Effectiveness of a Primary Care Multidisciplinary Treatment for Patients with Chronic Pain Compared with Treatment as Usual
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Sample Size
2.3. Participants
2.4. Data Management
2.5. Intervention Program
2.5.1. Diagnostic Process
- a.
- Pre-intake: After being referred for a multidisciplinary assessment, patients were asked to fill out patient-reported outcome measures (PROMS), assessing various outcomes, such as pain-related outcome measures and socio-demographic characteristics (described below in Section 2.6). PROMS were used to determine the predominant pain mechanism (i.e., central sensitization, neuropathic pain, or nociceptive pain) and adapt the intervention to the patients’ specific symptoms and needs. In this phase, specific attention was paid to patients showing the following symptoms of central sensitization:
- Pain NRS > 6
- Widespread Pain Index (WPI) ≥ 7
- Central Sensitization Inventory (CSI) > 40
- Pain Catastrophizing Scale (PCS) > 30
- b.
- Assessment: a 3 h multidisciplinary assessment according to a matched care model conducted by a physician, psychologist, and physiotherapist. For the assessment, a common assessment model was used in which pain symptoms, behavioral, somatic–medical, emotional, social, and cognitive factors, and the stages of change were determined. This model is based on the PSCEBSM model in which an appraisal is made of patients’ type of pain: (P), somatic and medical factors (S), cognitions and perceptions (C), emotional factors (E), behavioral factors (B), social factors (S), and motivation (M) [22]. Both the GP and the physiotherapist conducted a physical examination (both ±30 min in duration) to assess the patient’s physical status (i.e., determine pain type, mobility, and pain behavior). After the assessment, the multidisciplinary team discussed the patient’s pain type and constructed a biopsychosocial model of the patient, their pain, and the factors contributing to the current symptoms. This model served as a framework for pain education and further treatment.
2.5.2. Treatment Process
- a.
- Pain Neuroscience Education (PNE): This education consisted of two one-hour appointments, following the guidelines by Nijs et al. [26]. The first session was conducted by the general practitioner and during this session the medical and somatic aspects of the patients’ pain were discussed, and—in case of central sensitization (i.e., “Increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input” as defined by the IASP [27]), this phenomenon was explained [28]. During the second session, the psychologist and physiotherapist started by discussing the most essential information provided by the general practitioner and repeated the principles of central sensitization if applicable. The main goal during this session was to educate patients about the biopsychosocial factors related to/maintaining their altered pain sensitivity i.e., central sensitization. In addition, through shared decision making, a treatment plan was discussed in which these factors were addressed.
- b.
- Further treatment: Further possible treatment depended on the presence of patient-specific factors:
- Depression, anxiety, fear of movement → psychological treatment such as cognitive behavioral treatment, acceptance and commitment therapy, relaxation techniques, and/or exposure therapy. In case of more severe anxiety and/or depressive symptoms, medication such as antidepressants could be (temporarily) prescribed by the general practitioner.
- Post-traumatic stress disorder (PTSD) → psychological treatment such as trauma therapy (e.g., EMDR or imaginary exposure).
- Lack of exercise, avoidance behavior, and/or disuse → physical therapy such as graded activity, pacing programs and/or graded exposure, encouragement of healthy exercise habits (e.g., 30 min walk/cycle every day, daily activity program)
- Persistence behavior → daily schedule with alternating periods of activity and rest/relaxation. Helping patient decide where to set boundaries, usually under guidance of psychologist and physiotherapist.
- Use of medication such as opioids → tapering off medication by the general practitioner or switching to less harmful medication (such as antidepressants).
- Education about chronic pain and the biopsychosocial model
- Non-drug treatment: advise to seek distraction and support and to find a good balance between relaxation and activity. Possible referral to a psychologist in case of harmful cognitions, emotions, and behaviors; possible referral to a physiotherapist or remedial therapist for exercise programs aimed at an active lifestyle; and possible referral to social worker if social problems play a role.
- Drug treatment: avoid drug treatment if possible, especially avoid opioids if possible. When prescribing drugs, aim to prescribe for a short period.
- Referrals: referral to a rehabilitation if patient is experiencing a high degree of limitations due to pain; referral to medical specialist is there is a possible underlying cause that is treatable, etc.
2.6. Outcome Measures
2.7. Primary Outcome Measures
2.8. Secondary Outcome Measures
2.9. Post Hoc Analysis
2.10. Analytical Methods
3. Results
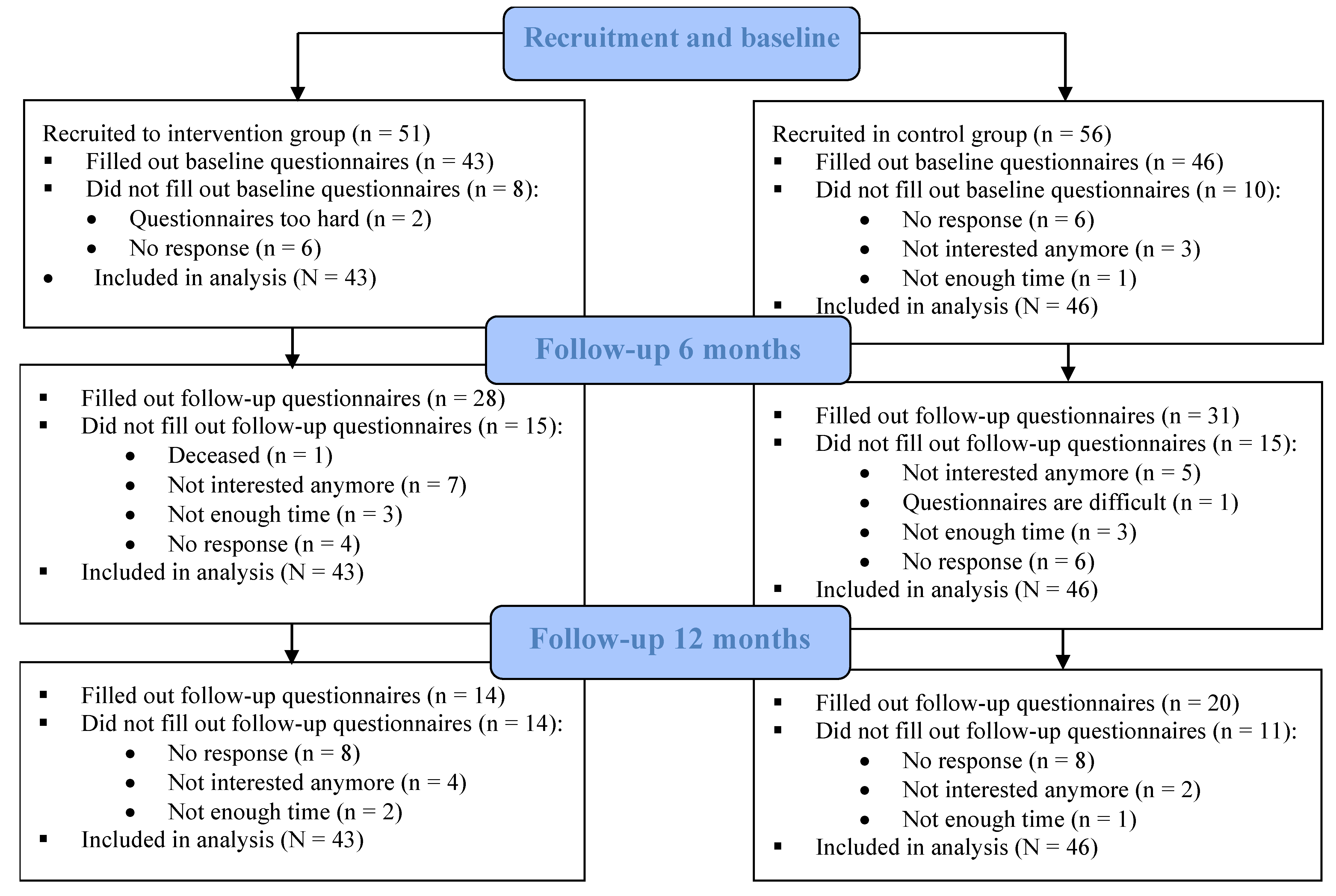
3.1. Participants
3.2. Participants’ Characteristics
3.3. Intention-to-Treat Analysis
3.4. Patients’ Satisfaction
3.5. Post Hoc Analysis: IPQ-B
4. Discussion
4.1. Comparison with Previous Studies
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef] [PubMed]
- Gatchel, R.J.; McGeary, D.D.; McGeary, C.A.; Lippe, B. Interdisciplinary chronic pain management: Past, present, and future. Am. Psychol. 2014, 69, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Gatchel, R.J.; Kishino, N.D.; Watts, L.; Bevers, K. The Biopsychosocial Model of the Assessment, Prevention, and Treatment of Chronic Pain. US Neurol. 2016, 12, 98–104. [Google Scholar] [CrossRef]
- Demyttenaere, K.; Bruffaerts, R.; Lee, S.; Posada-Villa, J.; Kovess, V.; Angermeyer, M.C.; Levinson, D.; de Girolamo, G.; Nakane, H.; Mneimneh, Z.; et al. Mental disorders among persons with chronic back or neck pain: Results from the world mental health surveys. Pain 2007, 129, 332–342. [Google Scholar] [CrossRef]
- Lerman, S.F.; Rudich, Z.; Brill, S.; Shalev, H.; Shahar, G. Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom. Med. 2015, 77, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Fydrich, T.; Turk, D.C. Efficacy of multidisciplinary pain treatment centers: A meta-analytic review. Pain 1992, 49, 221–230. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Okifuji, A. Evidence-Based Scientific Data Documenting the Treatment and Cost-Effectiveness of Comprehensive Pain Programs for Chronic Nonmalignant Pain. J. Pain 2006, 7, 779–793. [Google Scholar] [CrossRef]
- Karjalainen, K.A.; Malmivaara, A.; van Tulder, M.W.; Roine, R.; Jauhiainen, M.; Hurri, H.; Koes, B.W. Multidisciplinary rehabilitation for fibromyalgia and musculoskeletal pain in working age adults. Cochrane Database Syst. Rev. 2000. [Google Scholar] [CrossRef]
- Scascighini, L.; Toma, V.; Dober-Spielmann, S.; Sprott, H. Multidisciplinary treatment for chronic pain: A systematic review of interventions and outcomes. Rheumatology 2008, 47, 670–678. [Google Scholar] [CrossRef]
- Loeser, J.D.; Turk, D.C. Multidisciplinary Pain Management. Semin. Neurosurg. 2004, 15, 13–29. [Google Scholar] [CrossRef]
- Åsenlöf, P.; Denison, E.; Lindberg, P. Long-term follow-up of tailored behavioural treatment and exercise based physical therapy in persistent musculoskeletal pain: A randomized controlled trial in primary care. Eur. J. Pain 2009, 13, 1080–1088. [Google Scholar] [CrossRef]
- Stein, K.F.; Miclescu, A. Effectiveness of multidisciplinary rehabilitation treatment for patients with chronic pain in a primary health care unit. Scand. J. Pain 2013, 4, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Westman, A.; Linton, S.J.; Öhrvik, J.; Wahlén, P.; Theorell, T.; Leppert, J. Controlled 3-year follow-up of a multidisciplinary pain rehabilitation program in primary health care. Disabil. Rehabil. 2010, 32, 207–316. [Google Scholar] [CrossRef] [PubMed]
- De Jong, L.; Janssen, P.G.H.; Keizer, D.; Köke, A.J.A.; Schiere, S.; Van Bommel, M.; Van Coevorden, R.S.; Van de Vusse, A.; Van den Donk, M.; Van Es, A.; et al. NHG-Standaard Pijn. Huisarts Wet. 2015, 58, 472–485. [Google Scholar]
- American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: An updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology 2010, 112, 810–833. [Google Scholar] [CrossRef] [PubMed]
- DeBar, L.L.; Kindler, L.; Keefe, F.J.; Green, C.A.; Smith, D.H.; Deyo, R.A.; Ames, K.; Feldstein, A. A primary care-based interdisciplinary team approach to the treatment of chronic pain utilizing a pragmatic clinical trials framework. Transl. Behav. Med. 2012, 2, 523–530. [Google Scholar] [CrossRef]
- Upshur, C.C.; Luckmann, R.S.; Savageau, J.A. Primary care provider concerns about management of chronic pain in Community Clinic Populations. J. Gen. Intern Med. 2016, 21, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Ostelo, R.W.J.G.; Deyo, R.A.; Waddel, G.; Croft, P.; von Korff, M.; Bouter, L.M.; de Vet, H.C. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Herbert, R.D.; Ferreira, P.H.; Latimer, J.; Ostelo, R.W.; Grotle, M.; Barrett, B. The smallest worthwhile effect of nonsteroidal anti-inflammatory drugs and physiotherapy for chronic low back pain: A benefit-harm trade-off study. J. Clin. Epidemiol. 2013, 66, 1397–1404. [Google Scholar] [CrossRef]
- Christiansen, D.H.; de Vos Andersen, N.B.; Poulsen, P.H.; Ostelo, R.W. The smallest worthwhile effect of primary care physiotherapy did not differ across musculoskeletal pain sites. J. Clin. Epidemiol. 2018, 101, 44–52. [Google Scholar] [CrossRef]
- Becker, N.; Sjøgren, P.; Bech, P.; Olsen, A.K.; Eriksen, J. Treatment outcome of chronic non-malignant pain patients managed in a danish multidisciplinary pain centre compared to general practice: A randomised controlled trial. Pain 2000, 84, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Wijma, A.J.; van Wilgen, C.P.; Meeus, M.; Nijs, J. Clinical biopsychosocial physiotherapy assessment of patients with chronic pain: The first step in pain neuroscience education. Physiother. Theory Pract. 2016, 32, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Louw, A.; Schmidt, S.; Puentedura, E.; Zimney, K. Pain Neuroscience Education (Teaching People about Pain), 2nd ed.; OPTP: Minneapolis, MN, USA, 2018; p. 536. [Google Scholar]
- van Wilgen, C.P.; Nijs, J. Pijneducatie: Een Praktische Handleiding Voor (Para)Medici, 2nd ed.; Bohn Stafleu van Loghum: Houten, The Netherlands, 2018. [Google Scholar]
- van Wilgen, C.P.; Keizer, D. The sensitization model to explain how chronic pain exists without tissue damage. Pain Manag. Nurs. 2012, 13, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; van Wilgen, C.P.; Van Oosterwijck, J.; van Ittersum, M.; Meeus, M. How to explain central sensitization to patients with ‘unexplained’ chronic musculoskeletal pain: Practice guidelines. Man. Ther. 2011, 16, 413–418. [Google Scholar] [CrossRef]
- International Association for the Study of Pain. Terminology. Available online: https://www.iasp-pain.org/resources/terminology/?navItemNumber=576#Centralsensitization (accessed on 16 January 2023).
- Meeus, M.; Nijs, J. Central sensitization: A biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin. Rheumatol. 2007, 26, 465–473. [Google Scholar] [CrossRef]
- Downie, W.W.; Leatham, P.A.; Rhind, V.M.; Wright, V.; Branco, J.A.; Anderson, J.A. Studies with pain rating scales. Ann. Rheum. Dis. 1978, 37, 378–381. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2017, 62, 600–610. [Google Scholar] [CrossRef]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. The rand 36‐item health survey 1.0. Health Econ. 1993, 2, 217–227. [Google Scholar] [CrossRef]
- Vander Zee, K.I.; Sanderman, R.; Heyink, J.W.; de Haes, H. Psychometric qualities of the rand 36-item health survey 1.0: A multidimensional measure of general health status. Int. J. Behav. Med. 1996, 3, 104–122. [Google Scholar] [CrossRef]
- Brazier, J.; Roberts, J.; Deverill, M. The estimation of a preference-based measure of health from the SF-36. J. Health Econ. 2002, 21, 271–292. [Google Scholar] [CrossRef]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef]
- Kregel, J.; Vuijk, P.J.; Descheemaeker, F.; Keizer, D.; van der Noord, R.; Nijs, J.; Cagnie, B.; Meeus, M.; van Wilgen, P. The Dutch Central Sensitization Inventory (CSI): Factor Analysis, Discriminative Power and Test-Retest Reliability. Clin. J. Pain 2015, 32, 624–630. [Google Scholar] [CrossRef]
- Neblett, R.; Cohen, H.; Choi, Y.; Hartzell, M.M.; Williams, M.; Mayer, T.G.; Gatchel, R.J. The Central Sensitization Inventory (CSI): Establishing clinically-significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J. Pain 2013, 14, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Osman, A.; Barrios, F.X.; Gutierrez, P.M.; Kopper, B.A.; Merrifield, T.; Grittmann, L. The Pain Catastrophizing Scale: Further psychometric evaluation with adult samples. J. Behav. Med. 2000, 23, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Krol, M.; de Boer, D.; Pass, A.M.; Rademakers, J. CQ-Index Module Pijn: Meetinstrumentontwikkeling; NIVEL: Utrect, The Netherlands, 2013. [Google Scholar]
- Broadbent, E. The Brief Illness Perception Questionnaire Scoring Instructions. Available online: https://www.uib.no/ipq/ (accessed on 2 January 2022).
- Broadbent, E.; Petrie, K.J.; Main, J.; Weinman, J. The brief illness perception questionnaire. J. Psychosom. Res. 2006, 60, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Becker, S.O.; Ichino, A. Estimation of average treatment effects based on propensity scores. Stata J. 2002, 2, 358–377. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Katz, N.P.; Kerns, R.D.; Stucki, G.; Allen, R.R.; Bellamy, N.; et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005, 113, 9–19. [Google Scholar] [CrossRef]
- Adams, H.; Ellis, T.; Stanish, W.D.; Sullivan, M.J.L. Psychosocial factors related to return to work following rehabilitation of whiplash injuries. J. Occup. Rehabil. 2007, 17, 305–315. [Google Scholar] [CrossRef]
- Linton, S.J.; Flink, I.K.; Vlaeyen, J.W.S. Understanding the Etiology of Chronic Pain From a Psychological Perspective. Phys. Ther. 2018, 98, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.A.; Ryan, C.G.; Cooper, L.; Ellington, D.; Whittle, R.; Lavender, M.; Dixon, J.; Atkinson, G.; Cooper, K.; Martin, D.J. Pain Neuroscience Education for Adults With Chronic Musculoskeletal Pain: A Mixed-Methods Systematic Review and Meta-Analysis. J. Pain 2019, 20, 1140.E1–1140.E22. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Turner, J.A.; Romano, J.M. Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J. Consult. Clin. Psychol. 2001, 69, 655–662. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, A.; Roorda, L.D.; Otten, R.H.; van der Leeden, M.; Dekker, J.; Steultjens, M.P. Predictors of multidisciplinary treatment outcome in fibromyalgia:a systematic review. Disabil. Rehabil. 2013, 35, 437–449. [Google Scholar] [CrossRef]
- Sorrell, M.R.; Flanagan, W.; McCall, J.L. The Effect of Depression and Anxiety on the Success of Multidisciplinary Treatment of Chronic Resistant Myofascial Pain. J. Musculoskelet. Pain 2010, 11, 17–20. [Google Scholar] [CrossRef]
- Villemure, C.; Bushnell, C.M. Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain 2002, 95, 195–199. [Google Scholar] [CrossRef]
- Moss-Morris, R.; Humphrey, K.; Johnson, M.H.; Petrie, K.J. Patients’ perceptions of their pain condition across a multidisciplinary pain management program: Do they change and if so does it matter? Clin. J. Pain 2007, 23, 558–564. [Google Scholar] [CrossRef]
- Twisk, J.; de Boer, M.; de Vente, W.; Heymans, M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J. Clin. Epidemiol. 2013, 66, 1022–1028. [Google Scholar] [CrossRef]
- Acock, A.C. Working With Missing Values. J. Marriage Fam. 2005, 67, 1012–1028. [Google Scholar] [CrossRef]
- Crul, B.V.M.; Legemaate, J. Uitspraak Hoge Raad. Juridisering van richtlijnen en protocollen. Medisch Contact 2005, 60, 812–814. [Google Scholar]
| Control Group (n = 46) | Intervention Group (n = 43) | |
|---|---|---|
| Sex: male n (%) 1 | 3.0 (6.5) | 8.0 (18.6) |
| Age: mean (SD) | 44.8 (14.3) | 43.7 (13.9) |
| Marital status: n (%) | ||
| Married | 15.0 (32.6) | 19.0 (44.2) |
| Divorced | 4.0 (8.7) | 3.0 (7.0) |
| Cohabiting unmarried | 9.0 (19.6) | 6.0 (14.0) |
| Single | 9.0 (19.6) | 7.0 (16.3) |
| Living with parents | 1.0 (2.2) | 2.0 (4.7) |
| Widowed | 0.0 (0.0) | 1.0 (2.3) |
| Unknown | 8.0 (17.4) | 5.0 (11.6) |
| Children yes: n (%) 1 | 22.0 (47.8) | 25.0 (58.1) |
| Not answered | 8.0 (17.4) | 6.0 (14.0) |
| Education level: n (%) | ||
| No education | 0.0 (0.0) | 1.0 (2.3) |
| Primary school | 3.0 (6.5) | 1.0 (2.3) |
| Lower education | 8.0 (17.4) | 8.0 (18.6) |
| Middle education | 20.0 (43.5) | 20.0 (46.5) |
| Higher education | 7.0 (15.2) | 6.0 (14) |
| Unknown | 8.0 (17.4) | 7.0 (16.3) |
| Paid work yes: n (%) 1 | 13.0 (28.3) | 25.0 (58.1) |
| Unknown | 3.0 (6.5) | 5.0 (11.6) |
| Allowance: n (%) | 19.0 (43.3) | 12.0 (27.9) |
| Sickness benefit | 0.0 (0.0) | 6.0 (14.0) |
| Disability Benefits Act | 1.0 (2.2) | 1.0 (2.3) |
| WAJONG 2 | 6.0 (13) | 1.0 (2.3) |
| Unemployment Law | 5.0 (10.9) | 2.0 (4.7) |
| Welfare | 7.0 (15.2) | 2.0 (4.7) |
| Unknown | 8.0 (17.4) | 8.0 (16.3) |
| Smoker yes: n (%) 1 | 9.0 (19.6) | 10.0 (23.3) |
| Unknown | 8.0 (17.4) | 7.0 (16.3) |
| Pain duration in months: mean (SD) | 103.0 (113.0) | 98.0 (157.0) |
| Measurement (Range) | Control Group M (SD) | Intervention Group M (SD) | Average between-Group Difference (CI] 1 | p-Value |
|---|---|---|---|---|
| Pain NRS a (0–10) | ||||
| Baseline | 6.9 (1.5) | 6.4 (1.5) | ||
| 6 months | 6.3 (1.9) | 5.8 (1.9) | −0.4 (−1.4, 0.5) | 0.36 |
| 12 months | 6.6 (1.7) | 5.5 (1.9) | −0.9 (−1.9, 0.1) | 0.08 |
| Overall | −0.9 (−1.9, 0.05) | 0.06 | ||
| WPI b (1–21) | ||||
| Baseline | 8.6 (5.0) | 5.3 (3.6) | ||
| 6 months | 8.0 (4.9) | 4.8 (4.1) | −0.9 (−2.3, 0.6) | 0.25 |
| 12 months | 7.9 (4.8) | 5.6 (4.9) | −1.8 (−3.5, −0.2) | 0.03 * |
| Overall | −1.2 (−2.5, 0.1) | 0.06 | ||
| HR-QoL c (0–1) | ||||
| Baseline | 0.58 (0.08) | 0.60 (0.06) | ||
| 6 months | 0.58 (0.06) | 0.62 (0.07) | 0.01 (−0.03, 0.04) | 0.67 |
| 12 months | 0.64 (0.07) | 0.65 (0.07) | 0.05 (0.02, 0.08) | <0.01 * |
| Overall | 0.02 (−0.02, 0.05) | 0.33 |
| Measurement (Range) | Control Group M (SD) | Intervention Group M (SD) | Average between-Group Difference [CI] 1 | p-Value |
|---|---|---|---|---|
| RAND-36 Physical functioning (0–100) | ||||
| Baseline | 48.0 (22.1) | 61.0 (22.1) | ||
| 6 months | 50.2 (19.5) | 57.3 (24.7) | 1.4 [−9.5, 12.4] | 0.80 |
| 12 months | 53.0 (23.1) | 54.3 (27.5) | −3.0 [−14.6, 8.5] | 0.61 |
| Overall | −3.5 [−14.2, 7.1] | 0.51 | ||
| RAND-36 Role functioning: Physical (0–100) | ||||
| Baseline | 26.3 (40.2) | 19.1 (32.6) | ||
| 6 months | 29.3 (38.4) | 29.8 (43.0) | 12.7 [−6.3, 31.7] | 0.19 |
| 12 months | 28.6 (36.5) | 34.5 (40.7) | 10.4 [−10.0, 30.8] | 0.32 |
| Overall | 8.5 [−10.3, 27.3] | 0.37 | ||
| RAND-36 Energy/Fatigue (0–100) | ||||
| Baseline | 36.1 (17.5) | 46.3 (16.3) | ||
| 6 months | 40.7 (15.5) | 48.1 (20.2) | 2.7 (−5.6, 10.9] | 0.53 |
| 12 months | 39.5 (18.5) | 52.6 (19.7) | 3.8 [−5.2, 12.8] | 0.41 |
| Overall | 4.5 [−2.4, 11.3] | 0.20 | ||
| RAND-36 Emotional Wellbeing (0–100) | ||||
| Baseline | 62.2 (21.1) | 71.2 (16.1) | ||
| 6 months | 63.3 (17.1) | 66.8 (17.7) | 2.3 [−4.8, 9.5] | 0.52 |
| 12 months | 63.6 (18.7) | 69.5 (17.5) | 5.0 [−2.7, 12.7] | 0.21 |
| Overall | 5.6 [−0.5, 11.7] | 0.07 | ||
| RAND-36 Role functioning: Emotional (0–100) | ||||
| Baseline | 51.8 (48.8) | 66.7 (41.8) | ||
| 6 months | 49.4 (48.5) | 53.8 (49.1) | 0.7 [−24.0, 25.5] | 0.96 |
| 12 months | 66.7 (45.4) | 68.3 (44.1) | 5.5 [−17.4, 28.4] | 0.64 |
| Overall | 3.4 [−17.8, 24.6] | 0.75 | ||
| RAND-36 Social Functioning (0–100) | ||||
| Baseline | 40.0 (22.2) | 47.4 (22.1) | ||
| 6 months | 45.2 (20.1) | 47.3 (22.5) | 0.3 [−7.9, 8.5] | 0.95 |
| 12 months | 45.7 (22.8) | 45.7 (23.6) | 0.9 [−7.9, 9.7) | 0.85 |
| Overall | 0.4 [−7.2, 7.9] | 0.93 | ||
| RAND-36 Pain (0–100) | ||||
| Baseline | 35.8 (21.1) | 40.4 (17.2) | ||
| 6 months | 42.7 (15.5) | 47.6 (17.4) | 6.2 [−1.3, 14.8] | 0.11 |
| 12 months | 42.3 (17.7) | 47.0 (22.4) | 5.2 [−2.9, 13.3] | 0.21 |
| Overall | 3.9 [−3.4, 11.2] | 0.30 | ||
| RAND-36 General Health (0–100) | ||||
| Baseline | 34.8 (18.4) | 43.4 (13.3) | ||
| 6 months | 32.9 (16.7) | 46.0 (19.8) | 8.7 [0.07, 17.4] | <0.05 * |
| 12 months | 37.7 (16.9) | 40.7 (18.0) | 1.2 [−7.9, 10.4] | 0.79 |
| Overall | 4.6 [−3.0, 12.1] | 0.24 | ||
| RAND-36 Health change (0–100) | ||||
| Baseline | 31.6 (25.8) | 44.9 (29.4) | ||
| 6 months | 44.8 (24.4) | 59.6 (20.1) | 11.8 [−1.6, 25.3] | 0.09 |
| 12 months | 44.6 (24.9) | 58.3 (24.2) | 12.7 [−1.8, 27.3] | 0.09 |
| Overall | 14.2 [1.2, 27.1] | 0.03 * | ||
| CSI a (1–100) | ||||
| Baseline | 56.2 (16.2) | 40.0 (16.9) | ||
| 6 months | 53.5 (16.4) | 44.2 (20.2) | −0.0 [−5.6, 5.5] | 0.99 |
| 12 months | 50.7 (15.3) | 40.5 (19.4) | 0.8 [−5.1, 6.7] | 0.78 |
| Overall | −0.2 [−5.5, 5.0] | 0.93 | ||
| PCS b (0–52) | ||||
| Baseline | 33.2 (13.0) | 29.6 (10.3) | ||
| 6 months | 31.4 (13.1) | 27.8 (10.5) | −3.7 [−7.8, 0.4] | 0.08 |
| 12 months | 29.3 (12.5) | 24.9 (9.8) | −3.9 [8.3, 0.4] | 0.08 |
| Overall | −2.8 [−6.6, 1.1] | 0.16 |
| Item (Range) | Control Group M (SD) | Intervention Group M (SD) | Mean Difference [95% CI] | p-Value |
|---|---|---|---|---|
| Taken seriously (0–3) | 1.9 (0.8) | 2.3 (0.7) | −0.4 [−0.9, 0] | 0.04 * |
| Trust in expertise (0–3) | 1.9 (0.8) | 2.1 (0.7) | −0.2 [−0.7, 0.2] | 0.30 |
| Rating of result (0–3) | 1.6 (0.7) | 2.0 (1.0) | −0.4 [−0.9, 0.1] | 0.09 |
| Rating of care (0–10) | 6.5 (2.0) | 6.7 (2.1) | −0.1 [−1.3, 1.1] | 0.82 |
| Item (Range) | Control Group M (SD) | Intervention Group M (SD) | Average between-Group Difference [CI] 1 | p-Value |
|---|---|---|---|---|
| IPQ-B 1: Consequences (0–10) | ||||
| Baseline | 7.7 (1.9) | 8.1 (1.8) | ||
| 6 months | 7.8 (2.1) | 6.9 (2.4) | −1.4 [−2.4, −0.4] | <0.01 * |
| 12 months | 7.9 (2.2) | 6.9 (2.7) | −1.5 [−2.6, −0.5] | <0.01 * |
| Overall | −1.3 [−2.3, −0.3] | <0.01 * | ||
| IPQ-B 2: Timeline (0–10) | ||||
| Baseline | 8.5 (2.1) | 8.4 (2.0) | ||
| 6 months | 9.3 (1.6) | 7.9 (2.2) | −1.4 [−2.5, −0.3] | 0.01 * |
| 12 months | 9.0 (1.8) | 8.1 (2.7) | −1.5 [−2.5, −0.4] | <0.01 * |
| Overall | −1.4 [−2.6, −0.3] | 0.01 * | ||
| IPQ-B 3: Personal Control (0–10) | ||||
| Baseline | 4.5 (3.0) | 5.3 (3.0) | ||
| 6 months | 5.8 (2.7) | 5.7 (2.8) | −0.6 [−2.0, 0.8] | 0.41 |
| 12 months | 5.1 (2.5) | 5.9 (3.2) | 0.2 [−1.3, 1.8] | 0.78 |
| Overall | 0.3 [−0.9, 1.5] | 0.65 | ||
| IPQ-B 4: Treatment Control (0–10) | ||||
| Baseline | 4.9 (3.0) | 6.9 (1.7) | ||
| 6 months | 6.8 (2.6) | 6.6 (1.9) | −0.9 [−2.1, 0.4] | 0.17 |
| 12 months | 6.1 (2.2) | 6.0 (3.0) | −1.1 [−2.2, 0.2] | 0.10 |
| Overall | −0.4 [−1.5, 0.8] | 0.52 | ||
| IPQ-B 5: Identity (0–10) | ||||
| Baseline | 7.7 (2.0) | 8.1 (1.4) | ||
| 6 months | 7.6 (2.1) | 7.1 (2.4) | −1.1 [−2.2, 0.0] | 0.05 |
| 12 months | 7.7 (2.2) | 7.1 (2.6) | −1.3 [−2.5, −0.1] | <0.05 * |
| Overall | −1.0 [−2.2, 0.2] | 0.10 | ||
| IPQ-B 6: Concern (0–10) | ||||
| Baseline | 5.0 (3.2) | 7.0 (2.4) | ||
| 6 months | 5.9 (3.0) | 5.1 (3.0) | −3.0 [−4.2, −1.7] | <0.01 * |
| 12 months | 5.2 (3.2) | 5.5 (2.8) | −2.1 [−3.4, −0.8] | <0.01 * |
| Overall | −2.2 [−3.4, −1.0] | <0.01 * | ||
| IPQ-B 7: Understanding (0–10) | ||||
| Baseline | 6.2 (2.8) | 5.4 (2.3) | ||
| 6 months | 6.7 (2.6) | 6.5 (2.7) | 0.9 [−0.5, 2.3] | 0.22 |
| 12 months | 6.9 (2.8) | 6.1 (3.1) | 0.2 [−1.3, 1.7] | 0.83 |
| Overall | 0.4 [−0.9, 1.6] | 0.62 | ||
| IPQ-B 8: Emotional Response (0–10) | ||||
| Baseline | 7.1 (2.5) | 7.4 (2.4) | ||
| 6 months | 7.8 (2.4) | 6.9 (3.0) | −1.5 [−2.6, −0.4] | <0.01 * |
| 12 months | 7.1 (2.6) | 6.3 (2.8) | −1.1 [−2.3, 0.1] | 0.07 |
| Overall | −1.6 [−2.6, −0.5] | <0.01 * | ||
| HADS Anxiety (0–21) | ||||
| Baseline | 12.1 (2.8) | 13.0 (1.9) | ||
| 6 months | 11.9 (2.4) | 12.9 (2.1) | 0.1 [−0.8, 1.1] | 0.78 |
| 12 months | 12.1 (2.6) | 12.3 (2.0) | 0.6 [−0.3, 1.6] | 0.17 |
| Overall | 0.4 [−0.6, 1.3] | 0.45 | ||
| HADS Depression (0–21) | ||||
| Baseline | 8.1 (1.9) | 8.8 (1.6) | ||
| 6 months | 8.8 (1.9) | 9.2 (1.4) | 0.8 [−0.2, 1.8] | 0.11 |
| 12 months | 8.9 (1.9) | 10.0 (1.4) | 0.1 [−0.8, 1.1] | 0.76 |
| Overall | 0.2 [−0.6, 1.1] | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bults, R.M.; van Dongen, J.M.; Ostelo, R.W.J.G.; Nijs, J.; Keizer, D.; van Wilgen, C.P. Effectiveness of a Primary Care Multidisciplinary Treatment for Patients with Chronic Pain Compared with Treatment as Usual. J. Clin. Med. 2023, 12, 885. https://doi.org/10.3390/jcm12030885
Bults RM, van Dongen JM, Ostelo RWJG, Nijs J, Keizer D, van Wilgen CP. Effectiveness of a Primary Care Multidisciplinary Treatment for Patients with Chronic Pain Compared with Treatment as Usual. Journal of Clinical Medicine. 2023; 12(3):885. https://doi.org/10.3390/jcm12030885
Chicago/Turabian StyleBults, Rinske M., Johanna M. van Dongen, Raymond W. J. G. Ostelo, Jo Nijs, Doeke Keizer, and C. Paul van Wilgen. 2023. "Effectiveness of a Primary Care Multidisciplinary Treatment for Patients with Chronic Pain Compared with Treatment as Usual" Journal of Clinical Medicine 12, no. 3: 885. https://doi.org/10.3390/jcm12030885
APA StyleBults, R. M., van Dongen, J. M., Ostelo, R. W. J. G., Nijs, J., Keizer, D., & van Wilgen, C. P. (2023). Effectiveness of a Primary Care Multidisciplinary Treatment for Patients with Chronic Pain Compared with Treatment as Usual. Journal of Clinical Medicine, 12(3), 885. https://doi.org/10.3390/jcm12030885