018, 46, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Song, G.; Li, X.; Feng, H. The mechanism of “killer turn” causing residual laxity after transtibial posterior cruciate ligament reconstruction. Asia. Pac. J. Sports. Med. Arthrosc. Rehabil. Technol. 2016, 3, 13–18. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, X.; Ma, C.; Wu, H.; Li, R.; Wang, H.; Han, H.; Xia, Y. Evaluation of the permissible maximum angle of the tibial tunnel in transtibial anatomic posterior cruciate ligament reconstruction by computed tomography. Arch. Orthop. Trauma. Surg. 2019, 139, 547–552. [Google Scholar] [CrossRef]
- Kim, S.J.; Chang, J.H.; Kang, Y.H.; Song, D.H.; Park, K.Y. Clinical comparison of anteromedial versus anterolateral tibial tunnel direction for transtibial posterior cruciate ligament reconstruction: 2 to 8 years’ follow-up. Am. J. Sports. Med. 2009, 37, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, Z.; Zhang, K.; Pan, X.; Huang, X.; Li, J.; Li, Q. Lower Tibial Tunnel Placement in Isolated Posterior Cruciate Ligament Reconstruction: Clinical Outcomes and Quantitative Radiological Analysis of the Killer Turn. Orthop. J. Sports. Med. 2020, 8, 2325967120923950. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Wolfert, A.; Zantop, T.; Eggers, A.K.; Raschke, M.; Petersen, W. Reducing the “killer turn” in posterior cruciate ligament reconstruction by fixation level and smoothing the tibial aperture. Arthroscopy 2007, 23, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.J.; Ramezanpoor Asl, A.; Jafari, H.; Asgari, H.; Kaseb, M.H.; Dehghanifiroozabadi, M.J. Tibial Tunnel Preparation in Posterior Cruciate Ligament (PCL) Reconstruction. A Technical Tip to Lessen the Stress. Arch. Bone. Jt. Surg. 2019, 7, 463–468. [Google Scholar] [PubMed]
- Liu, F.; Zhang, S.; Xiao, Y.; Feng, X.; Liang, Z.; Leung, F.; Chen, B. Stenotic intercondylar notch is not a risk factor for posterior cruciate ligament rupture: A morphological analyses using magnetic resonance imaging. Knee. Surg. Sports Traumatol. Arthrosc. 2022, 30, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- van Kuijk, K.S.R.; Reijman, M.; Bierma-Zeinstra, S.M.A.; Waarsing, J.H.; Meuffels, D.E. Posterior cruciate ligament injury is influenced by intercondylar shape and size of tibial eminence. Bone Joint. J. 2019, 101, 1058–1062. [Google Scholar] [CrossRef]
- Bernhardson, A.S.; Aman, Z.S.; DePhillipo, N.N.; Dornan, G.J.; Storaci, H.W.; Brady, A.W.; Nakama, G.; LaPrade, R.F. Tibial Slope and Its Effect on Graft Force in Posterior Cruciate Ligament Reconstructions. Am. J. Sports Med. 2019, 47, 1168–1174. [Google Scholar] [CrossRef]
- Schatka, I.; Weiler, A.; Jung, T.M.; Walter, T.C.; Gwinner, C. High tibial slope correlates with increased posterior tibial translation in healthy knees. Knee. Surg. Sports Traumatol. Arthrosc. 2018, 26, 2697–2703. [Google Scholar] [CrossRef] [PubMed]
- Giffin, J.R.; Stabile, K.J.; Zantop, T.; Vogrin, T.M.; Woo, S.L.; Harner, C.D. Importance of tibial slope for stability of the posterior cruciate ligament deficient knee. Am. J. Sports Med. 2007, 35, 1443–1449. [Google Scholar] [CrossRef]
- Hees, T.; Petersen, W. Anterior Closing-Wedge Osteotomy for Posterior Slope Correction. Arthrosc. Tech. 2018, 7, e1079–e1087. [Google Scholar] [CrossRef] [PubMed]
- Queiros, C.M.; Abreu, F.G.; Moura, J.L.; de Abreu, G.V.; Vieira, T.D.; Helfer, L.; Sonnery-Cottet, B. Anterior Closing-Wedge Osteotomy for Posterior Slope Correction With Tibial Tubercle Preservation. Arthrosc. Tech. 2019, 8, e1105–e1109. [Google Scholar] [CrossRef] [PubMed]
- Kanakamedala, A.C.; Gipsman, A.; Lowe, D.T.; Strauss, E.J.; Alaia, M.J. Combined Anterior Opening-Wedge High Tibial Osteotomy and Tibial Tubercle Osteotomy with Posterior Cruciate Ligament Reconstruction. Arthrosc. Tech. 2022, 11, e601–e608. [Google Scholar] [CrossRef]
- Martineau, P.A.; Fening, S.D.; Miniaci, A. Anterior opening wedge high tibial osteotomy: The effect of increasing posterior tibial slope on ligament strain. Can. J. Surg. J. Can. De Chir. 2010, 53, 261–267. [Google Scholar]
- Kennedy, N.I.; LaPrade, R.F.; Goldsmith, M.T.; Faucett, S.C.; Rasmussen, M.T.; Coatney, G.A.; Engebretsen, L.; Wijdicks, C.A. Posterior cruciate ligament graft fixation angles, part 1: Biomechanical evaluation for anatomic single-bundle reconstruction. Am. J. Sports Med. 2014, 42, 2338–2345. [Google Scholar] [CrossRef]
- Okoroafor, U.C.; Saint-Preux, F.; Gill, S.W.; Bledsoe, G.; Kaar, S.G. Nonanatomic Tibial Tunnel Placement for Single-Bundle Posterior Cruciate Ligament Reconstruction Leads to Greater Posterior Tibial Translation in a Biomechanical Model. Arthroscopy 2016, 32, 1354–1358. [Google Scholar] [CrossRef]
- Johannsen, A.M.; Anderson, C.J.; Wijdicks, C.A.; Engebretsen, L.; LaPrade, R.F. Radiographic Landmarks for Tunnel Positioning in Posterior Cruciate Ligament Reconstructions. Am. J. Sports Med. 2013, 41, 35–42. [Google Scholar] [CrossRef]
- Van Hoof, T.; Cromheecke, M.; Tampere, T.; D’Herde, K.; Victor, J.; Verdonk, P.C. The posterior cruciate ligament: A study on its bony and soft tissue anatomy using novel 3D CT technology. Knee. Surg. Sports. Traumatol. Arthrosc. 2013, 21, 1005–1010. [Google Scholar] [CrossRef]
- Kim, C.; Baker, D.; Albers, B.; Kaar, S.G. An Anatomically Placed Tibial Tunnel does not Completely Surround a Simulated PCL Reconstruction Graft in the Proximal Tibia. J. Knee. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.B.; Wang, H.; Tie, K.; Mohammed, A.; Qi, Y.J. Arthroscopic fixation of an avulsion fracture of the tibia involving the posterior cruciate ligament: A modified technique in a series of 22 cases. Bone. Joint. J. 2015, 97-b, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Yi, A.; Kleiner, M.T.; Lorenzana, D.; Koniceck, J.; Charlton, T.; Rick Hatch, G.F., 3rd. Optimal femoral tunnel positioning in posterior cruciate ligament reconstruction using outside-in drilling. Arthroscopy 2015, 31, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.P.Y.; Merican, A.M.; Hashim, M.S.; Abbas, A.A.; Chan, C.K.; Mohamad, J.A. Three-Dimensional Computed Tomography Analysis of the Posterior Tibial Slope in 100 Knees. J. Arthroplast. 2017, 32, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.S.; Russe, K.; Lampakis, G.; Strobel, M.J. Reliability of stress radiography for evaluation of posterior knee laxity. Am. J. Sports Med. 2005, 33, 502–506. [Google Scholar] [CrossRef]
- Shelburne, K.B.; Kim, H.J.; Sterett, W.I.; Pandy, M.G. Effect of posterior tibial slope on knee biomechanics during functional activity. J. Orthop. Res. 2011, 29, 223–231. [Google Scholar] [CrossRef]
- Zhang, K.; Li, L.; Yang, L.; Shi, J.; Zhu, L.; Liang, H.; Wang, X.; Yang, X.; Jiang, Q. Effect of degenerative and radial tears of the meniscus and resultant meniscectomy on the knee joint: A finite element analysis. J. Orthop. Translat. 2019, 18, 20–31. [Google Scholar] [CrossRef]
- Weiss, J.A.; Maker, B.N.; Govindjee, S. Finite element implementation of incompressible, transversely isotropic hyperelasticity. Comput. Methods Appl. Mech. Eng. 1996, 135, 107–128. [Google Scholar] [CrossRef]
- Pena, E.; Martinez, M.A.; Calvo, B.; Palanca, D.; Doblare, M. A finite element simulation of the effect of graft stiffness and graft tensioning in ACL reconstruction. Clin. Biomech. 2005, 20, 636–644. [Google Scholar] [CrossRef]
- Bae, J.Y.; Kim, G.H.; Seon, J.K.; Jeon, I. Finite element study on the anatomic transtibial technique for single-bundle anterior cruciate ligament reconstruction. Med. Biol. Eng. Comput. 2016, 54, 811–820. [Google Scholar] [CrossRef]
- Li, G.; Zayontz, S.; Most, E.; DeFrate, L.E.; Suggs, J.F.; Rubash, H.E. In situ forces of the anterior and posterior cruciate ligaments in high knee flexion: An in vitro investigation. J. Orthop. Res. 2004, 22, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Shacham, S.; Castel, D.; Gefen, A. Measurements of the static friction coefficient between bone and muscle tissues. J. Biomech. Eng. 2010, 132, 084502. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Z.; Jiang, W.B.; Song, T.W.; Chi, Y.Y.; Xu, Q.; Liu, C.; Tang, W.; Xu, F.; Zhou, J.X.; Yu, S.B.; et al. Architecture of the cancellous bone in human proximal tibia based on P45 sectional plastinated specimens. Surg. Radiol. Anat. 2021, 43, 2055–2069. [Google Scholar] [CrossRef] [PubMed]
- Muggli, D.S.; Burkoth, A.K.; Anseth, K.S. Crosslinked polyanhydrides for use in orthopedic applications: Degradation behavior and mechanics. J. Biomed. Mater. Res. 1999, 46, 271–278. [Google Scholar] [CrossRef]
- Winkler, P.W.; Wagala, N.N.; Carrozzi, S.; Nazzal, E.M.; Fox, M.A.; Hughes, J.D.; Lesniak, B.P.; Vyas, D.; Rabuck, S.J.; Irrgang, J.J.; et al. Low posterior tibial slope is associated with increased risk of PCL graft failure. Knee. Surg. Sports Traumatol. Arthrosc. 2022, 30, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Youm, Y.S.; Cho, S.D.; Jung, S.H.; Bae, M.H.; Park, S.J.; Kim, H.W. Does Posterior Tibial Slope Affect Graft Rupture Following Anterior Cruciate Ligament Reconstruction? Arthroscopy 2018, 34, 2152–2155. [Google Scholar] [CrossRef] [PubMed]
- Bernhardson, A.S.; DePhillipo, N.N.; Daney, B.T.; Kennedy, M.I.; Aman, Z.S.; LaPrade, R.F. Posterior Tibial Slope and Risk of Posterior Cruciate Ligament Injury. Am. J. Sports Med. 2019, 47, 312–317. [Google Scholar] [CrossRef]
- Seo, S.S.; Kim, O.G.; Seo, J.H.; Kim, D.H.; Kim, Y.G.; Lee, I.S. Complications and Short-Term Outcomes of Medial Opening Wedge High Tibial Osteotomy Using a Locking Plate for Medial Osteoarthritis of the Knee. Knee. Surg. Relat. Res. 2016, 28, 289–296. [Google Scholar] [CrossRef]
- Tachibana, Y.; Tanaka, Y.; Kinugasa, K.; Hamada, M.; Horibe, S. Sequential Changes in Posterior Tibial Translation After Posterior Cruciate Ligament Reconstruction: Risk Factors for Residual Posterior Sagging. Orthop. J. Sports. Med. 2021, 9, 23259671211009805. [Google Scholar] [CrossRef]
- Kwon, J.H.; Han, J.H.; Jo, D.Y.; Park, H.J.; Lee, S.Y.; Bhandare, N.; Suh, D.W.; Nha, K.W. Tunnel volume enlargement after posterior cruciate ligament reconstruction: Comparison of achilles allograft with mixed autograft/allograft--a prospective computed tomography study. Arthroscopy 2014, 30, 326–334. [Google Scholar] [CrossRef]
- Wen, C.Y.; Qin, L.; Lee, K.M.; Wong, M.W.; Chan, K.M. Grafted tendon healing in tibial tunnel is inferior to healing in femoral tunnel after anterior cruciate ligament reconstruction: A histomorphometric study in rabbits. Arthroscopy 2010, 26, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.Z.; Zhang, J.; Song, G.Y.; Li, X.; Feng, H. What Role Does Low Bone Mineral Density Play in the “Killer Turn” Effect after Transtibial Posterior Cruciate Ligament Reconstruction? Orthop. Surg. 2016, 8, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, F.; Yoneta, K.; Miyaji, T.; Kidera, K.; Yonekura, A.; Osaki, M.; Gamada, K. Knee kinematics of severe medial knee osteoarthritis showed tibial posterior translation and external rotation: A cross-sectional study. Aging Clin. Exp. Res. 2020, 32, 1767–1775. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Yokoe, T.; Ouchi, K.; Tajima, T.; Chosa, E. Influence of the Tibial Tunnel Angle and Posterior Tibial Slope on “Killer Turn” during Posterior Cruciate Ligament Reconstruction: A Three-Dimensional Finite Element Analysis. J. Clin. Med. 2023, 12, 805. https://doi.org/10.3390/jcm12030805
Yang F, Yokoe T, Ouchi K, Tajima T, Chosa E. Influence of the Tibial Tunnel Angle and Posterior Tibial Slope on “Killer Turn” during Posterior Cruciate Ligament Reconstruction: A Three-Dimensional Finite Element Analysis. Journal of Clinical Medicine. 2023; 12(3):805. https://doi.org/10.3390/jcm12030805
Chicago/Turabian StyleYang, Fan, Takuji Yokoe, Koki Ouchi, Takuya Tajima, and Etsuo Chosa. 2023. "Influence of the Tibial Tunnel Angle and Posterior Tibial Slope on “Killer Turn” during Posterior Cruciate Ligament Reconstruction: A Three-Dimensional Finite Element Analysis" Journal of Clinical Medicine 12, no. 3: 805. https://doi.org/10.3390/jcm12030805
APA StyleYang, F., Yokoe, T., Ouchi, K., Tajima, T., & Chosa, E. (2023). Influence of the Tibial Tunnel Angle and Posterior Tibial Slope on “Killer Turn” during Posterior Cruciate Ligament Reconstruction: A Three-Dimensional Finite Element Analysis. Journal of Clinical Medicine, 12(3), 805. https://doi.org/10.3390/jcm12030805




