SARS-CoV-2 Vaccination and Clinical Presentation of COVID-19 in Patients Hospitalized during the Delta- and Omicron-Predominant Periods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
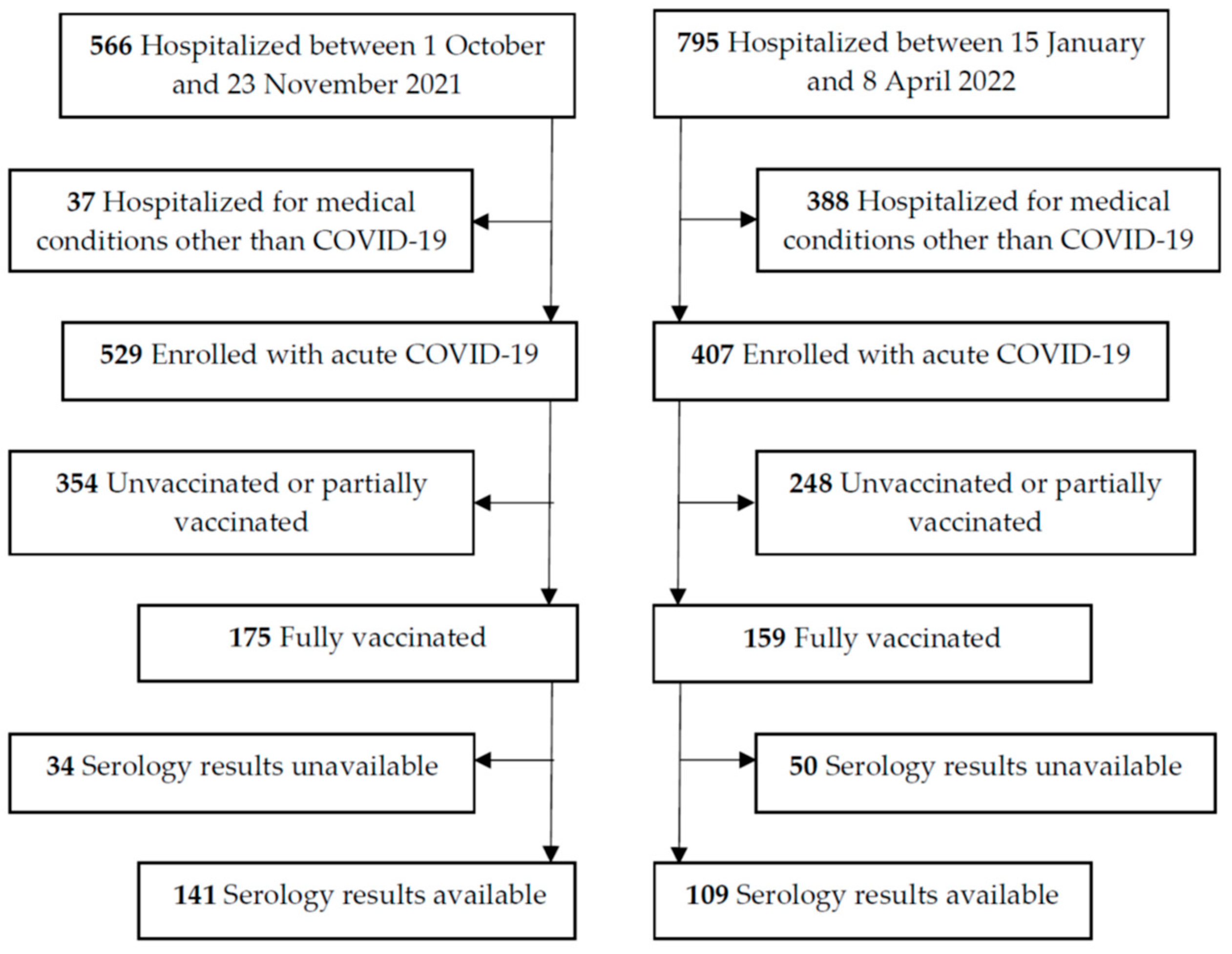
2.2. Participants
2.3. Data Collection
2.4. Laboratory Analysis
2.5. Classification of Vaccination Status and COVID-19 Severity
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Hospitalized Patients with COVID-19
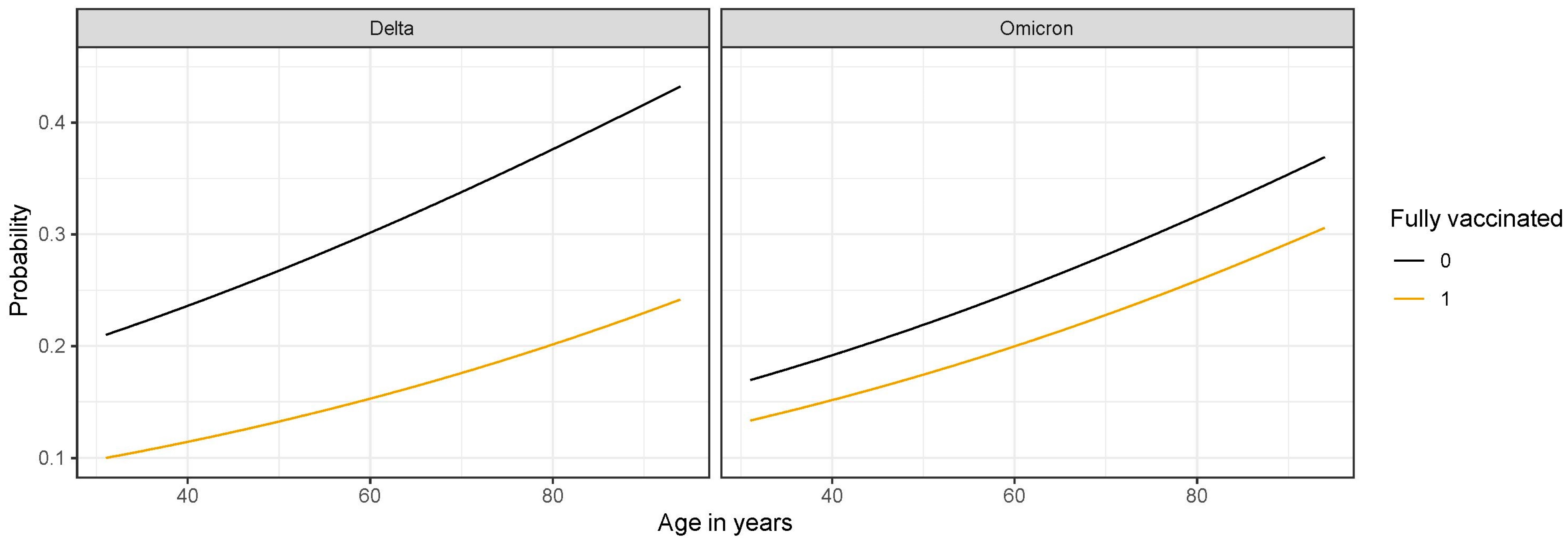
3.2. Association of SARS-CoV-2 Variant and Vaccination Status with Progression to Critically Severe Disease
3.3. COVID-19 in Vaccine Breakthrough Cases
3.4. SARS-CoV-2 Antibody Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrotri, M.; Krutikov, M.; Palmer, T.; Giddings, R.; Azmi, B.; Subbarao, S.; Fuller, C.; Irwin-Singer, A.; Davies, D.; Tut, G.; et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): A prospective cohort study. Lancet Infect. Dis. 2021, 21, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Juthani, P.V.; Gupta, A.; Borges, K.A.; Price, C.C.; Lee, A.I.; Won, C.H.; Chun, H.J. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect. Dis. 2021, 21, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Keehner, J.; Horton, L.E.; Pfeffer, M.A.; Longhurst, C.A.; Schooley, R.T.; Currier, J.S.; Abeles, S.R.; Torriani, F.J. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N. Engl. J. Med. 2021, 384, 1774–1775. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. J. Am. Med. Assoc. 2021, 326, 2043–2054. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit. Care. Med. 2020, 48, 440–469. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant—United States, December 1–8, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1731–1734. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern/ (accessed on 16 December 2022).
- Wolter, J.N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, G.D.; Everatt, J.; Bhiman, J.N.; Scheepers, C.; et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet 2022, 399, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Maslo, C.; Friedland, R.; Toubkin, M.; Anchen, L.; Akaloo, T.K.B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared with Previous Waves. J. Am. Med. Assoc. 2022, 327, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Ward, I.L.; Bermingham, C.; Ayoubkhani, D.; Gethings, O.J.; Pouwels, K.B.; Yates, T.; Khunti, K.; Hippisley-Cox, J.; Banerjee, A.; Walker, A.S.; et al. Risk of COVID-19 related deaths for SARS-CoV-2 omicron cohort study. Br. Med. J. 2022, 378, e070695. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Severity of Disease Associated with Omicron Variant as Compared with Delta Variant in Hospitalized Patients with Suspected or Confirmed SARS-CoV-2 Infection. Available online: https://www.who.int/publications/i/item/9789240051829 (accessed on 16 December 2022).
- Fall, A.; Eldesouki, R.E.; Sachithanandham, J.; Morris, C.P.; Norton, J.M.; Gaston, D.C.; Forman, M.; Abdullah, O.; Gallagher, N.; Li, M.; et al. The displacement of the SARS-CoV-2 variant Delta with Omicron: An investigation of hospital admissions and upper respiratory viral loads. eBioMedicine 2022, 79, 104008. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Kerr, S.; Woolhouse, M.; McMenamin, J.; Robertson, C.; on behalf of the EAVE II Collaborators. Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): A national cohort study with nested test-negative design. Lancet Infect. Dis. 2022, 22, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The incubation period of coronavirus disease 2019 (CoVID-19) from publicly reported confirmed cases: Estimation and application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Kmetič, P.; Valenčak, A.O.; Komloš, K.F.; Seme, K.; Sagadin, M.; Korva, M.; Poljak, M. Real-life head-to-head comparison of performance of two high-throughput automated assays for detection of SARS-CoV-2 RNA in nasopharyngeal swabs: The Alinity m and cobas 6800 SARS-CoV-2 assays. J. Mol. Diagn. 2021, 23, 920–928. [Google Scholar]
- Resman Rus, K.; Korva, M.; Knap, N.; Avšič Županc, T.; Poljak, M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J. Clin. Virol. 2021, 139, 104820. [Google Scholar] [CrossRef]
- European Medicines Agency. COVID-19 Vaccines: Authorised; EMA: Amsterdam, The Netherlands, 2021; Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised (accessed on 16 December 2022).
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Andersen, P.K.; Keiding, N. Multi-state models for event history analysis. Stat. Methods Med. Res. 2002, 11, 91–115. [Google Scholar] [CrossRef]
- World Health Organization; European Centre for Disease Prevention and Control. Joint ECDC-WHO Regional Office for Europe Weekly COVID-19 Surveillance Bulletin; WHO: Geneva, Switzerland; ECDC: Copenhagen, Denmark, 2021; Available online: https://worldhealthorg.shinyapps.io/euro-covid19/ (accessed on 16 December 2022).
- Tracking SARS-CoV-2 Epidemic in Slovenia. Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana, Slovenia. Available online: https://imi.si/novosti-kategorija-covid-19/ (accessed on 16 December 2022).
- Ulloa, A.C.; Buchan, S.A.; Daneman, N.B.K. Estimates of SARS-CoV-2 Omicron Variant Severity in Ontario, Canada. J. Am. Med. Assoc. 2022, 327, 1286–1288. [Google Scholar] [CrossRef]
- Bouzid, D.; Visseaux, B.; Kassasseya, C.; Daoud, A.; Fémy, F.; Hermand, C.; Truchot, J.; Beaune, S.; Javaud, N.; Peyrony, O.; et al. Comparison of Patients Infected with Delta Versus Omicron COVID-19 Variants Presenting to Paris Emergency Departments. Ann. Intern. Med. 2022, 175, 831–837. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Ho, J.C.W.; Cheung, M.; Ng, K.; Ching, R.H.H.; Lai, K.; Kam, T.T.; Gu, H.; Sit, K.; Hsin, M.K.Y.; et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022, 603, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Thompson, M.G.; Natarajan, K.; Irving, S.A.; Rowley, E.A. Effectiveness of a Third Dose of mRNA Vaccines against COVID-19—Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults during Periods of Delta and Omicron Variant Predominance. Morb. Mortal. Wkly. Rep. 2022, 71, 139–145. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Advice for the Public. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed on 16 December 2022).
- COVID-19 Tracker. Available online: https://covid-19.sledilnik.org/en/stats (accessed on 16 December 2022).
- Havers, F.P.; Pham, H.; Taylor, C.A.; Whitaker, M.; Patel, K.; Anglin, O.; Kambhampati, A.K.; Milucky, J.; Zell, E.; Moline, H.L.; et al. COVID-19-Associated Hospitalizations among Vaccinated and Unvaccinated Adults 18 Years or Older in 13 US States, January 2021 to April 2022. JAMA Intern. Med. 2022, 182, 1071–1081. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19 Vaccine Effectiveness Monthly Update. 2022. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccine-effectiveness (accessed on 16 December 2022).
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Ciesla, A.A.; Fleming-Dutra, K.E.; Smith, Z.R.; Britton, A.; Wiegand, R.E.; Miller, J.D.; Accorsi, E.K.; Schrag, S.J.; Verani, J.R.; et al. Effectiveness of Bivalent mRNA Vaccines in Preventing Symptomatic SARS-CoV-2 Infection—Increasing Community Access to Testing Program, United States, September–November 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1526–1530. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Xue, M.; Zheng, P.; Lyu, J.; Zhan, Z.; Hu, H.; Zhang, Y.; Zhang, X.D.; Sun, B. Factors affecting the antibody immunogenicity of vaccines against sars-cov-2: A focused review. Vaccines 2021, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Delta Period 1 | Omicron Period 1 | p-Value 2 |
|---|---|---|---|
| n = 529/566 (93.5%) 3 | n = 407/795 (51.9%) 3 | ||
| Age | 65 (53–78) | 75 (64–84) | <0.001 |
| Men | 309 (58.4) | 206 (50.6) | 0.02 |
| Charlson comorbidity index | 3 (1–4) | 4 (3–6) | <0.001 |
| Chronic illnesses 4 | |||
| Cardiovascular disease | 298 (56.3) | 281 (69.0) | <0.001 |
| Pulmonary disease | 67 (12.7) | 70 (17.2) | 0.062 |
| Asthma | 37 (7.0) | 19 (4.7) | 0.164 |
| COPD | 30 (5.7) | 38 (9.3) | 0.041 |
| Diabetes type II | 113 (21.4) | 102 (25.1) | 0.184 |
| Obesity (body mass index ≥ 30) | 183 (34.6) | 118 (29.0) | 0.078 |
| Immunocompromising condition 5 | 52 (9.8) | 36 (8.8) | 0.652 |
| One or more comorbidities | 396 (74.9) | 301 (74.0) | 0.763 |
| One comorbidity | 82 (15.5) | 58 (14.3) | 0.644 |
| Resident of long-term care facility | 18 (3.4) | 44 (10.8) | <0.001 |
| Fully vaccinated 6 | 175 (33.1) | 159 (39.1) | 0.063 |
| Received booster dose | 1 (0.2) | 55 (13.5) | 0.001 |
| Self-reported previous infection | 0 | 8 (2.0) | 0.009 |
| Primary vaccine received | |||
| BNT162b2 | 122 (69.7) | 130 (81.8) | 0.009 |
| ChAdOx-1S | 26 (14.9) | 13 (7.8) | — |
| Ad.26.COV2.S | 18 (10.3) | 6 (3.6) | — |
| mRNA-1273 | 9 (5.1) | 12 (7.2) | — |
| Therapy | |||
| Remdesivir | 55 (10.4) | 68 (16.7) | 0.006 |
| Any corticosteroids | 493 (93.2) | 342 (84.0) | <0.001 |
| Dexamethasone 8 | 398 (75.2) | 272 (66.8) | 0.005 |
| Methylprednisolone 8 | 202 (38.2) | 116 (28.5) | 0.002 |
| Tocilizumab | 14 (2.6) | 2 (0.5) | 0.011 |
| Monoclonal antibodies | 14 (2.6) | 18 (4.4) | 0.15 |
| Hypoxemic at admission | 485 (91.7) | 328 (80.6) | <0.001 |
| Critically severe disease (WHO score 7–10) 7 | 125 (23.6) | 107 (26.3) | 0.36 |
| Fully vaccinated | 33 (26.4) | 42 (39.3) | 0.048 |
| Unvaccinated | 92 (73.6) | 62 (57.9) | — |
| Death (WHO score 10) 7 | 72 (13.6) | 81 (19.9) | 0.012 |
| Fully vaccinated | 24 (33.3) | 35 (43.2) | 0.246 |
| Unvaccinated | 48 (66.7) | 46 (56.8) | — |
| Characteristics | Odds Ratio (95% CI) | p-Value 1 |
|---|---|---|
| Intercept | 0.08 (0.03–0.20) | <0.001 |
| Vaccination status (vaccinated vs. unvaccinated) | 0.42 (0.26–0.68) | <0.001 |
| SARS-CoV-2 variant (Omicron vs. Delta) | 0.77 (0.52–1.14) | 0.187 |
| Age | 1.02 (1.00–1.03) | 0.038 |
| Sex (male vs. female) | 1.33 (0.97–1.82) | 0.074 |
| Charlson comorbidity index | 1.11 (1.01–1.22) | 0.035 |
| Immunocompromising condition present (yes vs. no) | 1.32 (0.75–2.33) | 0.339 |
| Vaccine status—SARS-CoV-2 variant interaction | 1.80 (0.93–3.47) | 0.079 |
| Characteristics | Hazard Ratio (95% CI) | p-Value 1 |
|---|---|---|
| SARS-CoV-2 variant (Omicron vs. Delta) | 1.13 (0.94–1.36) | 0.186 |
| Vaccination status | 1.39 (1.13–1.72) | 0.002 |
| (vaccinated vs. unvaccinated) | ||
| Age | 0.99 (0.99–1.00) | 0.110 |
| Sex (male vs. female) | 0.91 (0.79–1.06) | 0.221 |
| Charlson comorbidity index | 0.96 (0.91–1.01) | 0.115 |
| Immunocompromising condition present (yes vs. no) | 0.70 (0.53–0.93) | 0.013 |
| Vaccine status—SARS-CoV-2 variant interaction | 0.92 (0.68–1.25) | 0.608 |
| Characteristic | Delta Period n = 175/529 (33.1%) | Omicron Period n = 159/407 (39.1%) | p-Value 1 |
|---|---|---|---|
| Age | 74 (64–83) | 78 (71–84.5) | 0.003 |
| Men | 110 (62.9) | 87 (54.7) | 0.148 |
| Charlson comorbidity index | 4 (3–6) | 5 (4–7) | <0.001 |
| Immunocompromising condition | 37 (21.1) | 20 (12.6) | 0.042 |
| Received booster dose | 1 (0.6) | 55 (34.6) | <0.001 |
| Critically severe disease (WHO score 7–10) 2 | 33 (18.9) | 42 (26.4) | 0.115 |
| Death (WHO score 10) 2 | 24 (13.7) | 35 (22) | 0.061 |
| Time since primary vaccination in weeks | 27 (21.3–33.5) | 37.9 (27–47.1) | <0.001 |
| Time since last vaccination in weeks | 27 (21.1–33.4) | 23.1 (16.5–35.7) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stupica, D.; Collinet-Adler, S.; Kejžar, N.; Poljak, M.; Štamol, T. SARS-CoV-2 Vaccination and Clinical Presentation of COVID-19 in Patients Hospitalized during the Delta- and Omicron-Predominant Periods. J. Clin. Med. 2023, 12, 961. https://doi.org/10.3390/jcm12030961
Stupica D, Collinet-Adler S, Kejžar N, Poljak M, Štamol T. SARS-CoV-2 Vaccination and Clinical Presentation of COVID-19 in Patients Hospitalized during the Delta- and Omicron-Predominant Periods. Journal of Clinical Medicine. 2023; 12(3):961. https://doi.org/10.3390/jcm12030961
Chicago/Turabian StyleStupica, Daša, Stefan Collinet-Adler, Nataša Kejžar, Mario Poljak, and Tina Štamol. 2023. "SARS-CoV-2 Vaccination and Clinical Presentation of COVID-19 in Patients Hospitalized during the Delta- and Omicron-Predominant Periods" Journal of Clinical Medicine 12, no. 3: 961. https://doi.org/10.3390/jcm12030961





