Effectiveness and Safety of Balloon Pulmonary Angioplasty for the Treatment of Patients with Persistent Pulmonary Hypertension after Pulmonary Endarterectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Balloon Pulmonary Angioplasty Procedure
2.3. Definitions
2.4. Clinical Assessment during Follow-Up and Complications
2.5. Statistical Analysis
3. Results
3.1. Effectiveness Analysis
3.2. Safety Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ende-Verhaar, Y.M.; Cannegieter, S.C.; Noordegraaf, A.V.; Delcroix, M.; Pruszczyk, P.; Mairuhu, A.T.; Huisman, M.V.; Klok, F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur. Respir. J. 2017, 49, 1601792. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.E.; Su, L.; Kiely, D.G.; Page, K.; Toshner, M.; Swietlik, E.; Treacy, C.; Ponnaberanam, A.; Condliffe, R.; Sheares, K.; et al. Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy. Circulation 2016, 133, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, M.; Albarrán, A.; Hernández, I.; López-Gude, M.J.; Sarnago, F.; Martín, R.; Arribas, F.; Escribano, P. Balloon Pulmonary Angioplasty for Inoperable Patients with Chronic Thromboembolic Pulmonary Hypertension. Observational Study in a Referral Unit. Rev. Esp. Cardiol. Engl. Ed. 2019, 72, 224–232. [Google Scholar] [CrossRef]
- Shimura, N.; Kataoka, M.; Inami, T.; Yanagisawa, R.; Ishiguro, H.; Kawakami, T.; Higuchi, Y.; Ando, M.; Fukuda, K.; Yoshino, H.; et al. Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int. J. Cardiol. 2015, 183, 138–142. [Google Scholar] [CrossRef]
- Yanaka, K.; Nakayama, K.; Shinke, T.; Shinkura, Y.; Taniguchi, Y.; Kinutani, H.; Tamada, N.; Onishi, H.; Tsuboi, Y.; Satomi-Kobayashi, S.; et al. Sequential Hybrid Therapy with Pulmonary Endarterectomy and Additional Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. J. Am. Heart Assoc. 2018, 7, e008838. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Darocha, S.; Pietrasik, A.; Pietura, R.; Jankiewicz, S.; Banaszkiewicz, M.; Sławek-Szmyt, S.; Biederman, A.; Mularek-Kubzdela, T.; Lesiak, M.; et al. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int. J. Cardiol. 2019, 278, 232–237. [Google Scholar] [CrossRef]
- Ito, R.; Yamashita, J.; Sasaki, Y.; Ikeda, S.; Suzuki, S.; Murata, N.; Ogino, H.; Chikamori, T. Efficacy and safety of balloon pulmonary angioplasty for residual pulmonary hypertension after pulmonary endarterectomy. Int. J. Cardiol. 2021, 334, 105–109. [Google Scholar] [CrossRef]
- Atas, H.; Mutlu, B.; Akaslan, D.; Kocakaya, D.; Kanar, B.; Inanc, N.; Karakurt, S.; Cimsit, C.; Yildizeli, B. Balloon Pulmonary Angioplasty in Patients with Inoperable or Recurrent/Residual Chronic Thromboembolic Pulmonary Hypertension: A Single-Centre Initial Experience. Heart Lung Circ. 2022, 31, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Hansen, J.V.; Dragsbaek, S.J.; Maeng, M.; Andersen, M.J.; Andersen, G.; Mellemjkaer, S.; Ilkjær, L.B.; Nielsen-Kudsk, J.E. Balloon pulmonary angioplasty for patients with chronic thromboembolic pulmonary hypertension previously operated by pulmonary endarterectomy. Pulm. Circ. 2022, 12, e12115. [Google Scholar] [CrossRef]
- Nishiyama, M.; Inoue, Y.; Sasaki, H.; Seike, Y.; Aoki, T.; Ueda, J.; Tsuji, A.; Ogo, T.; Matsuda, H.; Sakaguchi, T. Long-term outcomes of combined pulmonary endarterectomy and additional balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Gen. Thorac. Cardiovasc. Surg. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Madani, M.M.; Auger, W.R.; Pretorius, V.; Sakakibara, N.; Kerr, K.M.; Kim, N.H.; Fedullo, P.F.; Jamieson, S.W. Pulmonary Endarterectomy: Recent Changes in a Single Institution’s Experience of More than 2700 Patients. Ann. Thorac. Surg. 2012, 94, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, M.; Maneiro, N.; Lareo, A.; Albarrán, A.; Huertas, S.; Olazábal, A.; Delgado, J.; Alonso, S.; Sarnago, F.; Tejada, J.G.; et al. Selective Segmental Pulmonary Angiography: Anatomical, Technical and Safety Aspects of a Must-Learn Technique in Times of Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2021, 10, 3358. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.V.; González-Trevilla, A.A.; Subías, P.E. Fractional Flow Reserve-guided Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension. Rev. Esp. Cardiol. Engl. Ed. 2016, 69, 863. [Google Scholar] [CrossRef]
- Inami, T.; Kataoka, M.; Shimura, N.; Ishiguro, H.; Yanagisawa, R.; Taguchi, H.; Fukuda, K.; Yoshino, H.; Satoh, T. Pulmonary Edema Predictive Scoring Index (PEPSI), a New Index to Predict Risk of Reperfusion Pulmonary Edema and Improvement of Hemodynamics in Percutaneous Transluminal Pulmonary Angioplasty. JACC Cardiovasc. Interv. 2013, 6, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.V.; Melón, N.M.; González-Trevilla, A.A.; Cebada, F.S.; Nieto, S.H.; Cruz-Utrilla, A.; Hinojosa, W.; López-Gude, M.J.; Charterina, S.A.; Ostolaza, Y.R.; et al. Balloon pulmonary angioplasty can be an effective and safe therapeutic option in non-surgical elderly patients. Front. Cardiovasc. Med. 2022, 9, 1001518. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Jaïs, X.; Brenot, P.; Bouvaist, H.; Jevnikar, M.; Canuet, M.; Chabanne, C.; Chaouat, A.; Cottin, V.; De Groote, P.; Favrolt, N.; et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): A multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir. Med. 2022, 10, 961–971. [Google Scholar] [CrossRef]
- Freed, D.H.; Thomson, B.M.; Berman, M.; Tsui, S.S.; Dunning, J.; Sheares, K.K.; Pepke-Zaba, J.; Jenkins, D.P. Survival after pulmonary thromboendarterectomy: Effect of residual pulmonary hypertension. J. Thorac. Cardiovasc. Surg. 2011, 141, 383–387. [Google Scholar] [CrossRef]
- Matsuda, H.; Ogino, H.; Minatoya, K.; Sasaki, H.; Nakanishi, N.; Kyotani, S.; Kobayashi, J.; Yagihara, T.; Kitamura, S. Long-Term Recovery of Exercise Ability after Pulmonary Endarterectomy for Chronic Thromboembolic Pulmonary Hypertension. Ann. Thorac. Surg. 2006, 82, 1338–1343. [Google Scholar] [CrossRef]
- Karyofyllis, P.; Demerouti, E.; Papadopoulou, V.; Voudris, V.; Matsubara, H. Balloon Pulmonary Angioplasty as a Treatment in Chronic Thromboembolic Pulmonary Hypertension: Past, Present, and Future. Curr. Treat. Options Cardiovasc. Med. 2020, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ogawa, A.; Miyaji, K.; Mizoguchi, H.; Shimokawahara, H.; Naito, T.; Oka, T.; Yunoki, K.; Munemasa, M.; Matsubara, H. Novel Angiographic Classification of Each Vascular Lesion in Chronic Thromboembolic Pulmonary Hypertension Based on Selective Angiogram and Results of Balloon Pulmonary Angioplasty. Circ. Cardiovasc. Interv. 2016, 9, e003318. [Google Scholar] [CrossRef] [PubMed]



| Age (Years) | 49.2 ± 11.8 |
|---|---|
| Women (%) | 70.0 |
| Body mass index (Kg/m2) | 25.9 ± 2.8 |
| Thrombophilia (%) | 30.0 |
| Cancer history (%) | 40.0 |
| Hypothiroidism (%) | 20.0 |
| number of PH specific drugs | 2.2 ± 0.6 |
| Intravenous prostanoids (%) | 50.0 |
| Diuretics (%) | 100.0 |
| O2 (%) | 30.0 |
| N° of procedures/patient | 4.4 ± 2.0 |
| N° of treated lobes/procedure | 1.3 ± 0.6 |
| N° of treated segmentary arteries/procedure | 2.6 ± 1.2 |
| N° of treated subsegmentary arteries/procedure | 4.4 ± 2.4 |
| Balloon diameter (mm) | 2.6 ± 0.9 |
| Right atrium pressure (mmHg) | 8.1 ± 5.3 |
| Mean pulmonary artery pressure (mmHg) | 50.7 ± 15.3 |
| Cardiac index (L/min/m2) | 2.8 ± 0.4 |
| Pulmonary vascular resistances (W.U.) | 8.5 ± 3.6 |
| Pulmonay capilar wedge pressure (mmHg) | 10.7 ± 7.3 |
| Peripheric O2 saturation (%) | 94.2 ± 3.3 |
| Pulmonary artery O2 saturation (%) | 66.7 ± 4.3 |
| Before PEA | After PEA | Before BPA | p before vs. after PEA | p after PEA vs. before BPA | |
|---|---|---|---|---|---|
| Right atrium pressure (mmHg) | 11.0 ± 3.9 | 10.0 ± 4.3 | 8.1 ± 5.3 | 0.41 | 0.06 |
| Mean pulmonary pressure (mmHg) | 54.0 ± 8.4 | 51.2 ± 8.3 | 50.7 ± 15.3 | 0.23 | 0.89 |
| Cardiac index (l/min/m2) | 2.7 ± 0.5 | 2.7 ± 0.8 | 2.8 ± 0.4 | 0.88 | 0.75 |
| Pulmonary vascular resistances (W.U.) | 9.7 ± 4.4 | 8.6 ± 2.9 | 8.5 ± 3.6 | 0.42 | 0.96 |
| PEA: Pulmonary endarterectomy; BPA: Balloon pulmonary angioplasty. | |||||
| Before BPA | After BPA | p | |
|---|---|---|---|
| Right atrium pressure (mmHg) | 8.1 ± 5.3 | 8.3 ± 3.4 | 091 |
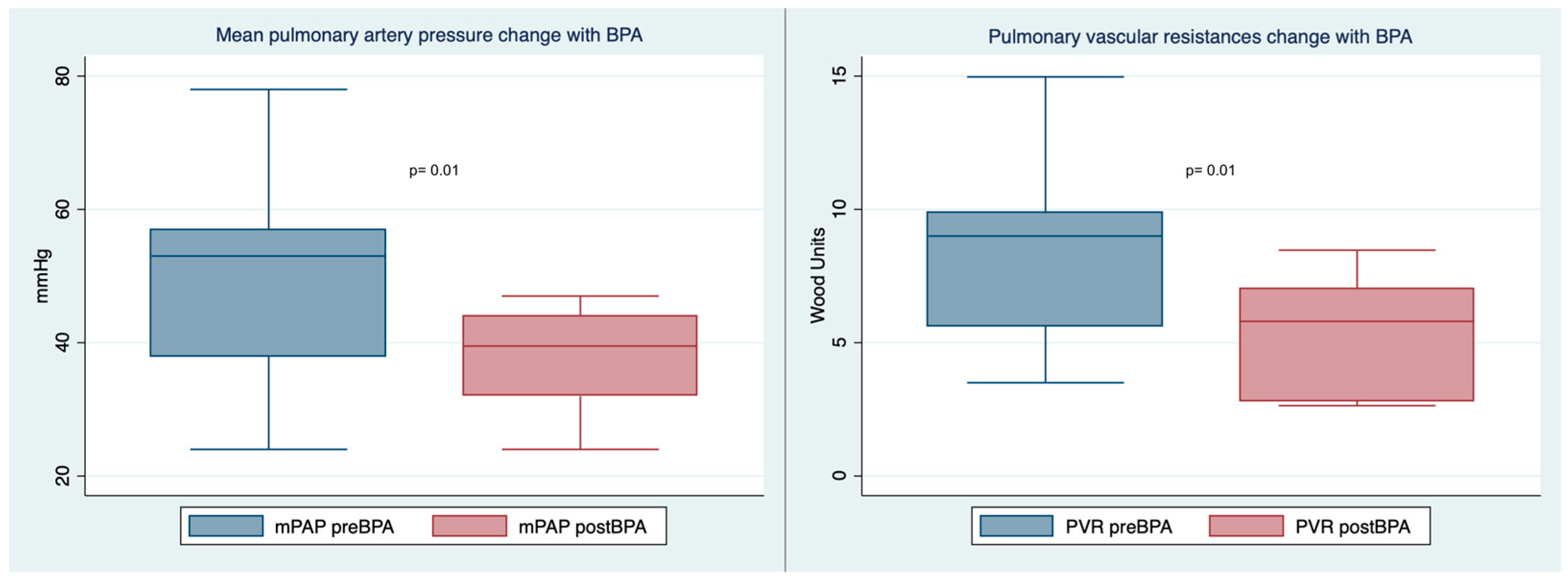
| Mean pulmonary artery pressure (mmHg) | 50.7 ± 15.3 | 38.0 ± 7.9 | 0.01 |
| Cardiac index (L/min/m2) | 2.8 ± 0.4 | 2.7 ± 0.7 | 0.75 |
| Pulmonay vascular resistances (W.U.) | 8.5 ± 3.6 | 5.3 ± 2.2 | 0.01 |
| Six-minute walk test (m) | 380 ± 118 | 407 ± 127 | 0.17 |
| NT-proBNP (pg/mL) | 680 (255–892) | 292 (122–755) | 0.21 |
| number of PH specific drugs | 2.2 ± 0.6 | 2.0 ± 0.8 | 0.51 |
| Intravenous prostanoids (%) | 50.0 | 0 | 0.02 |
| number of PH specific drugs (%) | |||
| 0 | 0 | 0 | |
| 1 | 10.0 | 30.0 | |
| 2 | 60.0 | 40.0 | |
| 3 | 30.0 | 30.0 | 1 |
| WHO functional class (%) | |||
| 1 | 0 | 10.0 | |
| 2 | 50.0 | 90.0 | |
| 3 | 40.0 | 0 | |
| 4 | 10.0 | 0 | 0.03 |
| Per Patient (n = 14) | Per Procedure (n = 50) | |
|---|---|---|
| Reperfusion edema (%) | 21.4 | 8.0 |
| grade 2 | 21.4 | 6.0 |
| grade 3 | 7.1 | 2.0 |
| grade 4 | 0 | 0 |
| grade 5 | 0 | 0 |
| Hemoptysis (%) | 28.6 | 14.0 |
| mild | 28.6 | 14.0 |
| severe | 0 | 0 |
| Vascular dissection (%) | 21.4 | 6.0 |
| Vascular perforation (%) | 0 | 0 |
| Contrast allergy (%) | 7.1 | 2.0 |
| Acute renal failure (%) | 0 | 0 |
| Periprocedural mortality (%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maneiro Melon, N.M.; Velazquez Martin, M.; Huertas Nieto, S.; Albarran Gonzalez-Trevilla, A.; Sarnago Cebada, F.; Cruz Utrilla, A.; Hinojosa Camargo, W.; Aguilar Colindres, R.; Melendo Viu, M.; Lopez Gude, M.J.; et al. Effectiveness and Safety of Balloon Pulmonary Angioplasty for the Treatment of Patients with Persistent Pulmonary Hypertension after Pulmonary Endarterectomy. J. Clin. Med. 2023, 12, 905. https://doi.org/10.3390/jcm12030905
Maneiro Melon NM, Velazquez Martin M, Huertas Nieto S, Albarran Gonzalez-Trevilla A, Sarnago Cebada F, Cruz Utrilla A, Hinojosa Camargo W, Aguilar Colindres R, Melendo Viu M, Lopez Gude MJ, et al. Effectiveness and Safety of Balloon Pulmonary Angioplasty for the Treatment of Patients with Persistent Pulmonary Hypertension after Pulmonary Endarterectomy. Journal of Clinical Medicine. 2023; 12(3):905. https://doi.org/10.3390/jcm12030905
Chicago/Turabian StyleManeiro Melon, Nicolas M., Maite Velazquez Martin, Sergio Huertas Nieto, Agustin Albarran Gonzalez-Trevilla, Fernando Sarnago Cebada, Alejandro Cruz Utrilla, Williams Hinojosa Camargo, Ricardo Aguilar Colindres, Maria Melendo Viu, Maria Jesus Lopez Gude, and et al. 2023. "Effectiveness and Safety of Balloon Pulmonary Angioplasty for the Treatment of Patients with Persistent Pulmonary Hypertension after Pulmonary Endarterectomy" Journal of Clinical Medicine 12, no. 3: 905. https://doi.org/10.3390/jcm12030905
APA StyleManeiro Melon, N. M., Velazquez Martin, M., Huertas Nieto, S., Albarran Gonzalez-Trevilla, A., Sarnago Cebada, F., Cruz Utrilla, A., Hinojosa Camargo, W., Aguilar Colindres, R., Melendo Viu, M., Lopez Gude, M. J., Morales Ruiz, R., Perez Nuñez, M., Arribas Ynsaurriaga, F., & Escribano Subias, P. (2023). Effectiveness and Safety of Balloon Pulmonary Angioplasty for the Treatment of Patients with Persistent Pulmonary Hypertension after Pulmonary Endarterectomy. Journal of Clinical Medicine, 12(3), 905. https://doi.org/10.3390/jcm12030905






