Variability of Low-Z Inhomogeneity Correction in IMRT/SBRT: A Multi-Institutional Collaborative Study
Abstract
1. Introduction
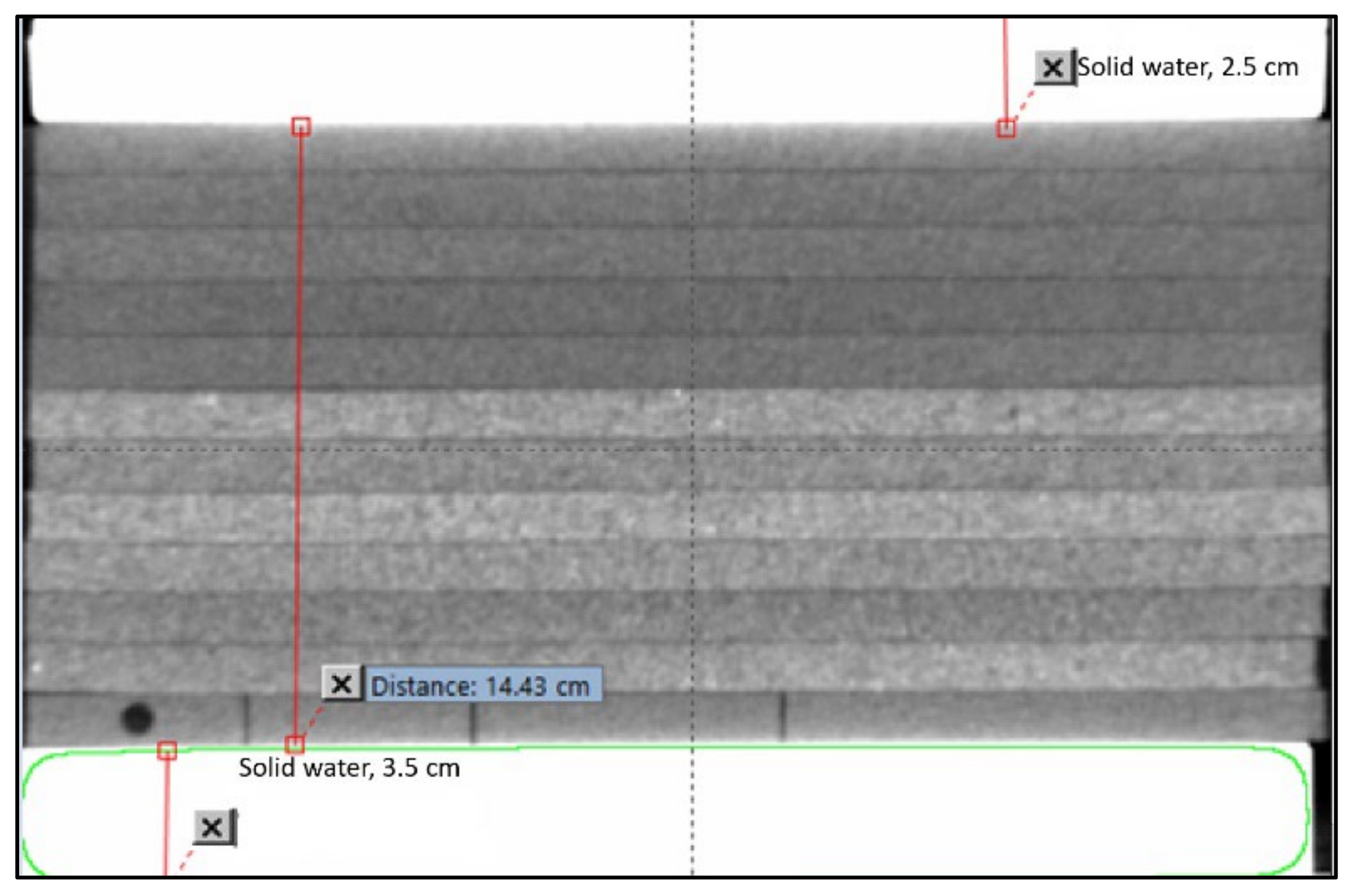
2. Materials and Methods
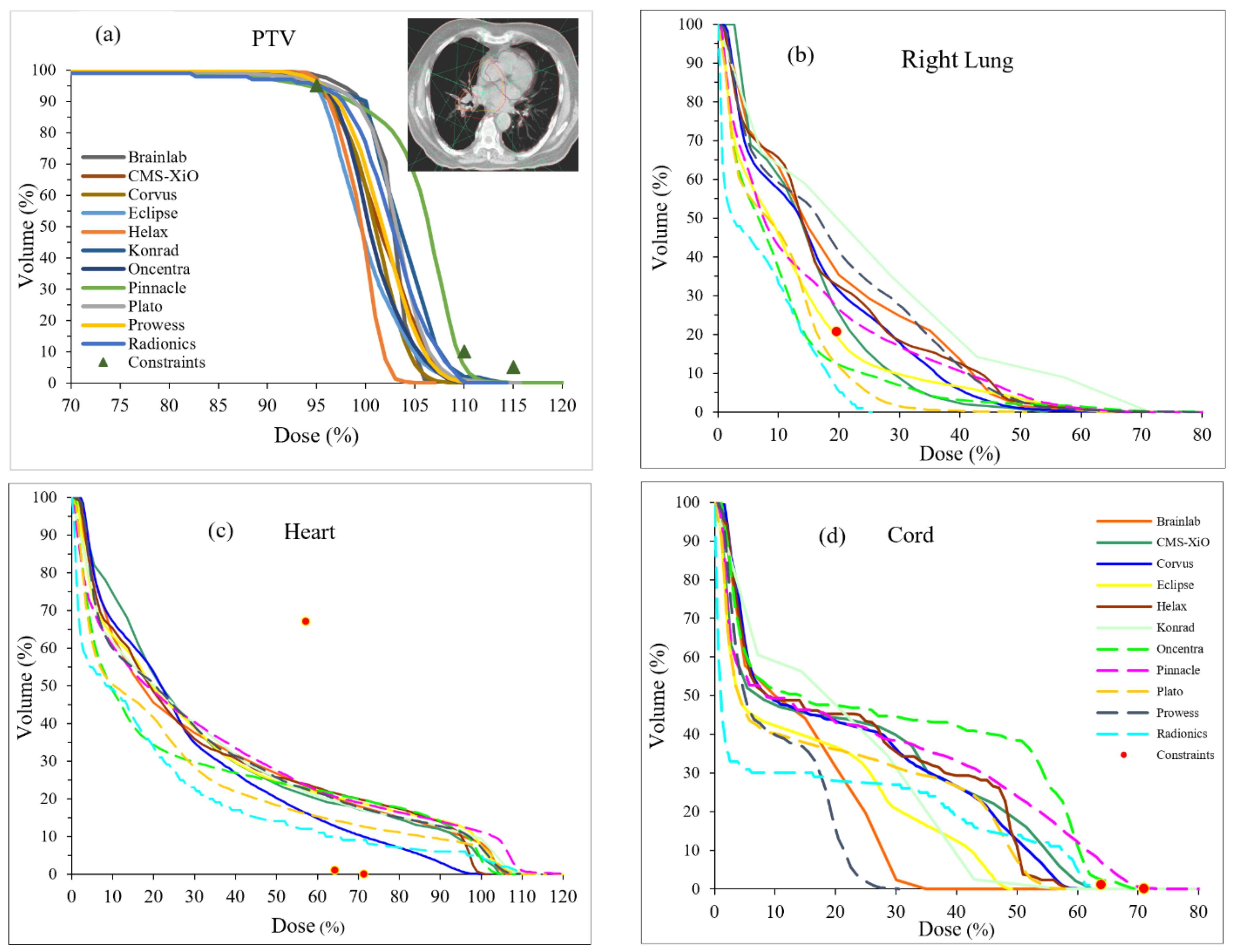
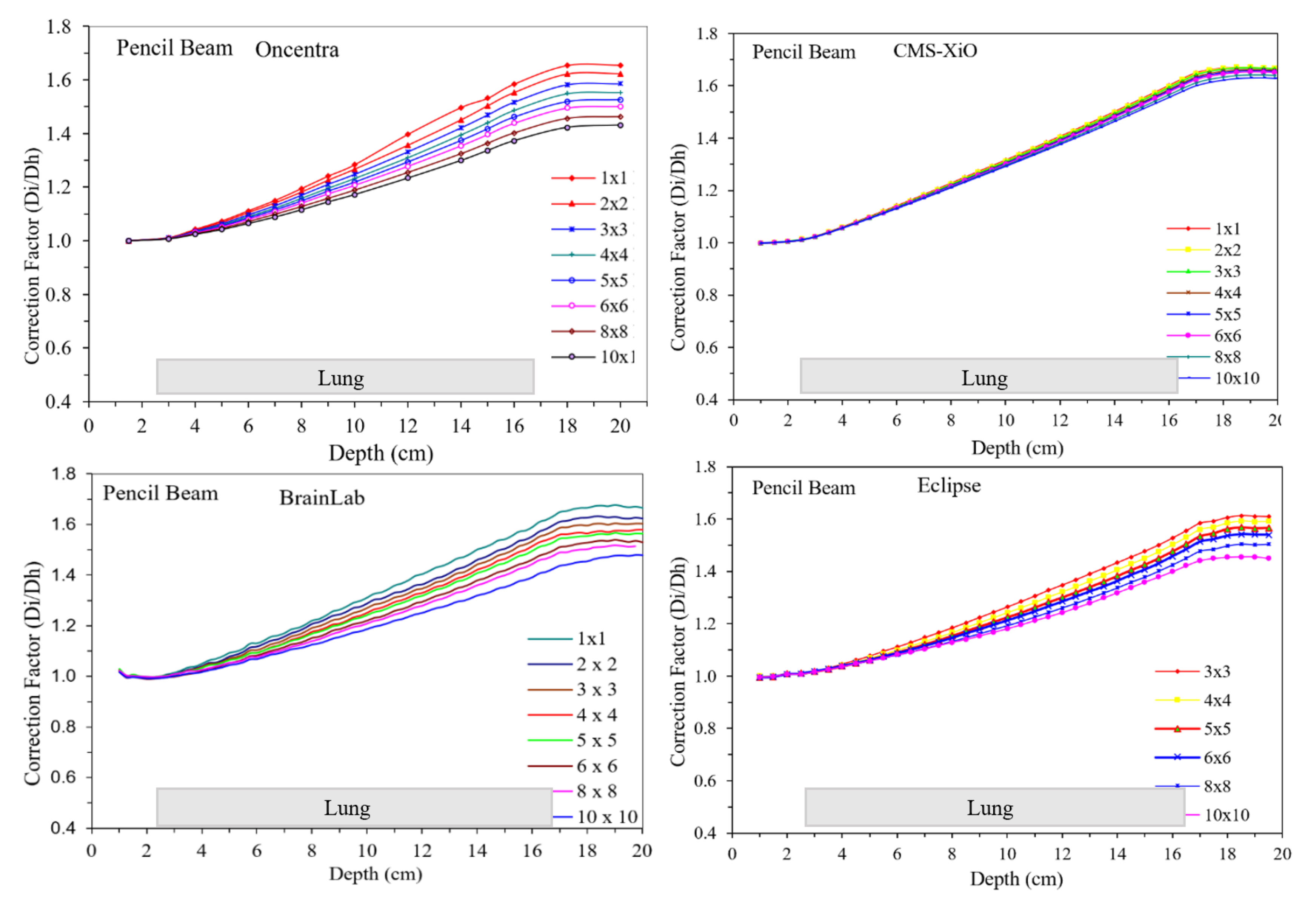
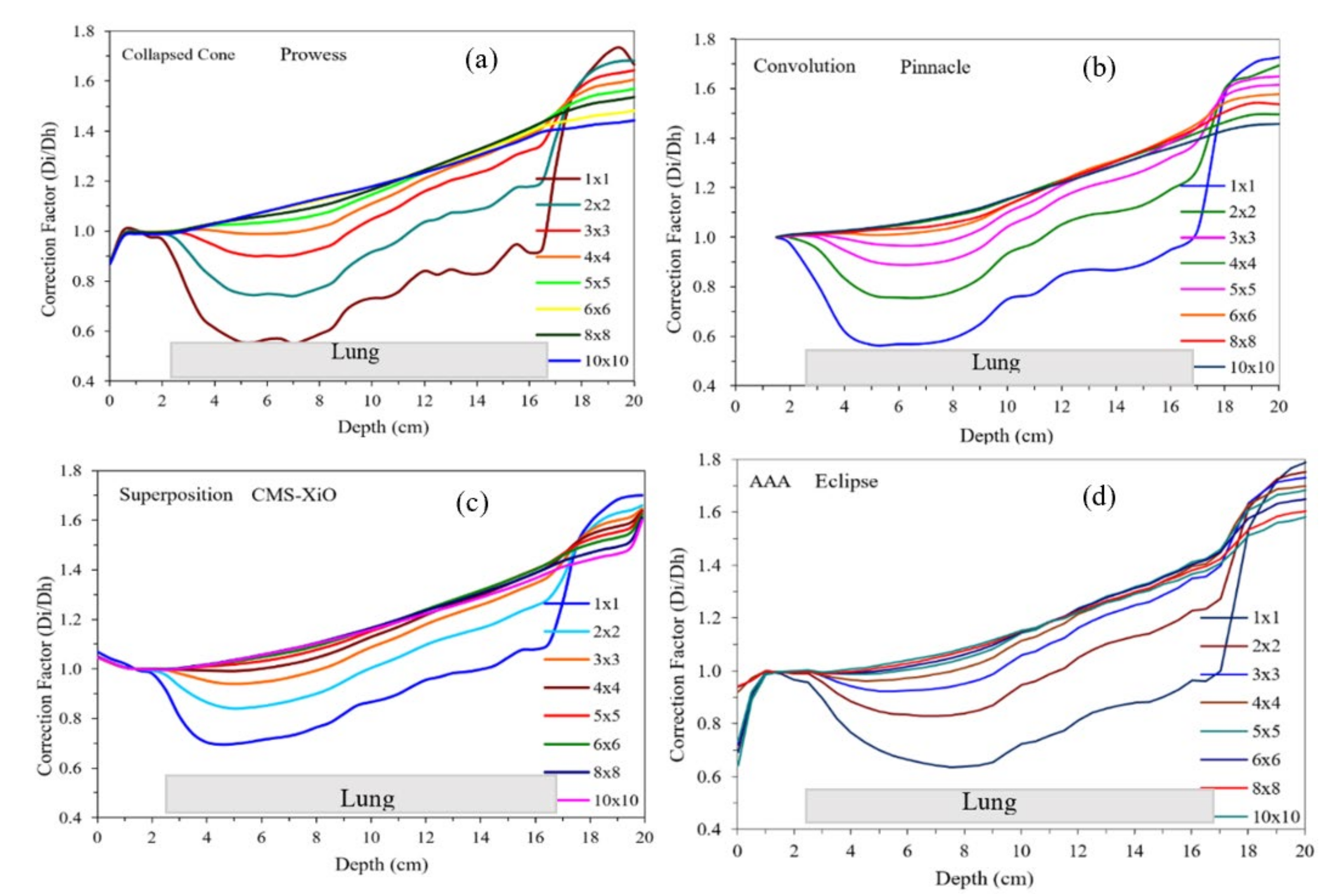
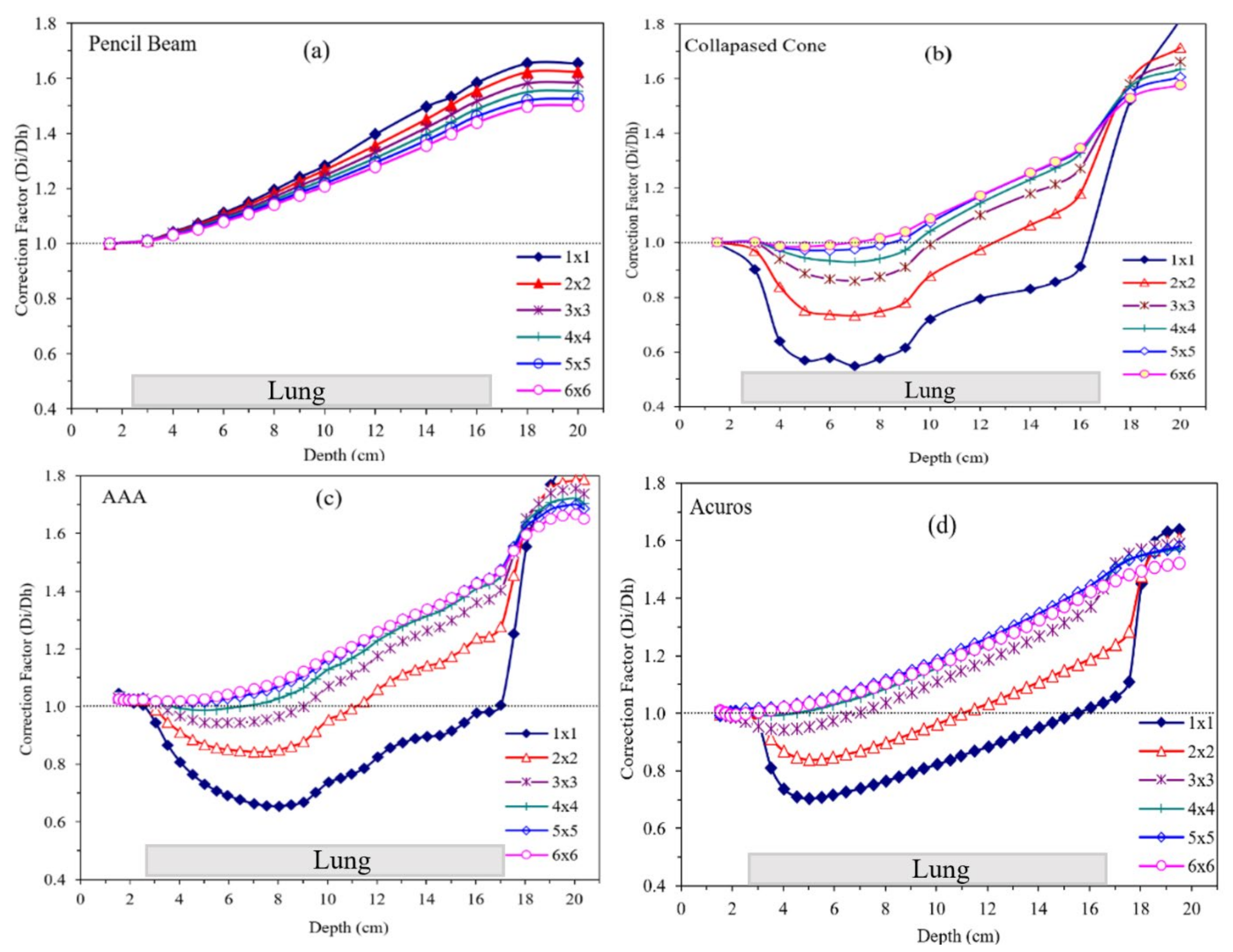
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ACS. American Cancer Society. Cancer Facts and Figures. 2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed on 21 August 2022).
- Shirvani, S.M.; Juloori, A.; Allen, P.K.; Komaki, R.; Liao, Z.; Gomez, D.; O’Reilly, M.; Welsh, J.; Papadimitrakopoulou, V.; Cox, J.D.; et al. Comparison of 2 common radiation therapy techniques for definitive treatment of small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Videtic, G.M.M.; Donington, J.; Giuliani, M.; Heinzerling, J.; Karas, T.Z.; Kelsey, C.R.; Lally, B.E.; Latzka, K.; Lo, S.S.; Moghanaki, D.; et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 295–301. [Google Scholar] [CrossRef]
- Timmerman, R.D.; Paulus, R.; Pass, H.I.; Gore, E.M.; Edelman, M.J.; Galvin, J.; Straube, W.L.; Nedzi, L.A.; McGarry, R.C.; Robinson, C.G.; et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol. 2018, 4, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, R.D.; Hu, C.; Michalski, J.M.; Bradley, J.C.; Galvin, J.; Johnstone, D.W.; Choy, H. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 1287–1288. [Google Scholar] [CrossRef] [PubMed]
- ICRU Report 83. In Prescribing, Recording, and Reporting Intensity-Modulated Photon-Beam Therapy (IMRT) (ICRU Report 83) Report; Report No.: ICRU Report 83; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 2010.
- ICRU Report 91. In Prescribing Recording, and Reporting of Stereotactic Treatments with Small Photon Beams Report; Report No.: ICRU Report 91; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 2017.
- Appel, S.; Bar, J.; Ben-Nun, A.; Perelman, M.; Alezra, D.; Urban, D.; Ben-Ayun, M.; Honig, N.; Ofek, E.; Katzman, T.; et al. Comparative effectiveness of intensity modulated radiation therapy to 3-dimensional conformal radiation in locally advanced lung cancer: Pathological and clinical outcomes. Br. J. Radiol. 2019, 92, 20180960. [Google Scholar] [CrossRef]
- Bradley, J.; Graham, M.V.; Winter, K.; Purdy, J.A.; Komaki, R.; Roa, W.H.; Ryu, J.K.; Bosch, W.; Emami, B. Toxicity and outcome results of RTOG 9311: A phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 318–328. [Google Scholar] [CrossRef]
- Furuse, K.; Kubota, K.; Kawahara, M.; Kodama, N.; Ogawara, M.; Akira, M.; Nakajima, S.; Takada, M.; Kusunoki, Y.; Negoro, S. Phase II study of concurrent radiotherapy and chemotherapy for unresectable stage III non-small-cell lung cancer. Southern Osaka Lung Cancer Study Group. J. Clin. Oncol. 1995, 13, 869–875. [Google Scholar] [CrossRef]
- Erdi, Y.E.; Rosenzweig, K.; Erdi, A.K.; Macapinlac, H.A.; Hu, Y.-C.; Braban, L.E.; Humm, J.L.; Squire, O.D.; Chui, C.-S.; Larson, S.M.; et al. Radiotherapy treatment planning for patients with non-small cell lung cancer using positron emission tomography (PET). Radiother. Oncol. 2002, 62, 51–60. [Google Scholar] [CrossRef]
- Giraud, P.; Antoine, M.; Larrouy, A.; Milleron, B.; Callard, P.; De Rycke, Y.; Carette, M.-F.; Rosenwald, J.-C.; Cosset, J.-M.; Housset, M.; et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1015–1024. [Google Scholar] [CrossRef]
- Narayanasamy, G.; Desai, D.; Morrill, S.; Zhang, X.; Galhardo, E.; Maraboyina, S.; Penagaricano, J. Technical Note: A planning technique to lower normal tissue toxicity in lung SBRT plans based on two likely dependent RTOG metrics. Med. Phys. 2018, 45, 2325–2328. [Google Scholar] [CrossRef]
- Popescu, C.C.; Olivotto, I.A.; Beckham, W.A.; Ansbacher, W.; Zavgorodni, S.; Shaffer, R.; Wai, E.S.; Otto, K. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 287–295. [Google Scholar] [CrossRef]
- Christian, J.A.; Bedford, J.L.; Webb, S.; Brada, M. Comparison of inverse-planned three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 735–741. [Google Scholar] [CrossRef]
- Fogliata, A.; Vanetti, E.; Albers, D.; Brink, C.; Clivio, A.; Knöös, T.; Nicolini, G.; Cozzi, L. On the dosimetric behaviour of photon dose calculation algorithms in the presence of simple geometric heterogeneities: Comparison with Monte Carlo calculations. Phys. Med. Biol. 2007, 52, 1363–1385. [Google Scholar] [CrossRef]
- Fogliata, A.; Nicolini, G.; Vanetti, E.; Clivio, A.; Cozzi, L. Dosimetric validation of the anisotropic analytical algorithm for photon dose calculation: Fundamental characterization in water. Phys. Med. Biol. 2006, 51, 1421–1438. [Google Scholar] [CrossRef]
- Knöös, T.; Wieslander, E.; Cozzi, L.; Brink, C.; Fogliata, A.; Albers, D.; Nyström, H.; Lassen, S. Comparison of dose calculation algorithms for treatment planning in external photon beam therapy for clinical situations. Phys. Med. Biol. 2006, 51, 5785–5807. [Google Scholar] [CrossRef]
- Fogliata, A.; Nicolini, G.; Clivio, A.; Vanetti, E.; Cozzi, L. Critical appraisal of Acuros XB and Anisotropic Analytic Algorithm dose calculation in advanced non-small-cell lung cancer treatments. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1587–1595. [Google Scholar] [CrossRef]
- Fogliata, A.; Nicolini, G.; Clivio, A.; Vanetti, E.; Cozzi, L. Accuracy of Acuros XB and AAA dose calculation for small fields with reference to RapidArc((R)) stereotactic treatments. Med. Phys. 2011, 38, 6228–6237. [Google Scholar] [CrossRef]
- Fogliata, A.; Nicolini, G.; Clivio, A.; Vanetti, E.; Cozzi, L. Dosimetric evaluation of Acuros XB Advanced Dose Calculation algorithm in heterogeneous media. Radiat. Oncol. 2011, 6, 82. [Google Scholar] [CrossRef]
- Yadav, P.; Chang, S.X.; Cheng, C.W.; DesRosiers, C.M.; Mitra, R.K.; Das, I.J. Dosimetric evaluation of high-Z inhomogeneity used for hip prosthesis: A multi-institutional collaborative study. Phys. Med. 2022, 95, 148–155. [Google Scholar] [CrossRef]
- Shiraishi, S.; Fong de Los Santos, L.E.; Antolak, J.A.; Olivier, K.R.; Garces, Y.I.; Park, S.S.; Grams, M.P. Phantom Verification of AAA and Acuros Dose Calculations for Lung Cancer: Do Tumor Size and Regression Matter? Pract. Radiat. Oncol. 2019, 9, 29–37. [Google Scholar] [CrossRef]
- Carrasco, P.; Jornet, N.; Dutch, M.A.; Weber, L.; Ginjaume, M.; Eudaldo, T.; Jurado, D.; Ruiz, A.; Ribas, M. Comparison of dose calculation algorithms in phantoms with lung equivalent hteregeneities under conditions of lateral electronic disequilibrium. Med. Phys. 2004, 31, 2899–2911. [Google Scholar] [CrossRef] [PubMed]
- Fogliata, A.; Cozzi, L. Dose calculation algorithm accuracy for small fields in non-homogeneous media: The lung SBRT case. Phys. Med. 2017, 44, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Ojala, J.J.; Kapanen, M.K.; Hyodynmaa, S.J.; Wigren, T.K.; Pitkanen, M.A. Performance of dose calculation algorithms from three generations in lung SBRT: Comparison with full Monte Carlo-based dose distributions. J. Appl. Clin. Med. Phys. 2014, 15, 4662. [Google Scholar] [CrossRef] [PubMed]
- Chetty, I.J.; Devpura, S.; Liu, D.; Chen, D.; Li, H.; Wen, N.; Kumar, S.; Fraser, C.; Siddiqui, M.S.; Ajlouni, M.; et al. Correlation of dose computed using different algorithms with local control following stereotactic ablative radiotherapy (SABR)-based treatment of non-small-cell lung cancer. Radiother. Oncol. 2013, 109, 498–504. [Google Scholar] [CrossRef]
- Orton, C.G.; Mondalek, P.M.; Spicka, J.T.; Herron, D.S.; Andres, L.I. Lung corrections in photon beam treatment planning: Are we ready? Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 2191–2199. [Google Scholar] [CrossRef]
- Xiao, Y.; Papiez, L.; Paulus, R.; Timmerman, R.; Straube, W.L.; Bosch, W.R.; Michalski, J.; Galvin, J.M. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: Stereotactic body tadiotherapy of inoperable stage I-II non–small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1235–1242. [Google Scholar] [CrossRef]
- Papanikolaou, N.; Battista, J.J.; Boyer, A.L.; Kappas, C.; Klein, E.; Mackie, T.; Sharpe, M.; Van Dyk, J. Tissue Inhomogeneity Corrections for Megavoltage Photon Beams: AAPM Report N0. 85; Medical Physics Publishing: Madison, WI, USA, 2004. [Google Scholar]
- Sempau, J.; Acosta, E.; Baró, J.; Fernandez-Vareá, J.M.; Salvat, F. An algorithm for Monte Carlo simulation of coupled electron-photon transport. Nucl. Inst. Methods B 1997, 132, 377–390. [Google Scholar] [CrossRef]
- Salvat, F.; Fernandez-Vareá, J.M.; Baró, J.; Sempau, J. PENELOPE: An Algorithm and Computer Code for Monte Carlo Simulation of Electron-Photon Showers; Report No.: 799; Informes Technicos Ciemat, Direccion de Techologia: Barcelona, Spain, 1996. [Google Scholar]
- IAEA. Dosimetry of Small Static Fields Used in External Beam Radiotherapy: An IAEA-AAPM International Code of Practice for Reference and Relative Dose Determination, Technical Report Series No. 483; International Atomic Energy Agency: Vienna, Austria, 2017. [Google Scholar]
- Das, I.J.; Francescon, P.; Moran, J.M.; Ahnesjö, A.; Aspradakis, M.M.; Cheng, C.; Ding, G.X.; Fenwick, J.D.; Huq, M.S.; Oldham, M.; et al. Report of AAPM Task Group 155: Megavoltage photon beam dosimetry in small fields and non-equilibrium conditions. Med. Phys. 2021, 48, e886–e921. [Google Scholar] [CrossRef]
- Das, I.J.; Sanfilippo, N.J.; Fogliata, A.; Luca Cozzi, L. Intensity Modulated Radiation Therapy: A Clinical Overview; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Das, I.J.; Moskvin, V.; Johnstone, P.A. Analysis of treatment planning time among systems and planners for intensity-modulated radiation therapy. J. Am. Coll. Radiol. 2009, 6, 514–517. [Google Scholar] [CrossRef]
- Sauer, O.A.; Shepard, D.M.; Mackie, T.R. Application of constrained optimization to radiotherapy planning. Med. Phys. 1999, 26, 2359–2366. [Google Scholar] [CrossRef]
- Nicolini, G.; Ghosh-Laskar, S.; Shrivastava, S.K.; Banerjee, S.; Chaudhary, S.; Agarwal, J.P.; Munshi, A.; Clivio, A.; Fogliata, A.; Mancosu, P.; et al. Volumetric modulation arc radiotherapy with flattening filter-free beams compared with static gantry IMRT and 3D conformal radiotherapy for advanced esophageal cancer: A feasibility study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 553–560. [Google Scholar] [CrossRef]
- Chetty, I.J.; Rosu, M.; McShan, D.L.; Fraass, B.A.; Ten Haken, R.K. The influence of beam model differences in the comparison of dose calculation algorithms for lung cancer treatment planning. Phys. Med. Biol. 2005, 50, 801–815. [Google Scholar] [CrossRef]
- Jones, A.O.; Das, I.J.; Jones, F.L. A Monte Carlo study of IMRT beamleats in homogeneous media. Med. Phys. 2003, 30, 296–300. [Google Scholar] [CrossRef]
- Jones, A.O.; Das, I.J. Comparison of inhomogeneity correction algorithms in small photon fields. Med Phys. 2005, 32, 766–776. [Google Scholar] [CrossRef]
- Galavis, P.E.; Hu, L.; Holmes, S.; Das, I.J. Characterization of the plastic scintillation detector Exradin W2 for small field dosimetry. Med. Phys. 2019, 46, 2468–2476. [Google Scholar] [CrossRef]
- Akino, Y.; Fujiwara, M.; Okamura, K.; Shiomi, H.; Mizuno, H.; Isohashi, F.; Suzuki, O.; Seo, Y.; Tamari, K.; Ogawa, K. Characterization of a microSilicon diode detector for small-field photon beam dosimetry. J. Radiat. Res. 2020, 61, 410–418. [Google Scholar] [CrossRef]
- Fogliata, A.; Cozzi, L.; Clivio, A.; Ibatici, A.; Mancosu, P.; Navarria, P.; Nicolini, G.; Santoro, A.; Vanetti, E.; Scorsetti, M. Preclinical assessment of volumetric modulated arc therapy for total marrow irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 628–636. [Google Scholar] [CrossRef]
- Ottosson, R.O.; Karlsson, A.; Behrens, C.F. Pareto front analysis of 6 and 15 MV dynamic IMRT for lung cancer using pencil beam, AAA and Monte Carlo. Phys. Med. Biol. 2010, 55, 4521–4533. [Google Scholar] [CrossRef]
- Kry, S.F.; Feygelman, V.; Balter, P.; Knoos, T.; Charlie Ma, C.M.; Snyder, M.; Tonner, B.; Vassiliev, O.N. AAPM Task Group 329: Reference dose specification for dose calculations: Dose-to-water or dose-to-muscle? Med. Phys. 2020, 47, e52–e64. [Google Scholar] [CrossRef]

| Structure | Constraints |
|---|---|
| PTV | 95% dose 95% volume |
| 110% dose < 10% volume | |
| 115% dose < 5% volume | |
| Lung | V20 < 20% |
| Heart | 50% dose < 70% volume |
| 65% dose < 2% volume | |
| 75% dose < 0 volume | |
| Spinal Cord | 60% dose < 2% volume |
| 70% dose < 0% volume |
| Vendor | TPS/Version | Algorithm | Treatment Machine |
|---|---|---|---|
| BrainLab | BrainScan/V5.31 | Pencil beam convolution | Varian 2100EX |
| CMS | XiO/V4.3.1 | Superposition/PBC | Varian Trilogy |
| Nomos * | Corvous V3 | Clarkson Integration | Siemens Oncor |
| Varian | Eclipse V13.2 | Pencil beam/AAA | Varian TrueBeam |
| MDS-Nordion * | Helax-TMS, V 6.0 | Pencil Beam | Siemens Primus |
| Siemens KonRad * | KonRad V 2.2 | Pencil Beam | Siemens Primus |
| Nucletron | Oncentra/V3.0 | Pencil beam/CC | Siemens Oncor |
| Philips | Pinnacle V 9.7 | Convolution/PBC | Elekta-Infinity |
| Nucletron * | Oncentra Plato V 13.7 | Pencil Beam | Elekta-SL |
| Prowess * | Panther/V5.2 | Convolution/PBC | Elekta-SL |
| Radionics | V RT4 | Pencil beam (modified) | Varian 2100EX |
| Univ. of North Carolina | PLanUNC | Modified Batho, PB | Siemens Primus |
| Monte Carlo | Penelope/2002 | Mixed MC Scheme | Varian Trilogy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, P.; DesRosiers, C.M.; Mitra, R.K.; Srivastava, S.P.; Das, I.J. Variability of Low-Z Inhomogeneity Correction in IMRT/SBRT: A Multi-Institutional Collaborative Study. J. Clin. Med. 2023, 12, 906. https://doi.org/10.3390/jcm12030906
Yadav P, DesRosiers CM, Mitra RK, Srivastava SP, Das IJ. Variability of Low-Z Inhomogeneity Correction in IMRT/SBRT: A Multi-Institutional Collaborative Study. Journal of Clinical Medicine. 2023; 12(3):906. https://doi.org/10.3390/jcm12030906
Chicago/Turabian StyleYadav, Poonam, Colleen M. DesRosiers, Raj K. Mitra, Shiv P. Srivastava, and Indra J. Das. 2023. "Variability of Low-Z Inhomogeneity Correction in IMRT/SBRT: A Multi-Institutional Collaborative Study" Journal of Clinical Medicine 12, no. 3: 906. https://doi.org/10.3390/jcm12030906
APA StyleYadav, P., DesRosiers, C. M., Mitra, R. K., Srivastava, S. P., & Das, I. J. (2023). Variability of Low-Z Inhomogeneity Correction in IMRT/SBRT: A Multi-Institutional Collaborative Study. Journal of Clinical Medicine, 12(3), 906. https://doi.org/10.3390/jcm12030906







