Computed Tomography Angiography for Detection of Pulmonary Embolism in Western Australia Shows Increasing Use with Decreasing Diagnostic Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohort and Data
- (1)
- Hospital morbidity data collection (HMDC) records from 2003 to 2015 for all discharges from any WA hospital (public or private) for all conditions excluding pregnancy.
- (2)
- Emergency Department Data Collection (EDDC) records 2003–2015 for all WA ED presentations.
- (3)
- PACS data for all CT scans in 2003–2015 undertaken in all WA public tertiary hospitals and some public secondary hospitals. Note that only secondary hospitals operating their own radiology departments (i.e., do not contract out radiology services) are included in the PACS data. Two secondary hospitals, Bentley Hospital and Armadale Health Service, are therefore excluded from the PACS. These hospitals account for approximately 6% of public hospital inpatient admissions in WA [31].
- (4)
- WA death registrations from 2003 to 2015.
2.2. Sub-Cohorts for Each Analysis
2.3. Outcomes
2.4. Analysis
2.5. Covariates
3. Results
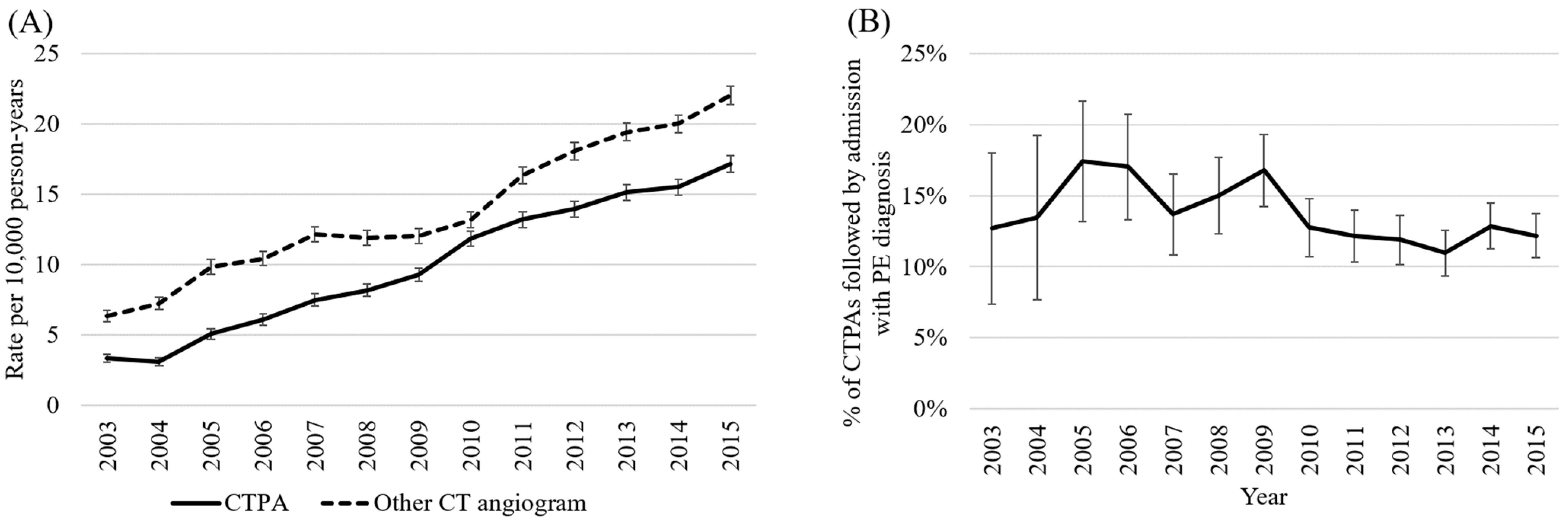
3.1. Trends in Use of CTPA (Aim 1a)
3.2. Diagnostic Yield of CTPA Performed Following ED Presentation (Aim 1b)
3.3. Trends in PE Hospitalisation and Death during PE Hospitalisation (Aim 2)
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 52, 543–603. [Google Scholar] [CrossRef] [Green Version]
- Doherty, S. Pulmonary embolism: An update. Aust. Fam. Physician. 2017, 46, 816–820. [Google Scholar] [PubMed]
- Molaee, S.; Ghanaati, H.; Safavi, E.; Foroumandi, M.; Peiman, S. Computed Tomography Pulmonary Angiography for Evaluation of Patients with Suspected Pulmonary Embolism: Use or Overuse. Iran. J. Radiol. 2015, 12, e22383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, A.J.E.; Wachsmann, J.; Chamarthy, M.R.; Panjikaran, L.; Tanabe, Y.; Rajiah, P. Imaging of acute pulmonary embolism: An update. Cardiovasc Diagn Ther. 2018, 8, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Salehi, L.; Phalpher, P.; Ossip, M.; Meaney, C.; Valani, R.; Mercuri, M. Variability in practice patterns among emergency physicians in the evaluation of patients with a suspected diagnosis of pulmonary embolism. Emerg. Radiol. 2019, 27, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.; Garrett, J.; Sarmiento, E.; Strachan, C.; Courtney, D. Over-Testing for Suspected Pulmonary Embolism in American Emergency Departments: The Continuing Epidemic. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e005753. [Google Scholar] [CrossRef] [PubMed]
- Gee, E.; Roberts, L.; Arya, R.; Tsianakas, V. Patient experience of pregnancy-related venous thrombosis: A phenomenological study. Thrombo. Res. 2019, 183, 86–90. [Google Scholar] [CrossRef]
- Gyftopoulos, S.; Smith, S.W.; Simon, E.; Kuznetsova, M.; Horwitz, L.I.; Makarov, D.V. Qualitative Study to Understand Ordering of CT Angiography to Diagnose Pulmonary Embolism in the Emergency Room Setting. J. Am. Coll. Radiol. 2018, 18, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Haubenreisser, H.; Schabel, C.; Leidecker, C.; Schmidt, B.; Schoenberg, S.O.; Henzler, T. CT pulmonary angiography in patients with acute or chronic renal insufficiency: Evaluation of a low dose contrast material protocol. Sci. Rep. 2018, 8, 1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, C.S. Radiation Dose Considerations in the Chest. Appl. Radiol. 2009. Available online: https://www.appliedradiology.com/articles/radiation-dose-considerations-in-the-chest (accessed on 13 January 2022).
- Ho, K.M.; Harahsheh, Y. Predicting contrast-induced nephropathy after CT pulmonary angiography in the critically ill: A retrospective cohort study. J. Intensive Care. 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Perelas, A.; Dimou, A.; Saenz, A.; Rhee, J.H.; Teerapuncharoen, K.; Rowden, A.; Eiger, G. CT Pulmonary Angiography Utilization in the Emergency Department: Diagnostic Yield and Adherence to Current Guidelines. Am. J. Med. Qual. 2015, 30, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.; Green, J. Over-testing: Why more is not better. Am. J. Med. 2014, 127, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.M.; Rogers, W.; Heath, I.; Degeling, C.; Doust, J.; Barratt, A. The challenge of overdiagnosis begins with its definition. BMJ 2015, 350, h869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American College of Emergency Physicians. CT Pulmonary Angiography in ED Patients; Choosing Wisely: Philadelphia, PA, USA, 2014; Available online: https://www.choosingwisely.org/clinician-lists/acep-ct-pulmonary-angiography-in-ed-patients/?highlight=ctpa (accessed on 16 December 2020).
- Choosing Wisely Australia. Recommendations: Internal Medicine Society of Australia and New Zealand; New South Wales: NPS MedicineWise; 2017. Available online: https://www.choosingwisely.org.au/recommendations/imsanz5 (accessed on 20 November 2020).
- Choosing Wisely Canada. CT Scans to Find a Blood Clot in the Lungs: When You Need One and When You Don’t; Choosing Wisely: Toronto, ON, Canada; Available online: https://choosingwiselycanada.org/ct-scans-find-blood-clot-lungs-need-one-dont/ (accessed on 16 December 2020).
- WA Department of Health. Diagnostic Imaging Pathways—Pulmonary Embolism (Haemodynamically Stable); WA Department of Health: Perth, Australia, 2018. Available online: http://www.imagingpathways.health.wa.gov.au/index.php/imaging-pathways/respiratory/pulmonary-embolism-haemodynamically-stable (accessed on 20 November 2020).
- Richardson, S.; Cohen, S.; Khan, S.; Zhang, M.; Qiu, G.; Oppenheim, M.I.; McGinn, T. Higher Imaging Yield When Clinical Decision Support Is Used. J. Am. Coll. Radiol. 2020, 17, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Deblois, S.; Chartrand-Lefebvre, C.; Toporowicz, K.; Chen, Z.; Lepanto, L. Interventions to Reduce the Overuse of Imaging for Pulmonary Embolism: A Systematic Review. J. Hosp. Med. 2018, 13, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakera, T.; Nagree, Y.; Song, S.; Jones, P. Bridging the communication gap between public and private radiology services. MJA 2009, 191, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.; Schwarts, L.; Woloshin, S. Time Trends in Pulmonary Embolism in the United States: Evidence of Overdiagnosis. Arch. Intern. Med. 2011, 171, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Germini, F.; Zarabi, S.; Eventov, M.; Turcotte, M.; Li, M.; de Wit, K. Pulmonary embolism prevalence among emergency department cohorts: A systematic review and meta-analysis by country of study. J. Thromb. Haemost. 2020. Epub ahead of print. [Google Scholar] [CrossRef]
- Segard, T.; Macdonald, W. Changing trends in venous thromboembolism-related imaging in Western Australian teaching hospital, 2001–2010. MJA 2013, 198, 100–103. [Google Scholar] [CrossRef]
- Mountain, D.; Keijzers, G.; Chu, K.; Joseph, A.; Read, C.; Blecher, G.; Furyk, J.; Bharat, C.; Velusamy, K.; Munro, A.; et al. RESPECT-ED: Rates of Pulmonary Emboli (PE) and Sub-Segmental PE with Modern Computed Tomographic Pulmonary Angiograms in Emergency Departments: A Multi-Center Observational Study Finds Significant Yield Variation, Uncorrelated with Use or Small PE Rates. PLoS ONE 2016, 11, e0166483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Computed Tomography (CT) Scanners (Indicator). 2021. Available online: https://data.oecd.org/healtheqt/computed-tomography-ct-scanners.htm#indicator-chart (accessed on 17 January 2022).
- Lam, J.H.; Pickles, K.; Stanaway, F.F.; Bell, K.J.L. Why clinicians overtest: Development of a thematic framework. BMC Health Serv. Res. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- WA Department of Health. Data Linkage Western Australia; WA Department of Health: Perth, Australia, 2021. Available online: https://www.datalinkage-wa.org.au/ (accessed on 18 February 2021).
- Holman, C.; Bass, A.; Rouse, I.; Hobbs, M. Population-based linkage of health records in Western Australia: Development of a health services research linked database. Aust. N. Zealand J. Public Health 1999, 23, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef]
- Department of Health. Admitted Patient Activity Summary; Government of Western Australia: Perth, Australia, 2022.
- Australian Bureau of Statistics. Estimated Resident Population by Single Year of Age, Western Australia; Australian Bureau of Statistics: Canberra, Australia, 2020.
- StataCorp. Stata Statistics/Data analysis, version 14.1, Standard Edition; StataCorp: College Station, TX, USA, 2015. [Google Scholar]
- Morgan, A.; Joshy, G.; Schaffer, A.; Laba, T.-L.; Litchfield, M.; Pearson, S.; Banks, E. Rapid and substantial increases in anticoagulant use and expenditure in Australia following the introduction of new types of oral anticoagulants. PLoS ONE 2018, 13, e0208824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Bureau of Statistics. Socio-Economic Indexes for Areas; Australian Bureau of Statistics: Canberra, Australia, 2018. Available online: https://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa (accessed on 8 December 2020).
- Hugo Centre for Population and Housing. Accessibility/Remoteness Index of Australia (ARIA); The University of Adelaide: Adelaide, Australia, 2020; Available online: https://www.adelaide.edu.au/hugo-centre/services/aria (accessed on 8 December 2020).
- Holman, C.; Preen, D.; Baynham, N.; Finn, J.; Semmens, J. A multipurpose comorbidity scoring system performed better than the Charlson index. J. Clin. Epidemiol. 2005, 58, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Quigley, A.; Brown, K.; Balasubramaniam, R. Appropriateness of Usage of Computed Tomography Pulmonary Angiography (CTPA) Investigation of Suspected Pulmonary Embolism; Royal College of Cardiologists: London, UK, 2010; Available online: https://www.rcr.ac.uk/audit/appropriateness-usage-computed-tomography-pulmonary-angiography-ctpa-investigation-suspected (accessed on 8 December 2020).
- Anjum, O.; Bleeker, H.; Ohle, R. Computed tomography for suspected pulmonary embolism results in a large number of non-significant incidental findings and follow-up investigations. Emerg Radiol. 2019, 26, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bariteau, A.; Stewart, L.; Emmett, T.; Kline, J. Systematic Review and Meta-analysis of Outcomes of Patients With Subsegmental Pulmonary Embolism With and Without Anticoagulation Treatment. Acad Emerg Med. 2018, 25, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Donato, A.; Khoche, S.; Santora, J.; Wagner, B. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb Res. 2010, 126, e266–e270. [Google Scholar] [CrossRef]
- Australian Government. Medicare Benefits Schedule Book; Commonwealth of Australia: Canberra, Australia, 2015.
- Independent Hospital Pricing Authority. National Hospital Cost Data Collection Australian Public Hospitals Cost Report 2012–2013; Commonwealth of Australia: Sydney, Australia, 2015.
- Carpernter, C.; Raja, A.; Brown, M. Overtesting and the Downstream Consequences of Overtreatment: Implications of “Preventing Overdiagnosis” for Emergency Medicine. Acad Emerg Med. 2015, 22, 1484–1492. [Google Scholar] [CrossRef] [Green Version]
- Bulliard, J.; Chiolero, A. Screening and overdiagnosis: Public health implications. Public Health Rev. 2015, 36, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bledsoe, J.R.; Kelly, C.; Stevens, S.M.; Woller, S.C.; Haug, P.; Bs, J.F.L.; Allen, T.L.; Ms, A.M.B.; Jacobs, J.R.; Elliott, C.G. Electronic pulmonary embolism clinical decision support and effect on yield of computerized tomographic pulmonary angiography: ePE-A pragmatic prospective cohort study. J. Am. Coll. Emerg. Physicians Open. 2021, 2, e12488. [Google Scholar] [CrossRef] [PubMed]
- Walen, S.; De Boer, E.; Edens, M.A.; Van Der Worp, C.A.J.; Boomsma, M.F.; Berg, J.W.K.V.D. Mandatory adherence to diagnostic protocol increases the yield of CTPA for pulmonary embolism. Insights Imaging 2016, 7, 727–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balague, F.; Cedraschi, C. Radiological examination in low back pain patients: Anxiety of the patient? Anxiety of the therapist? Jt. Bone Spine. 2006, 73, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Centres for Disease Control and Prevention. ALARA—As Low As Reasonably Acheivable; Centres for Disease Control and Prevention: Atlanta, GA, USA, 2015. Available online: https://www.cdc.gov/nceh/radiation/alara.html (accessed on 28 January 2021).
- Soffer, S.; Klang, E.; Shimon, O.; Barash, Y.; Cahan, N.; Greenspana, H.; Konen, E. Deep learning for pulmonary embolism detection on computed tomography pulmonary angiogram: A systematic review and meta-analysis. Sci Rep.11. 2021, 1, 15814. [Google Scholar] [CrossRef]

| Objective | 1a: Assess Overtesting by Describing Trends in Overall CTPA Utilization | 1b: Assess Overtesting by Assessing Diagnostic Yield of CTPA Requested by ED Physicians in Tertiary Hospitals | 2: Assess Overdiagnosis by Describing Population Trends in PE Hospitalization and In-Hospital Mortality |
|---|---|---|---|
| Cohort | WA population aged 20+ 2003–2016 | Those with CTPA performed day of or after an ED presentation at a tertiary hospital | 2016 WA population aged 20+ (trends in hospitalization) |
| Outcome | Annual count of CTPA examinations | Subsequent hospital episode with PE as primary or secondary diagnosis | PE hospitalization and death during PE hospitalization |
| Analysis | Age-sex standardized rates | Logistic regression | Age-sex standardized rates |
| N | Approx. 1.9 million 1 | 11,968 CTPAs among 10,707 people | Approx. 1.9 million 1 |
| Sex 1 | Male 49.8% Female 50.2% | Male 5362 (45.8%) Female 6336 (54.2%) | Male 49.8% Female 50.2% |
| Mean age | 46.9 | 61.5 | 46.9 |
| Proportion with outcome | 1525 (13.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youens, D.; Doust, J.; Ha, N.T.; O’Leary, P.; Wright, C.; Parizel, P.M.; Moorin, R. Computed Tomography Angiography for Detection of Pulmonary Embolism in Western Australia Shows Increasing Use with Decreasing Diagnostic Yield. J. Clin. Med. 2023, 12, 980. https://doi.org/10.3390/jcm12030980
Youens D, Doust J, Ha NT, O’Leary P, Wright C, Parizel PM, Moorin R. Computed Tomography Angiography for Detection of Pulmonary Embolism in Western Australia Shows Increasing Use with Decreasing Diagnostic Yield. Journal of Clinical Medicine. 2023; 12(3):980. https://doi.org/10.3390/jcm12030980
Chicago/Turabian StyleYouens, David, Jenny Doust, Ninh Thi Ha, Peter O’Leary, Cameron Wright, Paul M. Parizel, and Rachael Moorin. 2023. "Computed Tomography Angiography for Detection of Pulmonary Embolism in Western Australia Shows Increasing Use with Decreasing Diagnostic Yield" Journal of Clinical Medicine 12, no. 3: 980. https://doi.org/10.3390/jcm12030980





