Association of Anemia with Clinical Symptoms Commonly Attributed to Anemia—Analysis of Two Population-Based Cohorts
Abstract
:1. Introduction
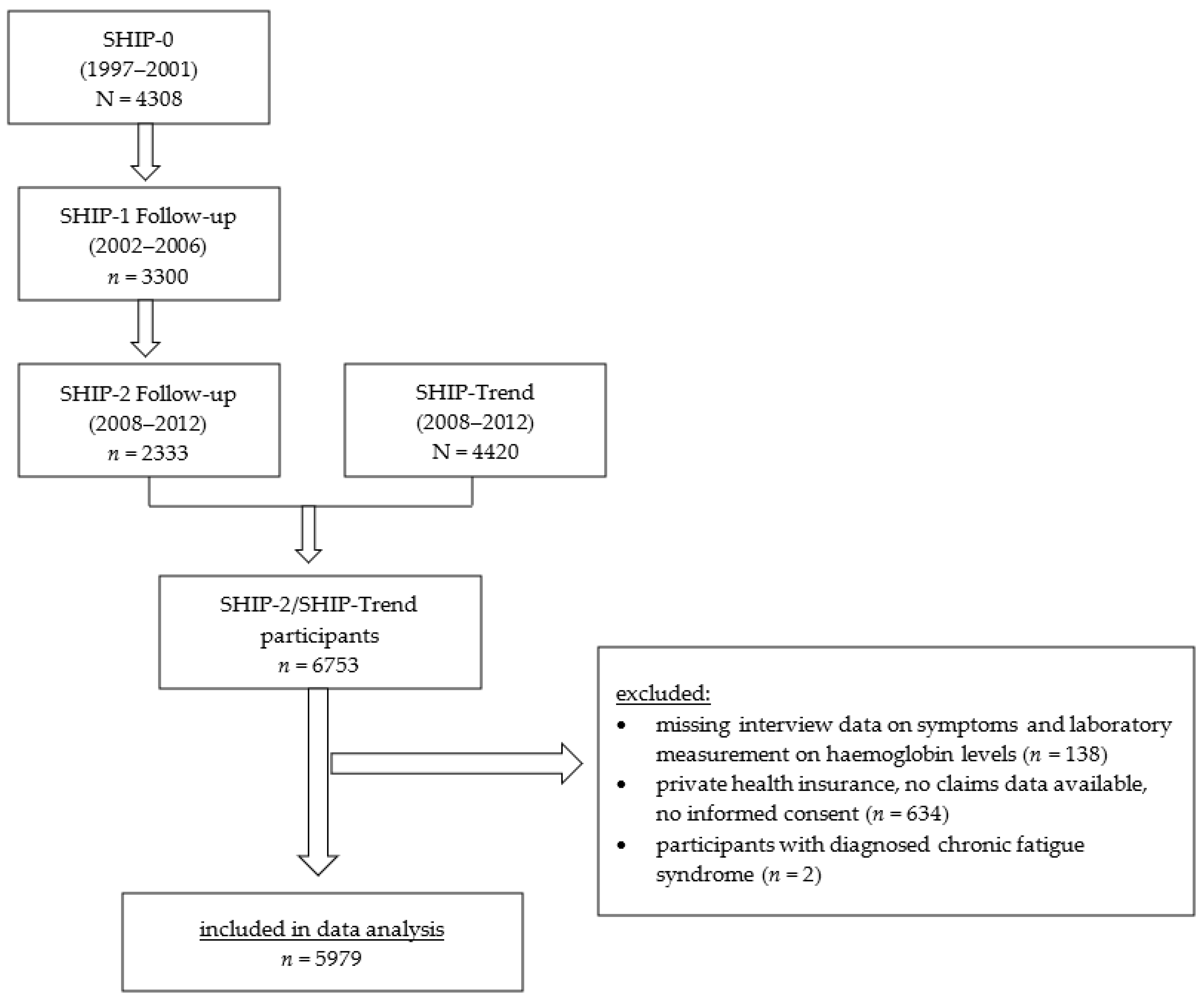
2. Materials and Methods
2.1. Design and Sample
2.2. Outcomes, Predictors, and Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Anemia
3.3. Association of Anemia and Symptoms
3.3.1. Fatigue
3.3.2. Lack of Energy
3.3.3. Lack of Concentration
3.3.4. Dyspnea
3.4. Positive Predictive Value of Symptoms for the Presence of Anemia
3.5. Association of Symptoms with Other Variables
4. Discussion
4.1. Summary of the Main Results
4.2. Comparison with Scientific Literature
4.3. Limitations
4.4. Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Anaemia. 2021. Available online: https://www.who.int/health-topics/anaemia#tab=tab_1 (accessed on 13 October 2021).
- Gómez Ramírez, S.; Remacha Sevilla, Á.F.; Muñoz Gómez, M. Anaemia in the elderly. Med. Clín. 2017, 149, 496–503. [Google Scholar] [CrossRef]
- Beghé, C.; Wilson, A.; Ershler, W.B. Prevalence and outcomes of anemia in geriatrics: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. 7A), 3S–10S. [Google Scholar] [CrossRef] [PubMed]
- Wouters, H.J.C.M.; van der Klauw, M.M.; de Witte, T. Association of anemia with health-related quality of life and survival: A large population-based cohort study. Haematologica 2019, 104, 468–476. [Google Scholar] [CrossRef]
- Leischker, A.H.; Fetscher, S.; Kolb, G.F. Anämie im Alter (Anaemia in the elderly). Dtsch. Med. Wochenschr. 2016, 141, 954–959. [Google Scholar] [CrossRef]
- Lanier, J.B.; Park, J.J.; Callahan, R.C. Anemia in Older Adults. Am. Fam. Physician 2018, 98, 437–442. [Google Scholar] [PubMed]
- Eaton, K.P.; Levy, K.; Soong, C.; Pahwa, A.K.; Petrilli, C.; Ziemba, J.B.; Cho, H.J.; Alban, R.; Blanck, J.F.; Parsons, A.S. Evidence-Based Guidelines to Eliminate Repetitive Laboratory Testing. JAMA Intern. Med. 2017, 177, 1833–1839. [Google Scholar] [CrossRef]
- Boennelykke, A.; Jensen, H.; Granfeldt Østgård, L.S.; Falborg, A.Z.; Christensen, K.S.; Hansen, A.T.; Emery, J.; Vedsted, P. Insufficient classification of anaemia in general practice: A Danish register-based observational study. Scand. J. Prim. Health Care 2021, 39, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.; Schafer, A.I.; La Cecil, R.F. (Eds.) Goldman-Cecil Medicine, 26th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Brunskill, S.J.; Millette, S.L.; Shokoohi, A.; Pulford, E.C.; Doree, C.; Murphy, M.F.; Stanworth, S. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst. Rev. 2015, 21, CD009699. [Google Scholar] [CrossRef]
- Weckmann, G.F.C.; Stracke, S.; Haase, A.; Spallek, J.; Ludwig, F.; Angelow, A.; Emmelkamp, J.M.; Mahner, M.; Chenot, J.-F. Diagnosis and management of non-dialysis chronic kidney disease in ambulatory care: A systematic review of clinical practice guidelines. BMC Nephrol. 2018, 19, 258. [Google Scholar] [CrossRef] [Green Version]
- Mansour, D.; Hofmann, A.; Gemzell-Danielsson, K. A Review of Clinical Guidelines on the Management of Iron Deficiency and Iron-Deficiency Anemia in Women with Heavy Menstrual Bleeding. Adv. Ther. 2021, 38, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Völzke, H.; Alte, D.; Schmidt, C.O.; Radke, D.; Lorbeer, R.; Friedrich, N.; Aumann, N.; Lau, K.; Piontek, M.; Born, G.; et al. Cohort profile: The study of health in Pomerania. Int. J. Epidemiol. 2011, 40, 294–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Völzke, H.; Schössow, J.; Schmidt, C.O.; Jürgens, C.; Richter, A.; Werner, A.; Werner, N.; Radke, D.; Teumer, A.; Ittermann, T.; et al. Cohort Profile Update: The Study of Health in Pomerania (SHIP). Int. J. Epid. 2022, 51, e372–e383. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity: Vitamin and Mineral Nutrition Information System. 2011. Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 17 February 2021).
- Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin (DEGAM) S3-Leitlinine Müdigkeit [S3-guideline Tiredness], Reg. Nr. 053-002. 2017. Available online: https://www.awmf.org/uploads/tx_szleitlinien/053-002m_S3_Muedigkeit_2018-05.pdf (accessed on 26 October 2022).
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.M.; Elwood, P.C. Symptoms of iron deficiency anaemia. A community survey. Br. J. Prev. Soc. Med. 1966, 20, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Stadje, R.; Dornieden, K.; Baum, E.; Becker, A.; Biroga, T.; Bösner, S.; Haasenritter, J.; Keunecke, C.; Viniol, A.; Donner-Banzhoff, N. The differential diagnosis of tiredness: A systematic review. BMC Fam. Pract. 2016, 17, 147. [Google Scholar] [CrossRef] [Green Version]
- Knottnerus, J.A.; Knipschild, P.G.; van Wersch, J.W.; Sijstermanns, A.H.J. Onverklaarde moeheid en hemoglobinegehalte; een onderzoek vanuit de huisartsenpraktijk (Unexplained fatigue and hemoglobin level; a study of family practice patients). Ned. Tijdschr. Geneeskd. 1986, 130, 402–405. [Google Scholar] [PubMed]
- Macher, S.; Herster, C.; Holter, M.; Moritz, M.; Matzhold, E.M.; Stojakovic, T.; Pieber, T.R.; Schlenke, P.; Drexler, C.; Amrein, K. The Effect of Parenteral or Oral Iron Supplementation on Fatigue, Sleep, Quality of Life and Restless Legs Syndrome in Iron-Deficient Blood Donors: A Secondary Analysis of the IronWoMan RCT. Nutrients 2020, 12, 1313. [Google Scholar] [CrossRef]
- Verdon, F.; Burnand, B.; Stubi, C.-L.F.; Bonard, C.; Graff, M.; Michaud, A.; Bischoff, T.; de Vevey, M.; Studer, J.-P.; Herzig, L.; et al. Iron supplementation for unexplained fatigue in non-anaemic women: Double blind randomised placebo controlled trial. BMJ 2003, 326, 1124. [Google Scholar] [CrossRef] [Green Version]
- Vaucher, P.; Druais, P.-L.; Waldvogel, S.; Favrat, B. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: A randomized controlled trial. CMAJ 2012, 184, 1247–1254. [Google Scholar] [CrossRef] [Green Version]
- Falkingham, M.; Abdelhamid, A.; Curtis, P.; Fairweather-Tait, S.; Dye, L.; Hooper, L. The effects of oral iron supplementation on cognition in older children and adults: A systematic review and meta-analysis. Nutr. J. 2010, 9, 4. [Google Scholar] [CrossRef]
- Johnstone, P.A.S.; Alla, R.; Yu, H.-H.M.; Portman, D.; Cheng, H.; Mitchell, R.; Jim, H. Patient-reported outcomes: Using ESAS to screen for anemia. Support. Care Cancer 2020, 28, 4141–4145. [Google Scholar] [CrossRef] [PubMed]
- Robalo Nunes, A.; Mairos, J.; Brilhante, D.; Marques, F.; Belo, A.; Cortez, J.; Fonseca, C. Screening for Anemia and Iron Deficiency in the Adult Portuguese Population. Anemia 2020, 2020, 1048283. [Google Scholar] [CrossRef] [PubMed]





| Disease | Billing Diagnosis ICD-10 GM | Time Frame and Kind of Diagnosis |
|---|---|---|
| at least one relevant and confirmed ICD diagnosis coded as …. prior to the SHIP-2/SHIP-Trend study entrance of the participants | ||
| Anemia | D50, D64, D55, D59, D53, D51, D61 |
|
| Heart Failure | I50 |
|
| Depression, Anxiety | F32, F33, F41, F31, F06, F20, F61, F40, F43, F34, F60 |
|
| Diabetes | E10, E11, E13, E14, E12 |
|
| Chronic Kidney Disease | N18 |
|
| Hypothyroidism | E01, E02, E03 |
|
| COPD | J44 |
|
| Asthma | J45 |
|
| Cancer | C0–C96 |
|
| Charlson Comorbidity Index | relevant diagnosis |
|
| Medication | ATC-CODES |
|---|---|
| Antineoplastic Agents | L01 |
| Benzodiazepine Derivatives | N05CD |
| Antidepressants | N06A |
| Antipsychotics | N05A |
| Antihistamine | R06A |
| Antihypertensives and Diuretics | C02, C03 |
| Opioids | N02A |
| Anti-Parkinson Drugs | N04 |
| All Participants (N = 5979; 100%) | Participants with Anemia (n = 379; 6.3%) | Participants without Anemia (n = 5600; 93.7%) | |
|---|---|---|---|
| Women | 3149 (52.7) | 183 (3.06) | 2966 (49.6) |
| Age | |||
| 20–29 | 327 (5.5) | 18 (0.3) | 309 (5.2) |
| 30–49 | 84 (1.4) | 1953 (32.7) | 2037 (34.1) |
| 50–69 | 2519 (42.1) | 138 (2.3) | 2381 (39.8) |
| >=70 | 1096 (18.3) | 139 (2.3) | 957 (16.0) |
| Fatigue | 1775 (29.7) | 128 (2.2) | 1647 (27.5) |
| Lack of energy | 968 (16.2) | 81 (1.4) | 887 (14.8) |
| Lack of concentration | 927 (15.5) | 70 (1.2) | 857 (14.3) |
| Shortness of breath/weakness | 1727 (28.9) | 145 (2.4) | 1582 (26.5) |
| Iron deficiency (serum ferritin) | 292 (5.0) (180 missing) | 95 (1.6) (13 missing) | 197 (3.4) (167 missing) |
| Depression /anxiety (SHIP-data) | 1273 (21.3) (22 missing) | 74 (1.2) (2 missing) | 1199 (20.1) (20 missing) |
| Depression / anxiety (claims data) | 715 (12.0) | 45 (0.8) | 670 (11.2) |
| Insomnia | 1453 (24.3) | 109 (1.8) | 1344 (22.5) |
| Hypertension | 569 (9.6) (30 missing) | 16 (0.3) (3 missing) | 553 (9.3) (27 missing) |
| Heart failure (claims data) | 121 (2.0) | 28 (0.4 | 93 (1.6) |
| Chronic kidney disease (claims data) | 165 (2.8) | 44 (0.8) | 121 (2.0) |
| Diabetes (claims data) | 794 (13.3) | 94 (1.6) | 700 (11.7) |
| Hypothyroidism (claims data) | 375 (6.3) | 27 (0.5) | 348 (5.8) |
| Cancer (claims data) | 92 (1.5) | 14 (0.2) | 78 (1.3) |
| Asthma (claims data) | 301 (5.0) | 20 (0.3) | 281 (4.7) |
| COPD (claims data) | 209 (3.5) | 28 (0.5) | 181 (3.0) |
| Charlson Comorbidity Index (claims data) | |||
| 0 | 4417 (73.9) | 216 (3.6) | 4201 (70.3) |
| 1 | 951 (15.9) | 63 (1.0) | 888 (14.9) |
| 2 | 324 (5.4) | 42 (0.7) | 282 (4.7) |
| 3–5 | 253 (4.2) | 49 (0.8) | 204 (3.4) |
| 6–9 | 34 (0.6) | 9 (0.2) | 25 (0.4) |
| Number of medications | |||
| 0 | 1656 (27.7) | 69 (1.2) | 1587 (26.5) |
| 1 | 1125 (18.8) | 48 (0.8) | 1077 (18.0) |
| 2 | 877 (14.7) | 40 (0.7) | 837 (14.0) |
| 3 | 607 (10.2) | 37 (0.7) | 570 (9.5) |
| 4 | 465 (7.8) | 29 (0.5) | 436 (7.3) |
| 5 | 385 (6.4) | 37 (0.6) | 348 (5.8) |
| >6 | 864 (14.4) | 119 (1.9) | 745 (12.5) |
| Medication with possible side-effect fatigue | 1061 (17.8) | 112 (1.9) | 949 (15.9) |
| Pregnant | 199 (3.3) | 4 (0.1) | 195 (3.2) |
| Anemia Severity | Women (n = 179) | Pregnant Women (n = 4) | Men (n = 196) |
|---|---|---|---|
| Mild | 110–119 g/L | 100–109 g/L | 110–129 g/L |
| 140 (78.4%) | 4 (100%) | 179 (92.2%) | |
| Moderate | 80–109 g/L | 70–99 g/L | 80–109 g/L |
| 39 (21.6%) | - | 15 (6.4%) | |
| Severe | <80 g/L | <70 g/L | <80 g/L |
| - | - | 2 (1.4%) |
| Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fatigue | Lack of Energy | Lack of Concentration | Dyspnea and/or Weakness | |||||
| Predictors | OR (95% CI) n = 5957 | OR (95% CI) n = 5957 | OR (95% CI) n = 5957 | OR (95% CI) n = 5957 | ||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Anemia | 1.15 (0.94; 1.40) | 1.19 (0.98; 1.46) | 1.41 (1.09; 1.81) | 1.45 (1.13; 1.86) | 1.12 (0.87; 1.44) | 1.13 (0.88; 1.46) | 1.04 (0.85; 1.29) | 1.11 (0.90; 1.37) |
| Women | 1.40 (1.26; 1.55) | 1.42 (1.28; 1.57) | 1.33 (1.16; 1.52) | 1.33 (1.16; 1.53) | 1.12 (0.98; 1.28) | 1.11 (0.97; 1.28) | 1.08 (0.97; 1.21) | 1.09 (0.98; 1.22) |
| Age (years) | 0.98 (0.97; 0.99) | 0.98 (0.98; 0.99) | 0.98 (0.97; 0.99) | 0.99 (0.98; 1.00) | 1.01 (1.00; 1.01) | 1.01 (1.00; 1.02) | 1.01 (1.01; 1.02) | 1.03 (1.02; 1.04) |
| Number of medications | 1.11 (1.08; 1.13) | - | 1.13 (1.09; 1.16) | - | 1.08 (1.05; 1.10) | - | 1.19 (1.17; 1.22) | - |
| Medication with possible side effect fatigue | - | 1.25 (1.10; 1.42) | - | 1.58 (1.36; 1.85) | - | 1.70 (1.47; 1.98) | - | 1.69 (1.49; 1.92) |
| Depression and/or anxiety (SHIP) | 3.44 (3.08; 3.84) | 3.49 (3.13; 3.91) | 8.22 (7.22; 9.36) | 8.13 (7.14; 9.26) | 6.79 (5.95; 7.74) | 6.58 (5.77; 7.51) | 2.66 (2.36; 2.99) | 2.68 (2.38; 3.01) |
| Insomnia (SHIP) | 2.24 (2.01; 2.49) | 2.31 (2.08; 2.57) | 2.06 (1.80; 2.35) | 2.12 (1.86; 2.43) | 1.76 (1.54; 2.02) | 1.78 (1.56; 2.04) | 1.65 (1.47; 1.84) | 1.72 (1.54; 1.93) |
| CCI (claims data) | 1.10 (1.04; 1.16) | 1.18 (1.12; 1.25) |
1.07 (0.99; 1.14) | 1.15 (1.08; 1.22) |
1.08 (1.01; 1.15) | 1.11 (1.04; 1.18) | 1.09 (1.03; 1.16) | 1.20 (1.14; 1.27) |
| Heart failure (claims data) | - | - | - | - | - | - | 2.66 (1.7; 4.00) | 3.03 (2.03; 4.53) |
| COPD (claims data) | - | - | - | - | - | - | 1.99 (1.49; 2.65) | 2.14 (1.61; 2.83) |
| Asthma (claims data) | - | - | - | - | - | - | 3.36 (2.67; 4.25) | 3.63 (2.88; 4.56) |
| Number of Symptoms | All Participants (n = 5979) (%) | Participants without Anemia (n = 5600) (%) | Participants with Anemia (n = 379) (%) | Positive Predictive Value % (95% CI) |
|---|---|---|---|---|
| Four Symptoms | 295 (4.9) | 271 (4.7) | 24 (6.6) | 8.4 (5.8–10.9) |
| Three Symptoms | 430 (7.2) | 387 (6.9) | 43 (10.5) | 8.9 (6.8–11.2) |
| Two Symptoms | 708 (11.5) | 658 (11.4) | 50 (13.1) | 6.9 (5.4–8.5) |
| One Symptom | 1511 (24.7) | 1412 (24.7) | 99 (24.6) | 6.1 (5.1–7.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weckmann, G.; Kiel, S.; Chenot, J.-F.; Angelow, A. Association of Anemia with Clinical Symptoms Commonly Attributed to Anemia—Analysis of Two Population-Based Cohorts. J. Clin. Med. 2023, 12, 921. https://doi.org/10.3390/jcm12030921
Weckmann G, Kiel S, Chenot J-F, Angelow A. Association of Anemia with Clinical Symptoms Commonly Attributed to Anemia—Analysis of Two Population-Based Cohorts. Journal of Clinical Medicine. 2023; 12(3):921. https://doi.org/10.3390/jcm12030921
Chicago/Turabian StyleWeckmann, Gesine, Simone Kiel, Jean-François Chenot, and Aniela Angelow. 2023. "Association of Anemia with Clinical Symptoms Commonly Attributed to Anemia—Analysis of Two Population-Based Cohorts" Journal of Clinical Medicine 12, no. 3: 921. https://doi.org/10.3390/jcm12030921
APA StyleWeckmann, G., Kiel, S., Chenot, J.-F., & Angelow, A. (2023). Association of Anemia with Clinical Symptoms Commonly Attributed to Anemia—Analysis of Two Population-Based Cohorts. Journal of Clinical Medicine, 12(3), 921. https://doi.org/10.3390/jcm12030921