Bronchoscopic Endobronchial Valve Therapy for Persistent Air Leaks in COVID-19 Patients Requiring Veno-Venous Extracorporeal Membrane Oxygenation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics and Complications
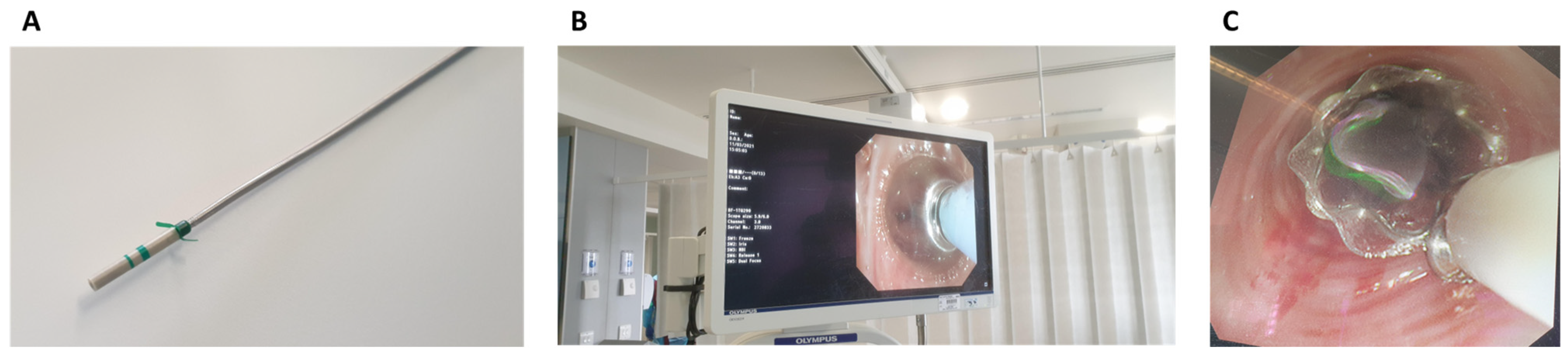
3.2. Characteristics of Air Leaks and Endobronchial Valve Deployment
3.3. Imaging and Patient Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Udwadia, Z.F.; Toraskar, K.K.; Pinto, L.; Mullerpatan, J.; Wagh, H.D.; Mascarenhas, J.M.; Gandhi, B.M.; Tripathi, A.; Sunavala, A.; Agrawal, U.; et al. Increased frequency of pneumothorax and pneumomediastinum in COVID-19 patients admitted in the ICU: A multicentre study from Mumbai, India. Clin. Med. 2021, 21, e615–e619. [Google Scholar] [CrossRef] [PubMed]
- Lemmers, D.H.L.; Abu Hilal, M.; Bnà, C.; Prezioso, C.; Cavallo, E.; Nencini, N.; Crisci, S.; Fusina, F.; Natalini, G. Pneumomediastinum and subcutaneous emphysema in COVID-19: Barotrauma or lung frailty? ERJ Open Res. 2020, 6, 00385–02020. [Google Scholar] [CrossRef] [PubMed]
- Melhorn, J.; Achaiah, A.; Conway, F.M.; Thompson, E.M.F.; Skyllberg, E.W.; Durrant, J.; Hasan, N.A.; Madani, Y.; Naran, P.; Vijayakumar, B.; et al. Pneumomediastinum in COVID-19: A phenotype of severe COVID-19 pneumonitis? The results of the United Kingdom (POETIC) survey. Eur. Respir. J. 2022, 60, 2102522. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, G.; Zhan, C.; Rosenberg, N.; Azour, L.; Wickstrom, M.; Mason, D.M.; Thomas, K.M.; Moore, W.H. Increased Incidence of Barotrauma in Patients with COVID-19 on Invasive Mechanical Ventilation. Radiology 2020, 297, E252–E262. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.R.; Watson, R.L.; Thetford, J.T.; Wong, J.I.; Kamangar, N. High Incidence of Barotrauma in Patients with Severe Coronavirus Disease 2019. J. Intensive Care Med. 2021, 36, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Rajdev, K.; Spanel, A.J.; McMillan, S.; Lahan, S.; Boer, B.; Birge, J.; Thi, M. Pulmonary Barotrauma in COVID-19 Patients with ARDS on Invasive and Non-Invasive Positive Pressure Ventilation. J. Intensive Care Med. 2021, 36, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Udi, J.; Lang, C.N.; Zotzmann, V.; Krueger, K.; Fluegler, A.; Bamberg, F.; Bode, C.; Duerschmied, D.; Wengenmayer, T.; Staudacher, D.L. Incidence of Barotrauma in Patients With COVID-19 Pneumonia During Prolonged Invasive Mechanical Ventilation—A Case-Control Study. J. Intensive Care Med. 2021, 36, 477–483. [Google Scholar] [CrossRef]
- Stewart, T.E.; Meade, M.O.; Cook, D.J.; Granton, J.T.; Hodder, R.V.; Lapinsky, S.E.; Mazer, C.D.; McLean, R.F.; Rogovein, T.S.; Schouten, B.D.; et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N. Engl. J. Med. 1998, 338, 355–361. [Google Scholar] [CrossRef]
- Anzueto, A.; Frutos–Vivar, F.; Esteban, A.; Alía, I.; Brochard, L.; Stewart, T.; Benito, S.; Tobin, M.J.; Elizalde, J.; Palizas, F.; et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004, 30, 612–619. [Google Scholar] [CrossRef]
- Brower, R.G.; Lanken, P.N.; MacIntyre, N.; Matthay, M.A.; Morris, A.; Ancukiewicz, M.; Schoenfeld, D.; Thompson, B.T. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2004, 351, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Gazivoda, V.P.; Ibrahim, M.; Kangas-Dick, A.; Sun, A.; Silver, M.; Wiesel, O. Outcomes of Barotrauma in Critically Ill COVID-19 Patients With Severe Pneumonia. J. Intensive Care Med. 2021, 36, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Das, K.M.; Lee, E.Y.; Al Jawder, S.E.; Enani, M.A.; Singh, R.; Skakni, L.; Al-Nakshabandi, N.; AlDossari, K.; Larsson, S.G. Acute Middle East Respiratory Syndrome Coronavirus: Temporal Lung Changes Observed on the Chest Radiographs of 55 Patients. AJR Am. J. Roentgenol. 2015, 205, W267–274. [Google Scholar] [CrossRef]
- Kao, H.-K.; Wang, J.-H.; Sung, C.-S.; Huang, Y.-C.; Lien, T.-C. Pneumothorax and mortality in the mechanically ventilated SARS patients: A prospective clinical study. Crit. Care 2005, 9, R440–R445. [Google Scholar] [CrossRef]
- Jolobe, O.M.P. Air leaks, pneumatoceles, and air spaces in Covid-19 pneumonia. Am. J. Emerg. Med. 2021, 46, 785. [Google Scholar] [CrossRef]
- Alhakeem, A.; Khan, M.M.; Soub, H.A.; Yousaf, Z. Case Report: COVID-19–Associated Bilateral Spontaneous Pneumothorax—A Literature Review. Am. J. Trop. Med. Hyg. 2020, 103, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Rohailla, S.; Ahmed, N.; Gough, K. SARS-CoV-2 infection associated with spontaneous pneumothorax. CMAJ Can. Med. Assoc. J. 2020, 192, E510. [Google Scholar] [CrossRef] [PubMed]
- González-Pacheco, H.; Gopar-Nieto, R.; Jiménez-Rodríguez, G.-M.; Manzur-Sandoval, D.; Sandoval, J.; Arias-Mendoza, A. Bilateral spontaneous pneumothorax in SARS-CoV-2 infection: A very rare, life-threatening complication. Am. J. Emerg. Med. 2021, 39, 258.e1–258.e3. [Google Scholar] [CrossRef]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Combes, A.; Agerstrand, C.; Annich, G.; Diaz, R.; Fan, E.; Hryniewicz, K.; Lorusso, R.; et al. Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet 2021, 398, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, A.; D’Andrilli, A.; Cascone, R.; Occhiati, L.; Anile, M.; Diso, D.; Cassiano, F.; Poggi, C.; Ibrahim, M.; Cusumano, G.; et al. Unidirectional endobronchial valves for management of persistent air-leaks: Results of a multicenter study. J. Thorac. Dis. 2018, 10, 6158–6167. [Google Scholar] [CrossRef]
- Ding, M.; Gao, Y.; Zeng, X.-T.; Guo, Y.; Yang, J. Endobronchial one-way valves for treatment of persistent air leaks: A systematic review. Respir. Res. 2017, 18, 186. [Google Scholar] [CrossRef] [Green Version]
- Reed, M.F.; Gilbert, C.R.; Taylor, M.D.; Toth, J.W. Endobronchial Valves for Challenging Air Leaks. Ann. Thorac. Surg. 2015, 100, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.R.; Casal, R.F.; Lee, H.J.; Feller-Kopman, D.; Frimpong, B.; Dincer, H.E.; Podgaetz, E.; Benzaquen, S.; Majid, A.; Folch, E.; et al. Use of One-Way Intrabronchial Valves in Air Leak Management After Tube Thoracostomy Drainage. Ann. Thorac. Surg. 2016, 101, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Bermea, R.S.; Miller, J.; Wilson, W.W.; Dugan, K.; Frye, L.; Murgu, S.; Hogarth, D.K. One-Way Endobronchial Valves as Management for Persistent Air Leaks: A Preview of What’s to Come? Am. J. Respir. Crit. Care Med. 2019, 200, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Brichon, P.-Y.; Poquet, C.; Arvieux, C.; Pison, C. Successful treatment of a life-threatening air leakage, complicating severe abdominal sepsis, with a one-way endobronchial valve. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Hodges, A.M.; Gillham, M.J.; Lewis, C.A. Bedside placement of an endobronchial valve to aid invasive ventilation and weaning from extracorporeal membrane oxygenation. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2015, 17, 219–222. [Google Scholar]
- Ghiani, A.; Hansen, M.; Tsitouras, K.; Neurohr, C. Endobronchial One-Way Valve Therapy Facilitates Weaning from Extracorporeal Membrane Oxygenation in a Patient with ARDS and Persistent Air Leak. Case Rep. Crit. Care 2018, 2018, 9736217. [Google Scholar] [CrossRef]
- Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [CrossRef]
- Ramanathan, K.; Shekar, K.; Ling, R.R.; Barbaro, R.P.; Wong, S.N.; Tan, C.S.; Rochwerg, B.; Fernando, S.M.; Takeda, S.; MacLaren, G.; et al. Extracorporeal membrane oxygenation for COVID-19: A systematic review and meta-analysis. Crit. Care 2021, 25, 211. [Google Scholar] [CrossRef]
- Available online: https://www.engage.england.nhs.uk/consultation/extra-corporeal-membrane-oxygenation/user_uploads/ecmo-service-specification-proposition-v1.pdf (accessed on 14 January 2022).
- Hu, Q.; Guan, H.; Sun, Z.; Huang, L.; Chen, C.; Ai, T.; Pan, Y.; Xia, L. Early CT features and temporal lung changes in COVID-19 pneumonia in Wuhan, China. Eur. J. Radiol. 2020, 128, 109017. [Google Scholar] [CrossRef]
- Weaver, L.; Das, A.; Saffaran, S.; Yehya, N.; Scott, T.E.; Chikhani, M.; Laffey, J.G.; Hardman, J.G.; Camporota, L.; Bates, D.G. High risk of patient self-inflicted lung injury in COVID-19 with frequently encountered spontaneous breathing patterns: A computational modelling study. Ann. Intensive Care 2021, 11, 109. [Google Scholar] [CrossRef]
- Thomas, J.; Kostousov, V.; Teruya, J. Bleeding and Thrombotic Complications in the Use of Extracorporeal Membrane Oxygenation. Semin. Thromb. Hemost. 2018, 44, 20–29. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Travaline, J.M.; McKenna, R.J.; De Giacomo, T.; Venuta, F.; Hazelrigg, S.R.; Boomer, M.; Criner, G.J. Treatment of Persistent Pulmonary Air Leaks Using Endobronchial Valves. Chest 2009, 136, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.K.; Verhoef, P.; Patel, S.B.; Carr, G.; Hogarth, D.K. Intrabronchial Valves. J. Bronchol. Interv. Pulmonol. 2012, 19, 137–141. [Google Scholar] [CrossRef]
- Kalatoudis, H.; Nikhil, M.; Zeid, F.; Shweihat, Y. Bronchopleural Fistula Resolution with Endobronchial Valve Placement and Liberation from Mechanical Ventilation in Acute Respiratory Distress Syndrome: A Case Series. Case Rep. Crit. Care 2017, 2017, 3092457. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, G.J.; Willemse, B.W.M.; Kneyber, M.C.J. Endobronchial valve placement for a severe pneumothorax in a child on ECLS. Pediatr. Pulmonol. 2019, 54, 1875–1877. [Google Scholar] [CrossRef]
- Criss, C.N.; Barbaro, R.; Bauman, K.A.; Folafoluwa, O.; Vellody, R.; Jarboe, M.D. Selective Management of Multiple Bronchopleural Fistulae in a Pediatric Patient on Extracorporeal Membrane Oxygenation: A Multidisciplinary Approach. J. Laparoendosc. Adv. Surg. Technol. 2018, 28, 1271–1274. [Google Scholar] [CrossRef]

| All (n = 10) | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 60% Male | Male | Female | Female | Male | Male | Female | Male | Male | Female | Male |
| Co-morbidities | - | Nil | Diverticulosis; GORD; anxiety; obesity | Nil | Nil | Pemphigus | Nil | Nil | Obesity; depression | Pre-eclampsia | Obesity; type 2DM |
| Primary problem | - | COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 |
| Concurrent problems | - | Massive PE; right haemothorax | Tubo-ovarian abscess; right basal segmental PE; left hydropneumothorax; right pneumothorax | Right pneumothorax; haemothorax; CMV reactivation | Right tension pneumothorax; loculated hydro-pneumothorax; CMV reactivation | Subsegmental PE; right haemothorax; CMV reactivation | Lower limb DVT; right pneumothorax; CMV reactivation | HIT; right apical pneumothorax; CMV reactivation | Bilateral multiple PE; renal, splenic, liver embolic infarcts; right and left pneumothorax | Bilateral multiple PE; multiple DVT; radial artery thrombus; CMV reactivation | Right tension hydro-pneumothorax; multiple PE left and right lower lobe segments; RV thrombus |
| Interventions | - | Thoracotomy; pleural drainage; percutaneous tracheostomy | Percutaneous tracheostomy; pleural drainage | Thoracotomy; single-lung ventilation; pleural drainage | Pleural drainage (CT-guided); intrapleural thrombolysis | Pleural drainage; endovascular coiling distal right phrenic artery | Emergency caesarean section; pleural drainage | Pleural drainage; percutaneous tracheostomy | Pleural drainage; percutaneous tracheostomy | Emergency caesarean section; pleural drainage; percutaneous tracheostomy | Pleural drainage; percutaneous tracheostomy; single-lung ventilation; pleural irrigation |
| Secondary pulmonary infection | 10 (100%) | K aerogenes; Prevotella oralis | P aeruginosa; E faecium | P aeruginosa; S marcescens; C striatum | C albicans; C koseri | C albicans; Acinetobacter nosocomialis; P aeruginosa | C albicans; P mirabilis | E cloacae; Aspergillus fumigatus; E faecium; S marcescens | S.aureus; Fusobacterium nucleatum; Prevotella nanceiensis; C albicans | S.aureus; E.coli | S.aureus; C albicans; Kytococcus schroeteri |
| Secondary pleural infection | 5 (50%) | K aerogenes; Prevotella oralis | P aeruginosa; E faecium; E faecalis | P aeruginosa; C striatum | No | C albicans; E faecium | No | No | No | E.coli | No |
| All (n = 10) | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Site of EBV placement | - | Right lower lobe basal segments | Left lower lobe posterior and lateral basal segments | Right lower lobe apical and basal segments | Right middle lobe bronchus | Right lower lobe basal segments | Right middle lobe bronchus | Right upper lobe all segments | Right middle lobe; apical and posterior basal segments of left lower lobe | Lingula, both segments; left upper lobe | Right lower lobe anterior, medial and lateral basal segments |
| Numbers of EBV (size) | 2.4 ± 0.8 | 2 (5.0 mm and 4.0 mm) | 2 (4.0 mm and 5.5 mm) | 3 (4.0 mm) | 1 (5.5 mm) | 3 (4.0 mm) | 1 (5.5 mm) | 3 (4.0 mm) | 3 (5.5 mm and 2 × 4.0 mm) | 3 (2 × 4.0 mm and 5.5 mm) | 3 (4.0 mm) |
| Cessation of leak at end of procedure | 10 (100%) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Immediate complications | 0 (0%) | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| VV-ECMO at time of EBV procedure | 9 (90%) | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Days from initial IMV to air leak | 14.5 ± 15.6 | 35 | 43 | 3 | −1 | 11 | 24 | −1 | 9 | 21 | 1 |
| Days from air leak to EBV | 18.5 ± 14.0 | 18 | 11 | 28 | 19 | 29 | 15 | 39 | 25 | 16 | 15 |
| Days of ECMO | 42.6 ± 19.6 | 43 * | 15 | 82 | 43 | 61 | 39 * | 43 | 52 | 22 | 26 |
| Recurrence of air leak | 4 (40%) | No | No | Yes | No | Yes | No | Yes | No | No | Yes |
| Days to removal of all pleural drains post EBV | 24.1 ± 23.5 $ | 4 | 7 | 70 | 28 | - | 10 | 37 | - | 13 | † |
| Alive (follow up time) | 8 (80%) | Yes (>1 year) | Yes (>1 year) | Yes (>1 year) | Yes (>1 year) | No | Yes (6 months) | Yes (6 months) | No | Yes (3 months) | Yes (3 months) |
| Functional status | Mildly reduced ET | Good ET | Reduced ET; ambulatory oxygen | Good ET | - | Reduced ET; ambulatory oxygen | Reduced ET; ambulatory oxygen | - | Reduced ET | Mildly reduced ET |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ficial, B.; Whebell, S.; Taylor, D.; Fernández-Garda, R.; Okiror, L.; Meadows, C.I.S. Bronchoscopic Endobronchial Valve Therapy for Persistent Air Leaks in COVID-19 Patients Requiring Veno-Venous Extracorporeal Membrane Oxygenation. J. Clin. Med. 2023, 12, 1348. https://doi.org/10.3390/jcm12041348
Ficial B, Whebell S, Taylor D, Fernández-Garda R, Okiror L, Meadows CIS. Bronchoscopic Endobronchial Valve Therapy for Persistent Air Leaks in COVID-19 Patients Requiring Veno-Venous Extracorporeal Membrane Oxygenation. Journal of Clinical Medicine. 2023; 12(4):1348. https://doi.org/10.3390/jcm12041348
Chicago/Turabian StyleFicial, Barbara, Stephen Whebell, Daniel Taylor, Rita Fernández-Garda, Lawrence Okiror, and Christopher I. S. Meadows. 2023. "Bronchoscopic Endobronchial Valve Therapy for Persistent Air Leaks in COVID-19 Patients Requiring Veno-Venous Extracorporeal Membrane Oxygenation" Journal of Clinical Medicine 12, no. 4: 1348. https://doi.org/10.3390/jcm12041348






