Impact of Arterial Calcification of the Lower Limbs on Long-Term Clinical Outcomes in Patients on Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Enrollment and Purpose of This Study
2.2. Lower Limbs’ Arterial Calcification
2.3. Measurement of Ankle–Brachial Pressure Index and Toe–Brachial Pressure Index
2.4. Patient Assessment and Group Assignment
2.5. Clinical and Laboratory Parameters
2.6. Treatment Strategy for LEAD
2.7. Patient Outcomes
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics and Fontaine Group Assignment
3.2. Treatment of LEAD
3.3. Patient Outcome
3.4. Risk Factors for 3-Year and 10-Year Clinical Outcomes
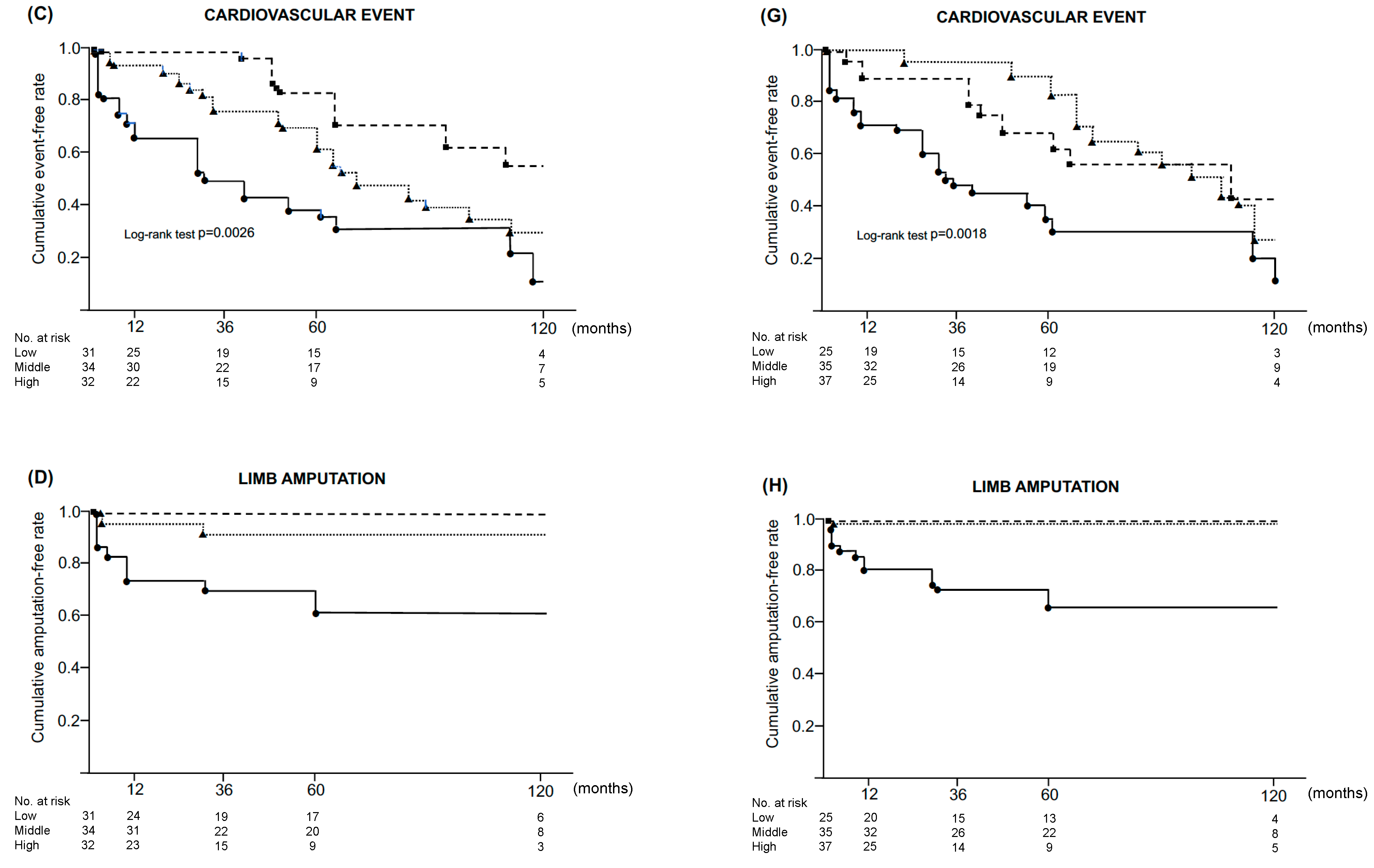
3.5. Impact of SFACS and BKACS on 10-Year Clinical Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindner, A.; Charra, B.; Sherrard, D.J.; Scribner, B.H. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N. Engl. J. Med. 1974, 290, 697–701. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, A.M.; Bertenthal, D.; Shlipak, M.G.; Sen, S.; Chren, M.M. Impact of renal insufficiency on mortality in advanced lower extremity peripheral arterial disease. J. Am. Soc. Nephrol. 2005, 16, 514–519. [Google Scholar] [CrossRef]
- Aulivola, B.; Hile, C.N.; Hamdan, A.D.; Sheahan, M.G.; Velardi, J.R.; Skillman, J.J.; Campbell, D.R.; Scovell, S.D.; LoGerfo, F.W.; Pomposelli, F.B., Jr. Major lower extremity amputation: Outcome of a modern series. Arch. Surg. 2004, 139, 395–399. [Google Scholar] [CrossRef]
- Herzog, C.A.; Littrell, K.; Arko, C.; Frederick, P.D.; Blaney, M. Clinical characteristics of dialysis patients with acute myocardial infarction in the United States: A collaborative10 project of the United States Renal Data System and the National Registry of Myocardial infarction. Circulation 2007, 116, 1465–1472. [Google Scholar] [CrossRef]
- Kobayashi, S. Cardiovascular events in chronic kidney disease (CKD)—An importance of vascular calcification and microcirculatory impairment. Ren. Replace. Ther. 2016, 2, 55. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ikeda, T.; Morita, H.; Ohtake, T.; Kumagai, H. Asymptomatic cerebral lacunae in patients with chronic kidney disease. Am. J. Kidney Dis. 2004, 44, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Kobayashi, S.; Moriya, H.; Negishi, K.; Okamoto, K.; Maesato, K.; Saito, S. High prevalence of occult coronary artery stenosis in patients with chronic kidney disease at the initiation of renal replacement therapy: An angiographic examination. J. Am. Soc. Nephrol. 2005, 16, 1141–1148. [Google Scholar] [CrossRef]
- Ohtake, T.; Oka, M.; Ikee, R.; Mochida, Y.; Ishioka, K.; Moriya, H.; Hidaka, S.; Kobayashi, S. Impact of lower limbs’ arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J. Vasc. Surg. 2011, 53, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Möncheberg, J.G. Uber die reine Mediaverkalkung der Extremitätenarterien und ihr verhalten zur Arterosklerose. Virchow. Arch. Pathol. Anat. 1903, 171, 141–167. [Google Scholar] [CrossRef]
- Ohtake, T.; Ishioka, K.; Honda, K.; Oka, M.; Maesato, K.; Mano, T.; Ikee, R.; Moriya, H.; Hidaka, S.; Kobayashi, S. Impact of coronary artery calcification in hemodialysis patients: Risk factors and associations with prognosis. Hemodial. Int. 2010, 14, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001, 38, 938–942. [Google Scholar] [CrossRef]
- London, G.M. Cardiovascular calcification in uremic patients: Clinical impact on cardiovascular function. J. Am. Soc. Nephrol. 2003, 14, S305–S309. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Raggi, P.; Bellasi, A.; Kooienga, L.; Spiegel, D.M. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007, 71, 438–441. [Google Scholar] [CrossRef]
- Mastuoka, M.; Iseki, K.; Tamashiro, M.; Fujimoto, N.; Higa, N.; Touma, T.; Takishita, S. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin. Exp. Nephrol. 2004, 8, 54–58. [Google Scholar]
- Shimoyama, Y.; Tsuruta, Y.; Niwa, T. Coronary artery calcification score is associated with mortality in Japanese hemodialysis patients. J. Ren. Nutr. 2012, 22, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Shantouf, A.S.; Budoff, M.J.; Ahmadi, N.; Ghaffari, A.; Flores, F.; Gopal, A.; Noori, N.; Jing, J.; Kovesdy, C.P.; Kalantar-Zadeh, K. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am. J. Nephrol. 2010, 31, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Okuno, S.; Ishimura, E.; Kitatani, K.; Fujino, Y.; Kohno, K.; Maeno, Y.; Maekawa, K.; Yamakawa, T.; Imanishi, Y.; Inaba, M.; et al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2007, 49, 417–425. [Google Scholar] [CrossRef]
- Verbeke, F.; Van Biesen, W.; Honkanen, E.; Wikström, B.; Jensen, P.B.; Krzesinski, J.M.; Rasmussen, M.; Vanholder, R.; Rensma, P.L. CORD Study Investigators: Prognostic value of aortic stiffness and calcification dor cardiovascular events and mortality in hemodialysis patients: Outcome of the calcification outcome in renal disease (CORD) study. Clin. J. Am. Soc. Nephrol. 2011, 6, 153–159. [Google Scholar] [CrossRef]
- Noordzij, M.; Cranenburg, E.M.; Engelsman, L.F.; Hermans, M.M.; Boeschoten, E.W.; Brandenburg, V.M.; Bos, W.J.W.; Kooman, J.P.; Dekker, F.W.; Ketteler, M.; et al. Progression of aortic calcification is associated with disprders of mineral metabolism and mortality in chronic dialysis patients. Nephrol. Dial. Transplant. 2011, 26, 1662–1669. [Google Scholar] [CrossRef]
- Inoue, T.; Ogawa, T.; Ishida, H.; Ando, Y.; Nitta, K. Aortic arch calcification evaluated on chest X-ray is a strong independent predictor of cardiovascular events in chronic hemodialysis patients. Heart Vessel. 2012, 27, 135–142. [Google Scholar] [CrossRef]
- Ohya, M.; Otani, H.; Kimura, K.; Saika, Y.; Fujii, R.; Yukawa, S.; Shigematsu, T. Vascular calcification estimated by aortic calcification area index is a significant predictive parameter of cardiovascular mortality in hemodialysis patients. Clin. Exp. Nephrol. 2011, 15, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Okazaki, M.; Tsuchiya, K.; Kawaguchi, H.; Nitta, K. Aortic arch calcification predicts cardiovascular and all-cause mortality in maintenance hemodialysis patients. Kidney Blood Press. Res. 2014, 39, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, M.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronay artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Bird, C.E.; Criqui, M.H.; Fronek, A.; Denenberg, J.O.; Klauber, M.R.; Langer, R.D. Quantitative and qualitative progression of peripheral arterial disease by non-invasive testing. Vasc. Med. 1999, 4, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, R.; Kim, M.; Kieny, R. Surgical treatment of peripheral circulation disorders. Helv. Chir. Acta 1954, 21, 499–533. [Google Scholar] [PubMed]
- Norgan, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkers, F.G.R.; Rutherford, R.B.; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease. Int. Angiol. 2007, 26, 81–157. [Google Scholar]
- Mizuiri, S.; Nishizawa, Y.; Yamashita, K.; Mizuno, K.; Ishine, M.; Doi, S.; Masaki, T.; Shigemoto, K. Coronary artery calcification score and common iliac artery calcification score in non-dialysis CKD patients. Nephrology 2018, 23, 837–845. [Google Scholar] [CrossRef]
- Wasmuth, S.; Baumgartner, I.; Do, D.-D.; Willenberg, T.; Saguner, A.; Zwahlen, M.; Diehm, N. Renal insufficiency is independently associated with a distal distribution pattern of symptomatic lower limb atherosclerosis. Eur. J. Endovasc. Surg. 2010, 39, 591–596. [Google Scholar] [CrossRef]
- Tintut, Y.; Patel, J.; Parhami, F.; Demer, L.L. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000, 102, 2636–2642. [Google Scholar] [CrossRef]
- Ohtake, T.; Kobayashi, S.; Oka, M.; Furuya, R.; Iwagami, M.; Tsutsumi, D.; Mochida, Y.; Maesato, K.; Ishioka, K.; Hidekazu, M.; et al. Lanthanum carbonate delays progression of coronary artery calcification compared with calcium-based phosphate binders in patients on hemodialysis: A pilot study. J. Cardiovasc. Pharm. Ther. 2013, 18, 439–446. [Google Scholar] [CrossRef]
- Chertow, G.M.; Burke, S.K.; Raggi, P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002, 62, 245–252. [Google Scholar] [CrossRef]
- Block, G.A.; Spiegel, D.M.; Ehrich, J.; Mehta, R.; Lindbergh, J.; Dreisbach, A.; Raggi, P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005, 68, 1815–1824. [Google Scholar] [CrossRef]
- Shantouf, R.; Ahmadi, N.; Flores, F.; Tiano, J.; Gopal, A.; Kalantar-Zadeh, K.; Budoff, M.J. Impact of phosphate binder type on coronary artery calcification in hemodialysis patients. Clin. Nephrol. 2010, 74, 12–18. [Google Scholar] [CrossRef]
- Toussaint, N.D.; Lau, K.K.; Polkinghorne, K.R.; Kerr, P.G. Attenuation of aortic calcification with lanthanum carbonate versus calcium-based phosphate binders in haemodialysis: A pilot randomized controlled trial. Nephrology 2011, 16, 290–298. [Google Scholar] [CrossRef]
- Jamal, S.A.; Vandermeer, B.; Raggi, P.; Mendelssohn, D.C.; Charterley, T.; Dorgan, M.; Lok, C.E.; Fitchett, D.; Tsuyuki, R.T. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: An updated systematic review and meta-analysis. Lancet 2013, 382, 1268–1277. [Google Scholar] [CrossRef]
- Isaka, Y.; Hamano, T.; Fujii, H.; Tsujimoto, Y.; Koiwa, F.; Sakaguchi, Y.; Tanaka, R.; Tomiyama, N.; Tatsugami, F.; Teramukai, S. Optimal phosphate control related to coronary artery calcification in dialysis patients. J. Am. Soc. Nephrol. 2021, 32, 723–735. [Google Scholar] [CrossRef]
- Raggi, P.; Chertow, G.M.; Torres, P.U.; Csiky, B.; Naso, A.; Nossuli, K.; Moustafa, M.; Goodman, W.G.; Lopez, N.; Downey, G.; et al. The ADVANCE study: A randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol. Dial. Transplant. 2011, 268, 1327–1339. [Google Scholar] [CrossRef]
- Ok, E.; Asci, G.; Bayraktaroglu, S.; Toz, H.; Ozkahya, M.; Yilmaz, M.; Kircelli, F.; Ok, E.S.; Ceyman, N.; Duman, S.; et al. Reduction of dialysate calcium level reduces progression of coronary artery calcification and improves low bone turnover in patients on hemodialysis. J. Am. Soc. Nephrol. 2016, 27, 2475–2486. [Google Scholar] [CrossRef]
- Ogata, M.; Fukagawa, M.; Hirakata, H.; Kagimura, T.; Fukushima, M.; Aizawa, T. LANDMARK Investigators and Committees. Effect of treating hyperphosphatemia with lanthanum carbonate vs calcium carbonate on cardiovascular events in patients with chronic kidney disease undergoing hemodialysis: The LANDMARK Randomized Clinical Trial. JAMA 2021, 325, 1946–1954. [Google Scholar] [CrossRef]
- Chertow, G.M.; Block, G.A.; Correa-Rotter, R.; Drueke, T.B.; Florge, J.; Goodman, W.G.; Herzog, C.A.; Kubo, Y.; London, G.M.; Mahaffey, K.W.; et al. Effect of cinacalcet on cardiovascular disease in patients on hemodialysis. N. Engl. J. Med. 2013, 367, 2482–2494. [Google Scholar]
- Kayssi, A.; Al-Atassi, T.; Oreopoulos, G.; Roche-Nagle, G.; Tan, K.T.; Rajan, D.K. Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for peripheral arterial disease of the lower limbs. Cochrane Database Syst. Rev. 2016, CD011319. [Google Scholar] [CrossRef]
- Liistro, F.; Angioli, P.; Ventoruzzo, G.; Ducci, K.; Reccia, M.R.; Falsini, G.; Scatena, A.; Pieroni, M.; Bolognese, L. Randomized controlled trial of Acotec drug-eluting balloon versus plain balloon for below-the-knee angioplasty. JACC Cardiovasc. Interv. 2020, 13, 2277–2286. [Google Scholar] [CrossRef]
- Heidemann, F.; Peters, F.; Kuchenbecker, J.; Krestzburg, T.; Sedrakyan, A.; Marschall, U.; L’Hoest, H.; Debus, R.; Behrendt, C.A. Long term outcomes after revascularizations below the knee with paclitaxel coated devices: A propensity score matched cohort analysis. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Teymen, B.; Akturk, S. Drug-eluting balloon angioplasty for below the knee lesions in end stage renal disease patients with critical limb ischemia: Midterm results. J. Interv. Cardiol. 2017, 30, 93–100. [Google Scholar] [CrossRef]

| LEAD (-) | LEAD (+) Fontaine Category | |||
|---|---|---|---|---|
| (n = 47) | I (n = 31) | II (n = 8) | IV (n = 11) | |
| Age (yr) | 63.0 ± 13.4 | 72.5 ± 9.0 ** | 71.4 ± 6.6 | 73.1 ± 10.5 * |
| Male/female (n) | 31:16 | 17:14 | 8:0 | 7:4 |
| HD duration (months) | 53.6 ± 51.3 | 74.5 ± 70.1 | 171.1 ± 144.7 | 74.9 ± 70.8 |
| Risk factors n, (%) | ||||
| Diabetes | 13 (27.7) | 12 (38.7) | 4 (50.0) | 9 (81.8) ** |
| Hypertension | 44 (93.6) | 28 (90.3) | 8 (100) | 11 (100) |
| Dyslipidemia | 5 (10.6) | 8 (25.8) | 0 (0) | 5 (45.5) * |
| Smoking | 8 (17.0) | 4 (12.9) | 1 (12.5) | 1 (9.0) |
| Comorbidity n, (%) | ||||
| IHD | 3 (6.4) | 17 (54.8) ** | 4 (50.0) * | 6 (54.5) ** |
| Stroke | 1 (2.1) | 7 (22.6) * | 3 (37.5) * | 4 (36.3) * |
| Blood pressure (mmHg) | ||||
| Systolic | 138.1 ± 18.3 | 143.0 ± 21.4 | 128.2 ± 198.9 | 136.7 ± 20.6 |
| Diastolic | 79.5 ± 12.3 | 78.3 ± 12.5 | 68.9 ± 8.8 | 72.6 ± 20.6 |
| Laboratory valuables | ||||
| CRP (mg/dL) | 0.18 ± 0.31 | 0.42 ± 0.53 | 0.70 ± 1.43 * | 6.91 ± 16.5 ** |
| Albumin (g/dL) | 3.7 ± 0.5 | 3.7 ± 0.5 | 3.6 ± 0.2 | 3.3 ± 0.4 * |
| Fibrinogen (mg/dL) | 319.1 ± 88.2 | 329.8 ± 91.6 | 312.2 ± 84.8 | 424.5 ± 91.5 ** |
| Ca (mg/dL) | 9.0 ± 0.8 | 9.3 ± 0.7 | 9.2 ± 1.1 | 9.2 ± 0.9 |
| Pi (mg/dL) | 5.7 ± 1.5 | 5.4 ± 1.4 | 4.9 ± 1.2 | 6.0 ± 2.0 |
| T.Chol (mg/dL) | 166.6 ± 44.8 | 162.9 ± 41.7 | 136.8 ± 20.1 | 144.0 ± 35.1 |
| TG (mg/dL) | 95.4 ± 44.8 | 106.4 ± 86.1 | 104.1 ± 41.3 | 89.8 ± 27.6 |
| HDL-C (mg/dL) | 50.1 ± 17.9 | 48.3 ± 13.3 | 39.4 ± 11.9 | 44.8 ± 12.8 |
| LDL-C (mg/dL) | 84.7 ± 33.3 | 79.0 ± 28.6 | 65.9 ± 17.7 | 74.4 ± 32.3 |
| ABI | 1.21 ± 0.09 | 0.89 ± 0.31 ** | 0.82 ± 0.27 | 0.82 ± 0.26 |
| TBI | 0.82 ± 0.19 | 0.44 ± 0.22 ** | 0.45 ± 0.25 | 0.16 ± 0.13 *# |
| Calcification score | ||||
| SFACS | 865.1 ± 2279.6 | 4307.3 ± 4471.5 *** | 4680.3 ± 4682.7 *** | 9579.7 ± 5856.9 ***# |
| BKACS | 619.9 ± 1437.1 | 2035.9 ± 2993.7 *** | 7151.1 ± 7438.7 *** | 7565.9 ± 5808.0 ***# |
| Medication | ||||
| Antiplatelet drugs, n (%) | 3 (6.4) | 16 (51.6) ** | 8 (100) ** | 11 (100) ** |
| Prostaglandin I2 analogue | 0 (0) | 6 (19.5) ** | 1 (12.5) | 5 (54.5) ** |
| ARB | 39 (83.0) | 15 (48.4) | 5 (62.5) | 4 (36.4) * |
| ACE-I | 3 (6.4) | 2 (6.5) | 0 (0) | 0 (0) |
| Statin | 5 (10.6) | 8 (3.2) | 0 (0) | 5 (45.5) * |
| Phosphate binder | 0 (0) | 6 (19.4) | 1 (12.5) | 5 (54.5) |
| Calcium carbonate | 33 (70.2) | 18 (58.1) | 5 (62.5) | 6 (54.5) |
| Sevelamer | 10 (21.3) | 8 (25.8) | 6 (75.0) ** | 3 (27.3) |
| Vitamin D | 24 (51.0) | 18 (58.1) | 5 (62.5) | 7 (63.6) |
| LEAD (-) | LEAD (+) Fontaine Category | |||
|---|---|---|---|---|
| (n = 47) | I (n = 31) | II (n = 8) | IV (n = 11) | |
| 3-year death, n, (%) | 9 (19.1) | 8 (25.8) ** | 2 (25.0) **## | 8 (72.7) **## |
| Cardiovascular | 4 (8.5) | 4 (12.8) | 0 (0) | 6 (54.5) ***## |
| Infection | 0 (0) | 2 (6.5) | 1 (12.5) | 1 (9.1) |
| Other | 5 (10.6) | 2 (6.5) | 1 (12.5) | 1 (9.1) |
| 10-year death, n, (%) | 24 (51.1) | 21 (67.7) | 4 (50.0) | 9 (81.8) **# |
| Cardiovascular | 15 (31.9) | 11 (35.4) | 3 (37.5) | 8 (72.7) *# |
| Infection | 4 (8.5) | 6 (19.4) | 0 (0) | 0 (0) |
| Other | 5 (10.6) | 4 (12.9) | 1 (12.5) | 1 (9.1) |
| 3-year cardiovascular event, n, (%) | 8 (17.2) | 6 (19.4) | 2 (25.0) | 9 (81.9) ***## |
| Heart failure | ||||
| AMI | 0 (0) | 0 (0) | 0 (0) | 1 (9.1) |
| Sudden death | 2 (4.3) | 1 (3.2) | 0 (0) | 1 (9.1) |
| Stroke | 2 (4.3) | 1 (3.2) | 0 (0) | 1 (9.1) |
| Arrythmia | 2 (4.3) | 2 (6.5) | 2 (25.0) | 3 (27.3) * |
| 10-year cardiovascular event, n, (%) | 24 (50.9) | 14 (45.3) | 8 (100) * | 10(90.9) *## |
| Heart failure | 5 (10.6) | 3 (9.7) | 0 (0) | 3 (27.2) |
| AMI | 0 (0) | 2 (6.5) | 1 (12.5) | 2 (18.2) |
| Sudden death | 5 (10.6) | 2 (6.5) | 1 (12.5) | 1 (9.1) |
| Stroke | 5 (10.6) | 2 (6.5) | 2 (25.0) | 2 (18.2) |
| Arrythmia | 9 (19.1) | 5 (16.1) | 4 (50.0) | 2 (18.2) |
| 3-year amputation, n, (%) | 0 (0) | 4 (12.9) ** | 1 (12.5) **## | 4 (36.4) **## |
| Major | 0 (0) | 2 (6.4) | 0 (0) | 2 (18.2) |
| Minor | 0 (0) | 2 (6.5) | 1 (12.5) | 2 (18.2) |
| 10-year amputation, n, (%) | 1 (2.1) | 4 (12.9) ** | 1 (12.5) **## | 7 (63.6) **## |
| Major | 0 (0) | 2 (6.4) | 0 (0) | 4 (36.4) |
| Minor | 1 (2.1) | 2 (6.5) | 1 (12.5) | 3 (27.2) |
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||||
| HR | 95% CI | p | HR | 95% CI | P | HR | 95% CI | p | |
| All-cause mortality | |||||||||
| CRP | 4.16 | 2.16–8.01 | <0.001 | 2.82 | 1.72–4.64 | <0.001 | 2.66 | 1.56–4.49 | 0.003 |
| Albumin | 0.33 | 0.17–0.66 | 0.002 | ||||||
| Age | 1.06 | 1.02–1.103 | 0.008 | 1.06 | 1.01–1.11 | 0.023 | 1.06 | 1.01–1.11 | 0.021 |
| Diabetes | 1.19 | 0.54–2.62 | 0.667 | ||||||
| IHD | 2.16 | 0.99–4.74 | 0.054 | ||||||
| SFACS | 1.29 | 0.90–1.86 | 0.164 | ||||||
| BKACS | 1.61 | 1.05–2.49 | 0.03 | ||||||
| CLTI | 5.06 | 2.00–12.79 | <0.001 | ||||||
| Cardiovascular mortality | |||||||||
| CRP | 3.09 | 1.34–7.137 | 0.008 | ||||||
| Albumin | 0.45 | 0.17–1.24 | 0.122 | ||||||
| Age | 1.05 | 0.99–1.11 | 0.078 | ||||||
| Diabetes | 2.72 | 0.91–8.13 | 0.076 | ||||||
| IHD | 7.25 | 2.02–26.01 | 0.002 | 5.48 | 1.30–23.06 | 0.021 | 5.59 | 1.32–23.63 | 0.019 |
| SFACS | 1.83 | 0.98–3.40 | 0.057 | ||||||
| BKACS | 2.28 | 1.19–4.36 | 0.013 | ||||||
| CLTI | 9.11 | 3.01–27.56 | <0.001 | ||||||
| Cardiovascular event | |||||||||
| CRP | 5.36 | 2.64–10.90 | <0.001 | 2.13 | 1.37–3.34 | <0.001 | 1.59 | 1.07–2.37 | 0.022 |
| Albumin | 0.42 | 0.19–0.92 | 0.03 | ||||||
| Age | 1.06 | 1.01–1.11 | 0.014 | 1.06 | 1.01–1.12 | 0.026 | 1.06 | 1.00–1.11 | 0.034 |
| Diabetes | 20.1 | 0.84–4.74 | 0.117 | ||||||
| IHD | 2.15 | 0.91–5.06 | 0.803 | ||||||
| SFACS | 2.03 | 1.18–3.49 | 0.01 | 1.72 | 1.05–2.81 | 0.031 | |||
| BKACS | 2.29 | 1.35–3.90 | 0.002 | ||||||
| CLTI | 9.27 | 3.74–22.94 | <0.001 | ||||||
| Limb amputation | |||||||||
| CRP | 3.44 | 1.09–10.87 | 0.035 | ||||||
| Albumin | 0.34 | 0.09–1.24 | 0.103 | ||||||
| Age | 1.02 | 0.96–1.09 | 0.522 | ||||||
| Diabetes | 3.79 | 0.73–19.54 | 0.112 | ||||||
| IHD | 2.61 | 0.58–11.62 | 0.211 | ||||||
| SFACS | 3.76 | 1.00–14.19 | 0.05 | ||||||
| BKACS | 4.14 | 1.30–13.14 | 0.016 | ||||||
| CLTI | 18.61 | 3.96–87.32 | <0.001 | 12.98 | 1.63–103.39 | 0.016 | 13.94 | 2.06–94.39 | 0.007 |
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| All-cause mortality | |||||||||
| CRP | 2.99 | 1.88–4.76 | <0.001 | 2.24 | 1.46–3.44 | <0.001 | 2.26 | 1.48–3.46 | <0.001 |
| Albumin | 0.49 | 0.27–0.91 | 0.023 | ||||||
| Age | 1.05 | 1.03–1.08 | <0.001 | 1.06 | 1.02–1.08 | <0.001 | 1.06 | 1.03–1.09 | <0.001 |
| Diabetes | 1.98 | 1.18–3.32 | 0.01 | ||||||
| IHD | 1.76 | 1.05–2.97 | 0.033 | ||||||
| SFACS | 1.28 | 1.02–1.61 | 0.034 | ||||||
| BKACS | 1.35 | 1.03–1.78 | 0.033 | ||||||
| CLTI | 5.92 | 2.74–12.75 | <0.001 | ||||||
| Cardiovascular mortality | |||||||||
| CRP | 3.26 | 1.82–5.84 | <0.001 | 1.78 | 1.09–2.92 | 0.023 | 1.66 | 1.04–2.66 | 0.033 |
| Albumin | 0.87 | 0.37–2.04 | 0.747 | ||||||
| Age | 1.04 | 1.01–1.07 | 0.02 | 1.04 | 1.00–1.07 | 0.041 | |||
| Diabetes | 3.35 | 1.68–6.68 | <0.001 | ||||||
| IHD | 3.21 | 1.67–6.19 | <0.001 | 2.44 | 1.06–5.58 | 0.035 | |||
| SFACS | 1.73 | 1.22–2.46 | 0.002 | ||||||
| BKACS | 1.71 | 1.20–2.45 | 0.003 | ||||||
| CLTI | 12.96 | 5.43–30.91 | <0.001 | 4.59 | 1.10–19.12 | 0.036 | 7.06 | 1.72–28.99 | 0.007 |
| Cardiovascular event | |||||||||
| CRP | 2.68 | 1.65–4.36 | <0.001 | 1.74 | 1.19–2.54 | 0.004 | 1.56 | 1.10–2.21 | 0.012 |
| Albumin | 0.94 | 0.46–1.91 | 0.867 | ||||||
| Age | 1.05 | 1.02–1.08 | 0.001 | 1.04 | 1.01–1.07 | 0.014 | 1.04 | 1.01–1.08 | 0.005 |
| Diabetes | 2.01 | 1.14–3.52 | 0.016 | ||||||
| IHD | 1.91 | 1.08–3.37 | 0.025 | ||||||
| SFACS | 1.54 | 1.17–2.01 | 0.002 | 1.42 | 1.01–2.17 | 0.045 | |||
| BKACS | 1.59 | 1.18–2.14 | 0.003 | ||||||
| CLTI | 11.22 | 5.00–25.21 | <0.001 | 3.84 | 1.13–13.11 | 0.032 | |||
| Limb amputation | |||||||||
| CRP | 4.63 | 1.75–12.28 | 0.002 | ||||||
| Albumin | 0.55 | 0.17–1.83 | 0.332 | ||||||
| Age | 1.01 | 0.96–1.06 | 0.782 | ||||||
| Diabetes | 6.87 | 1.48–31.83 | 0.014 | ||||||
| IHD | 1.69 | 0.52–5.55 | 0.386 | ||||||
| SFACS | 11.21 | 2.42–51.87 | 0.002 | 5.87 | 1.14–30.18 | 0.034 | |||
| BKACS | 4.23 | 1.76–10.47 | 0.002 | ||||||
| CLTI | 22.95 | 6.15–85.66 | <0.001 | 7.74 | 1.32–45.32 | 0.023 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohtake, T.; Mitomo, A.; Yamano, M.; Shimizu, T.; Mochida, Y.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S.; et al. Impact of Arterial Calcification of the Lower Limbs on Long-Term Clinical Outcomes in Patients on Hemodialysis. J. Clin. Med. 2023, 12, 1299. https://doi.org/10.3390/jcm12041299
Ohtake T, Mitomo A, Yamano M, Shimizu T, Mochida Y, Ishioka K, Oka M, Maesato K, Moriya H, Hidaka S, et al. Impact of Arterial Calcification of the Lower Limbs on Long-Term Clinical Outcomes in Patients on Hemodialysis. Journal of Clinical Medicine. 2023; 12(4):1299. https://doi.org/10.3390/jcm12041299
Chicago/Turabian StyleOhtake, Takayasu, Ayaka Mitomo, Mizuki Yamano, Toshihiro Shimizu, Yasuhiro Mochida, Kunihiro Ishioka, Machiko Oka, Kyoko Maesato, Hidekazu Moriya, Sumi Hidaka, and et al. 2023. "Impact of Arterial Calcification of the Lower Limbs on Long-Term Clinical Outcomes in Patients on Hemodialysis" Journal of Clinical Medicine 12, no. 4: 1299. https://doi.org/10.3390/jcm12041299
APA StyleOhtake, T., Mitomo, A., Yamano, M., Shimizu, T., Mochida, Y., Ishioka, K., Oka, M., Maesato, K., Moriya, H., Hidaka, S., Mwanatambwe, M., & Kobayashi, S. (2023). Impact of Arterial Calcification of the Lower Limbs on Long-Term Clinical Outcomes in Patients on Hemodialysis. Journal of Clinical Medicine, 12(4), 1299. https://doi.org/10.3390/jcm12041299








