Osteopontin and Regulatory T Cells in Effector Phase of Allergic Contact Dermatitis
Abstract
1. Introduction
2. Material and Methods
2.1. Evaluation of Peripheral Blood Morphology
2.2. The Evaluation of T Lymphocytes with Expression of Intracellular Osteopontin in Peripheral Blood by the Method of Flow Cytometry
2.3. The Evaluation of CD4, CD4CD25, CD4CD25high, and CD4CD25highCD127low T Lymphocytes in Peripheral Blood by the Method of Flow Cytometry
2.4. Statistical Analysis
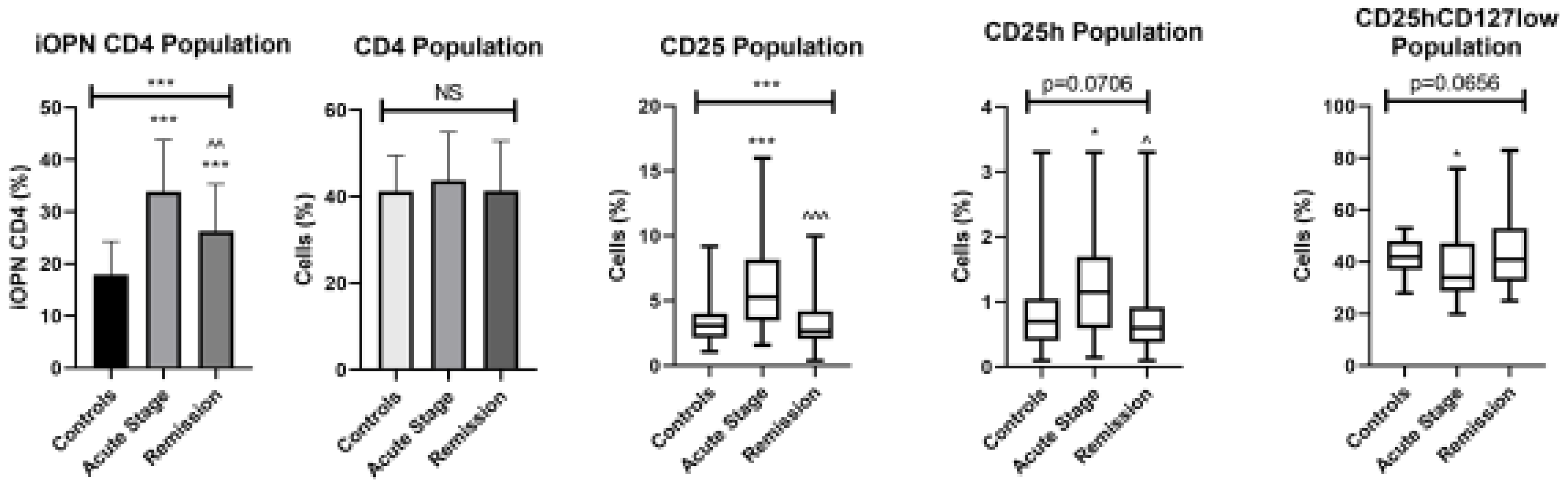
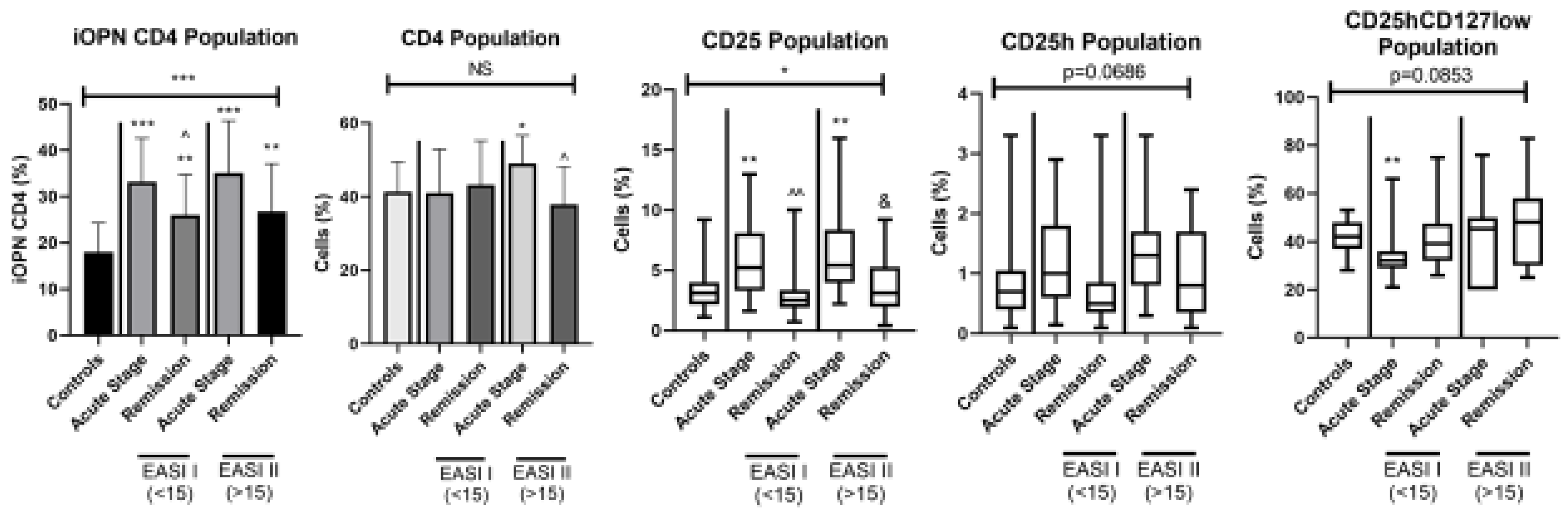
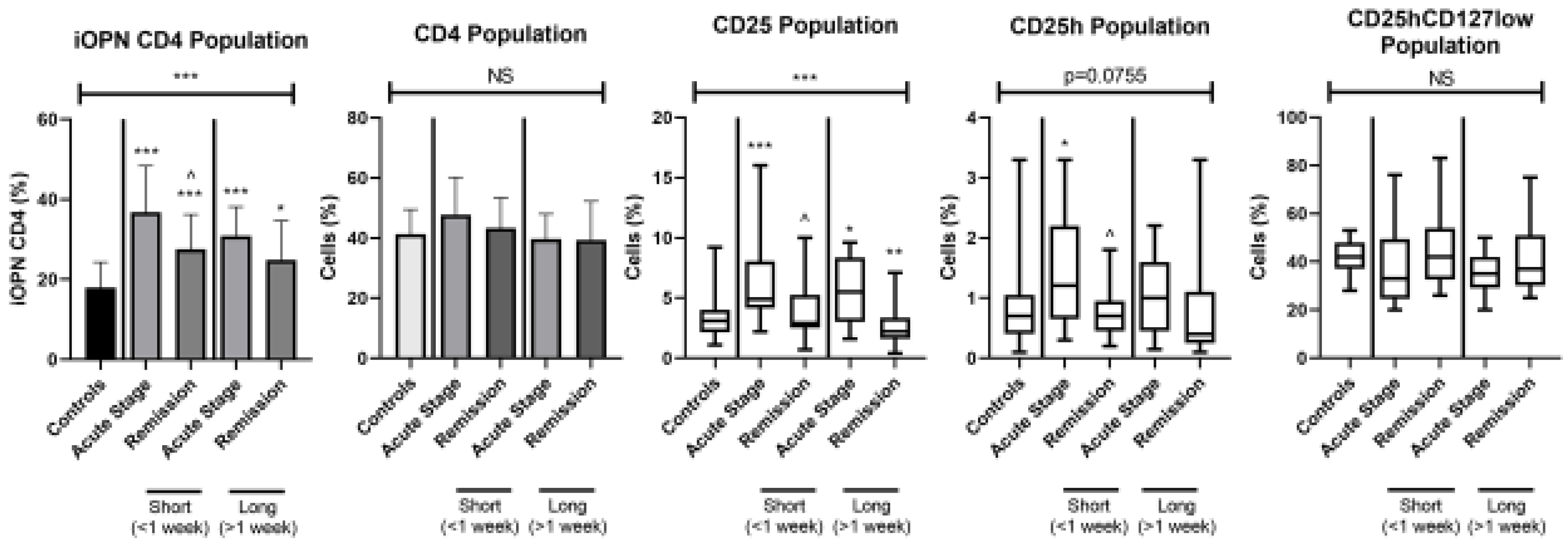
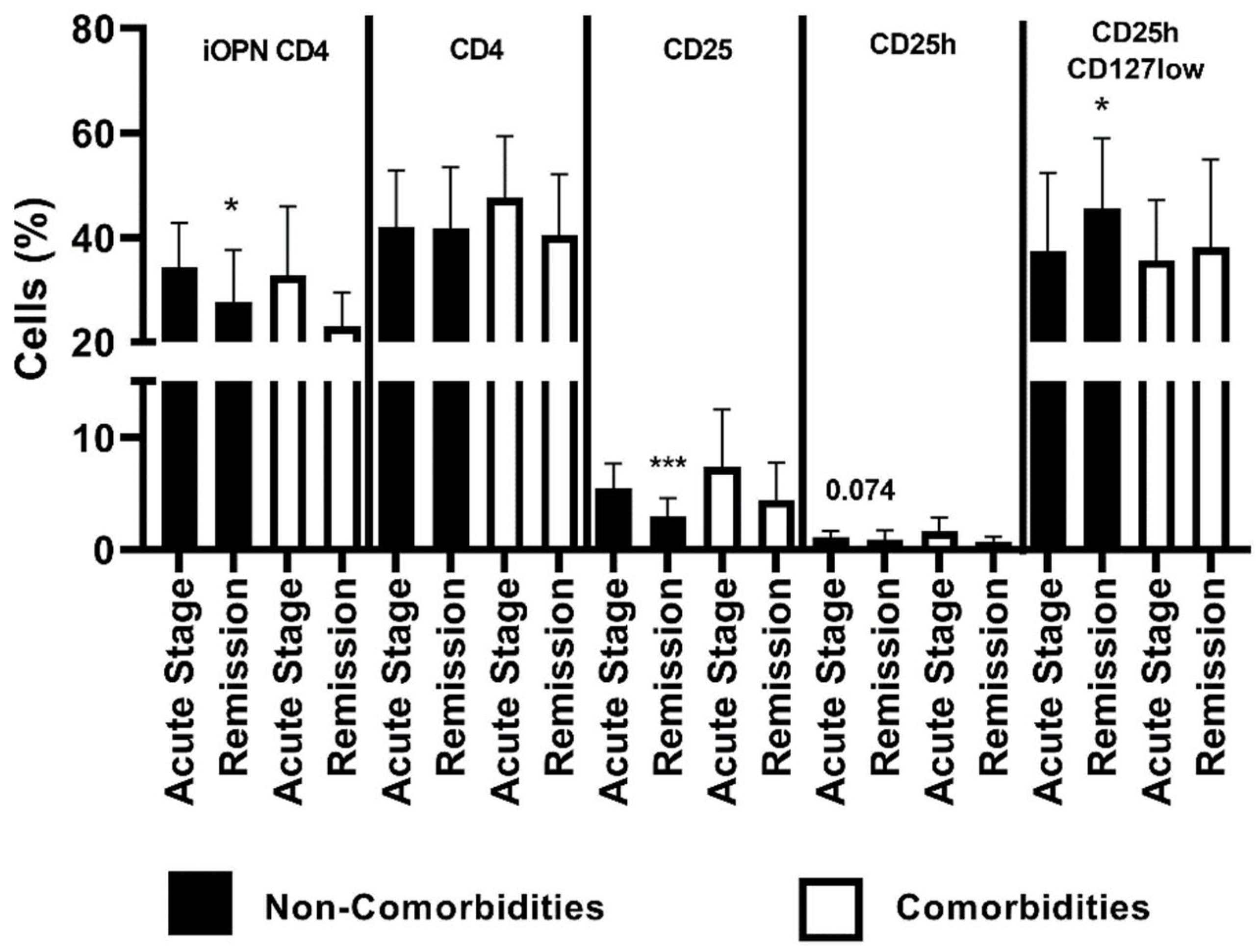
3. Results
4. Discussion
5. Conclusions
- The increase in the T cell population with intracellular expression of osteopontin can indicate its participation in acute ACD. Taking into account previous studies showing an increase sOPN level in the serum of patients with acute ACD could indicate the origin of sOPN from blood lymphocytes and the possibility of transformation of iOPN into sOPN.
- These results can also confirm the pro-inflammatory effect of OPN in acute ACD. An increased percentage of T lymphocytes with iOPN expression during remission of skin lesions can indicate the prolonged presence of these cells in the blood, however, there is no OPN secretion from T lymphocytes.
- The decreased percentage of regulatory T lymphocytes in the blood of patients with acute ACD in comparision with results in the healthy group may be related to the transformation of Tregs into CD4CD25 T cells, of which increased percentage was found in the acute stage of contact dermatitis. It may also indicate their increased recruitment to the skin.
- The positive correlation between the percentage of CD4CD25 T lymphocytes and the EASI index may be indirect evidence for the importance of activated lymphocytes with the CD4CD25 phenotype (in addition to CD8 lymphocytes) as effector cells in allergic contact dermatitis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peiser, M.; Tralau, T.; Heidler, J.; Api, A.M.; Arts, J.H.E.; Basketter, D.A.; English, J.; Diepgen, T.L.; Fuhlbrigge, R.C.; Gaspari, A.A.; et al. Allergic contact dermatitis: Epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Cell. Mol. Life Sci. 2012, 69, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, A.B.; Foss-Skiftesvik, M.H.; Thyssen, J.P.; Deleuran, M.; Mortz, C.G.; Zachariae, C.; Skov, L.; Osterballe, M.; Funding, A.; Avnstorp, C.; et al. Contact allergy in Danish children: Current trends. Contact Dermat. 2018, 79, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu. Rev. Immunol. 2007, 25, 821–852. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of Immune Events in the Murine Contact Hypersensitivity Model: Toward the Understanding of Allergic Contact Dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef]
- Waller, A.H.; Sanchez-Ross, M.; Kaluski, E.; Klapholz, M. Osteopontin in cardiovascular disease: A potential therapeutic target. Cardiol. Rev. 2010, 18, 125–131. [Google Scholar] [CrossRef]
- Scatena, M.; Liaw, L.; Giachelli, C.M. Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2302–2309. [Google Scholar] [CrossRef]
- Zhao, M.; Liang, F.; Zhang, B.; Yan, W.; Zhang, J. The impact of osteopontin on prognosis and clinicopathology of colorectal cancer patients: A systematic meta-analysis. Sci. Rep. 2015, 5, 12713. [Google Scholar] [CrossRef]
- Uede, T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol. Int. 2011, 61, 265–280. [Google Scholar] [CrossRef]
- Buback, F.; Renkl, A.C.; Schulz, G.; Weiss, J.M. Osteopontin and the skin: Multiple emerging roles in cutaneous biology and pathology. Exp. Dermatol. 2009, 18, 750–759. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Kim, H.J.; Kim, J.H.; Garcia, V.A.; Cantor, H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc. Natl. Acad. Sci. USA 2008, 105, 7235–7239. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Kim, J.H.; Garcia, V.A.; Cantor, H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: Role of intracellular osteopontin. Immunity 2008, 29, 68–78. [Google Scholar] [CrossRef]
- Cantor, H.; Shinohara, M.L. Regulation of T-helper-cell lineage development by osteopontin: The inside story. Nat. Rev. Immunol. 2009, 9, 137–141. [Google Scholar] [CrossRef]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef]
- Bartosińska, J.; Przepiórka-Kosińska, J.; Sarecka-Hujar, B.; Raczkiewicz, D.; Kowal, M.; Chyl-Surdacka, K.; Bartosiński, J.; Kosiński, J.; Krasowska, D.; Chodorowska, G. Osteopontin Serum Concentration and Metabolic Syndrome in Male Psoriatic Patients. J. Clin. Med. 2021, 10, 755. [Google Scholar] [CrossRef]
- Buommino, E.; Tufano, M.A.; Balato, N.; Canozo, N.; Donnarumma, M.; Gallo, L.; Balato, A.; Ayala, F. Osteopontin: A new emerging role in psoriasis. Arch. Dermatol. Res. 2009, 301, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Shen, J.L.; Wu, C.Y.; Chang, Y.T.; Chen, C.M.; Lee, F.Y. Elevated plasma osteopontin level is associated with occurrence of psoriasis and is an unfavorable cardiovascular risk factor in patients with psoriasis. J. Am. Acad. Dermatol. 2009, 60, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Lavi, H.; Assayag, M.; Schwartz, A.; Arish, N.; Fridlender, Z.G.; Berkman, N. The association between osteopontin gene polymorphisms, osteopontin expression and sarcoidosis. PLoS ONE 2017, 12, e0171945. [Google Scholar] [CrossRef] [PubMed]
- Soheila, N.; Behzad, I.; Mehdi, G.; Fahimeh, A.; Niloufar, N. The influence of osteopontin on the pathogenesis of alopecia areata and its association with disease severity. Iran. J. Dermatol. 2018, 21, 43–47. [Google Scholar]
- Akelma, A.Z.; Cizmeci, M.N.; Kanburoglu, M.K.; Bozkaya, D.; Catal, F.; Mete, E.; Kutukoglu, I.; Namuslu, M. Elevated level of serum osteopontin in school-age children with asthma. Allergol. Immunopathol. 2014, 42, 275–281. [Google Scholar] [CrossRef]
- Masuoka, M.; Shiraishi, H.; Ohta, S.; Suzuki, S.; Arima, K.; Aoki, S.; Toda, S.; Inagaki, N.; Kurihara, Y.; Hayashida, S.; et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J. Clin. Investig. 2012, 122, 2590–2600. [Google Scholar] [CrossRef]
- Bassyouni, R.H.; Ibrehem, E.G.; El Raheem, T.A.A.; El-Malek, M.A. The role of osteopontin in skin diseases. Glob. Vaccines Immunol. 2016, 1, 48–50. [Google Scholar] [CrossRef]
- Seier, A.M.; Renkl, A.C.; Schulz, G.; Uebele, T.; Sindrilaru, A.; Iben, S.; Liaw, L.; Kon, S.; Uede, T.; Weiss, J.M. Antigen-specific induction of osteopontin contributes to the chronification of allergic contact dermatitis. Am. J. Pathol. 2010, 176, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Renkl, A.C.; Maier, C.S.; Kimmig, M.; Liaw, L.; Ahrens, T.; Kon, S.; Maeda, M.; Hotta, H.; Uede, T.; et al. Osteopontin Is Involved in the Initiation of Cutaneous Contact Hypersensitivity by Inducing Langerhans and Dendritic Cell Migration to Lymph Nodes. J. Exp. Med. 2001, 194, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Iyonaga, K.; Ichiyasu, H.; Nagano, J.; Suga, M.; Sasaki, Y. Differentiation, maturation, and survival of dendritic cells by osteopontin regulation. Clin. Diagn. Lab. Immunol. 2005, 12, 206–212. [Google Scholar] [CrossRef]
- Reduta, T.; Śniecińska, M.; Pawłoś, A.; Sulkiewicz, A.; Sokołowska, M. Serum osteopontin levels in disseminated allergic contact dermatitis. Adv. Med. Sci. 2015, 60, 273–276. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakagouchi, S. Control of regulatory cell development by the transcription factor Fox3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Nomura, I.; Goleva, E.; Howell, M.D.; Hamid, Q.A.; Ong, P.Y.; Hall, C.F.; Darst, M.A.; Gao, B.; Boguniewicz, M.; Travers, J.B.; et al. Cytokine Milieu of Atopic Dermatitis, as Compared to Psoriasis, Skin Prevents Induction of Innate Immune Response Genes. J. Immunol. 2003, 171, 3262–3269. [Google Scholar] [CrossRef]
- Sakagouchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and not-self. Nat. Immunol. 2005, 6, 345–352. [Google Scholar] [CrossRef]
- Ng, W.F.; Duggan, P.J.; Ponchel, F.; Matarese, G.; Lombardi, G.; Edwards, A.D.; Isaacs, J.D.; Lechler, R.I. Human CD4+CD25+ cells: A naturally occurring population of regulatory T cells. Blood 2001, 98, 2736–2744. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Brown, J.A.; Freeman, G.J.; Hafler, D.A. CD4+CD25high Regulatory Cells in Human Peripheral Blood. J. Immunol. 2001, 167, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F. FOXP3: Of mice and men. Annu. Rev. Immunol. 2006, 24, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; Fazekas de St Groth, B.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4 Treg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Di Caro, V.; D’Anneo, A.; Phillips, B.; Engman, C.; Harnaha, J.; Lakomy, R.; Styche, A.; Trucco, M.; Giannoukakis, N. Interleukin-7 matures suppressive CD127(+) forkhead box P3 (FoxP3)(+) T cells into CD127(-) CD25(high) FoxP3(+) regulatory T cells. Clin. Exp. Immunol. 2011, 165, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.S.; Pauli, M.L.; Neuhaus, I.M.; Yu, S.S.; Arron, S.; Harris, H.W.; Yang, S.H.-Y.; Anthony, B.A.; Sverdrup, F.; Krow-Lucal, E.; et al. Memory regulatory T cells reside in human skin. J. Clin. Investig. 2014, 124, 1027–1036. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J.; et al. A vawe of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 2015, 43, 1011–1021. [Google Scholar] [CrossRef]
- Chow, Z.; Mueller, S.N.; Deane, J.A.; Hickey, M.J. Dermal regulatory T cells display distinct migratory behavior that is modulated during adaptive and innate inflammation. J. Immunol. 2013, 191, 3049–3056. [Google Scholar] [CrossRef]
- Gratz, I.K.; Truong, H.A.; Yang, S.H.; Maurano, M.M.; Lee, K.; Abbas, A.K.; Rosenblum, M.D. Memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 2013, 190, 4483–4487. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Vasquez, K.S.; Pauli, M.L.; Leitner, E.G.; Chu, K.; Truong, H.-A.; Lowe, M.M.; Rodriguez, R.S.; Ali, N.; Laszik, Z.G.; et al. Commensal Microbes and Hair Follicle Morphogenesis Coordinately Drive Treg Migration into Neonatal Skin. Cell Host Microbe 2017, 21, 467–477.e5. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Ono, M.; Setoguchi, R.; Yagi, H.; Hori, S.; Fehervari, Z.; Shimizu, J.; Takahashi, T.; Nomura, T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006, 212, 8–27. [Google Scholar] [CrossRef]
- Von Boehmer, H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005, 5, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Askenasy, N.; Kaminitz, A.; Yarkoni, S. Mechanisms of T regulatory cell function. Autoimmun. Rev. 2008, 7, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M.; Thurston, M.; Omoto, M.; Cherill, R.; Tofte, S.J.; Graeber, M. The Easi Evaluator Group The eczema area and severity index (EASI): Assessment of reliability in atopic dermatitis. Exp. Dermatol. 2001, 10, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Suzuki, K.; Goldberg, H.A.; Rittling, S.R.; Denhardt, D.T.; McCulloch, C.A.; Sodek, J. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: Evidence of a role for an intracellular form of osteopontin. J. Cell. Physiol. 2004, 198, 155–167. [Google Scholar] [CrossRef]
- Zohar, R.; Suzuki, N.; Suzuki, K.; Arora, P.; Glogauer, M.; McCulloch, C.A.; Sodek, J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J. Cell. Physiol. 2000, 184, 118–130. [Google Scholar] [CrossRef]
- Suzuki, K.; Takeyama, S.; Sakai, Y.; Yamada, S.; Shinoda, H. Current topics in pharmacological research on bone metabolism: Inhibitory effects of bisphosphonates on the differentiation and activity of osteoclasts. J. Pharmacol. Sci. 2006, 100, 189–194. [Google Scholar] [CrossRef]
- Junaid, A.; Moon, M.C.; Harding, G.E.; Zahradka, P. Osteopontin localizes to the nucleus of 293 cells and associates with polo-like kinase-1. Am. J. Physiol. Cell. Physiol. 2007, 292, 919–926. [Google Scholar] [CrossRef]
- Inoue, M.; Shinohara, M.L. Intracellular osteopontin (iOPN) and immunity. Immunol. Res. 2011, 49, 160–172. [Google Scholar] [CrossRef]
- Leavenworth, J.W.; Verbinnen, B.; Wang, Q.; Shen, E.; Cantor, H. Intracellular osteopontin regulates homeostasis and function of natural killer cells. Proc. Natl. Acad. Sci. USA 2015, 112, 494–499. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Lu, L.; Bu, J.; Werneck, M.B.; Kobayashi, K.S.; Glimcher, L.H.; Cantor, H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat. Immunol. 2006, 7, 498–506. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Jansson, M.; Hwang, E.S.; Werneck, M.B.; Glimcher, L.H.; Cantor, H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc. Natl. Acad. Sci. USA 2005, 102, 17101–17106. [Google Scholar] [CrossRef] [PubMed]
- Zinkevičienė, A.; Kainov, D.; Lastauskienė, E.; Kvedarienė, V.; Bychkov, D.; Byrne, M.; Girkontaitė, I. Serum Biomarkers of Allergic Contact Dermatitis: A Pilot Study. Int. Arch. Allergy Immunol. 2015, 168, 161–164. [Google Scholar] [CrossRef]
- Kehren, J.; Desvignes, C.; Krasteva, M.; Ducluzeau, M.T.; Assossou, O.; Horand, F.; Hahne, M.; Kägi, D.; Kaiserlian, D.; Nicolas, J.F. Cytotoxicity is mandatory for CD8(+) T cell-mediated contact hypersensitivity. J. Exp. Med. 1999, 189, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, F.; Kanzaki, H.; Fuji, K.; Arata, J.; Skiba, H.; Tsujii, K.; Iwatsuki, K. Initaial recruitment of interferon-gamma-producing CD8+ effector cells, followed by infiltration of CD4+ cells in 2,4,6-trinitro-1-chlorobenzene (TNCB)-induced murine contact hypersensitivity reactions. J. Dermatol. 2002, 29, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Kagen, M.H.; McCormick, T.S.; Cooper, K.D. Regulatory T cells in psoriasis. Ernst. Schering. Res. Found. Workshop 2006, 56, 193–209. [Google Scholar]
- Soler, D.C.; McCormick, T.S. The Dark Side of Regulatory T Cells in Psoriasis. J. Investig. Dermatol. 2011, 131, 1785–1786. [Google Scholar] [CrossRef]
- Yang, L.; Li, B.; Dang, E.; Jin, L.; Fan, X.; Wang, G. Impaired function of regulatory T cells in patients with psoriasis is mediated by phosphorylation of STAT3. J. Dermatol. Sci. 2016, 81, 85–92. [Google Scholar] [CrossRef]
- Shin, B.S.; Furuhashi, T.; Nakamura, M.; Torii, K.; Morita, A. Impaired inhibitory function of circulating CD4+CD25+ regulatory T cells in alopecia areata. J. Dermatol. Sci. 2013, 70, 141–143. [Google Scholar] [CrossRef]
- Tembhre, M.K.; Sharma, V.K. T-helper and regulatory T-cell cytokines in the peripheral blood of patients with active alopecia areata. Br. J. Dermatol. 2013, 169, 543–548. [Google Scholar] [CrossRef]
- Kubo, R.; Muramatsu, S.; Sagawa, Y.; Saito, C.; Kasuya, S.; Nishioka, A.; Nishida, E.; Morita, A. Activated regulatory T cells are increased in patients with alopecia areata for suppressing disease acitivity. J. Dermatol. Sci. 2017, 86, e27–e28. [Google Scholar] [CrossRef]
- Ou, L.S.; Goleva, E.; Hall, C.; Leung, D.Y. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J. Allergy Clin. Immunol. 2004, 113, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Reefer, A.J.; Satinover, S.M.; Solga, M.D.; Lannigan, J.A.; Nguyen, J.T.; Wilson, B.B.; Woodfolk, J.A. Analysis of CD25hiCD4+ “regulatory” T-cell subtypes in atopic dermatitis reveals a novel T(H)2-like population. J. Allergy Clin. Immunol. 2008, 121, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Wisniewski, J.A.; Woodfolk, J.A. The Role of Regulatory T Cells in Atopic Dermatitis. Curr. Probl. Dermatol. 2011, 41, 112–124. [Google Scholar]
- Szegedi, A.; Baráth, S.; Nagy, G.; Szodoray, P.; Gál, M.; Sipka, S.; Bagdi, E.; Banham, A.H.; Krenács, L. Regulatory T cells in atopic dermatitis: Epidermal dendritic cell clusters may contribute to their local expansion. Br. J. Dermatol. 2009, 160, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Cavani, A.; Mei, D.; Guerra, E.; Corinti, S.; Giani, M.; Pirrotta, L.; Puddu, P.; Girolomoni, G. Patients with allergic contact dermatitis to nickel and nonallergic individuals display different nickel-specific T cell responses. Evidence for the presence of effector CD8+ and regulatory CD4+ T cells. J. Investig. Dermatol. 1998, 111, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Cavani, A.; Nasorri, F.; Ottaviani, C.; Sebastiani, S.; de Pita, O.; Girolomoni, G. Human CD25+ regulatory T cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J. Immunol. 2003, 17, 5760–5768. [Google Scholar] [CrossRef]
- Hirahara, K.; Liu, J.; Clarc, R.A.; Yamanaha, K.; Fuhlbrigge, R.C.; Kupper, T.S. The majority of human peripheral blood CD4 CD25h Foxp3 regulatory T cells bear functional skin-homing receptors. J. Immunol. 2006, 177, 4488–4491. [Google Scholar] [CrossRef]
- Overstreet, M.G.; Gaylo, A.; Angermann, B.R.; Hughson, A.; Hyun, Y.M.; Lambert, K.; Acharya, M.; Billroth-Maclurg, A.C.; Rosenberg, A.F.; Topham, D.J.; et al. Inflammation-induced interstitial migration of effector CD4+ T cells is dependent on integrin αV. Nat. Immunol. 2013, 14, 949–958. [Google Scholar] [CrossRef]
- Rosenblum, M.D.; Way, S.S.; Abbas, A.K. Regulatory T cell memory. Nat. Rev. Immunol. 2016, 16, 90–101. [Google Scholar] [CrossRef]
- Reduta, T.; Stasiak-Barmuta, A.; Laudańska, H. CD4+CD25+ and CD4+CD2+high regulatory T cells in disseminated and localized forms of allergic contact dermatitis: Relation to specific cytokines. Folia. Histochem. Cytobiol. 2011, 49, 255–262. [Google Scholar] [CrossRef]
- Wolak, T. Osteopontin- a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 2014, 236, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Rosenblum, M.D. Regulatory T cells in skin. Immunology 2017, 152, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Maser, R.E.; Lenhard, M.J.; Pohlig, R.T.; Balagopal, P.B. Osteopontin and clusterin levels in type 2 diabetes mellitus: Differential association with peripheral autonomic nerve function. Neurol. Sci. 2017, 38, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Maneechotesuwan, K.; Kasetsinsombat, K.; Wongkajornsilp, A.; Barnes, P.J. Simvastatin up-regulates adenosine deaminase and suppresses osteopontin expression in COPD patients through an IL-13-dependent mechanism. Respir. Res. 2016, 17, 104. [Google Scholar] [CrossRef]
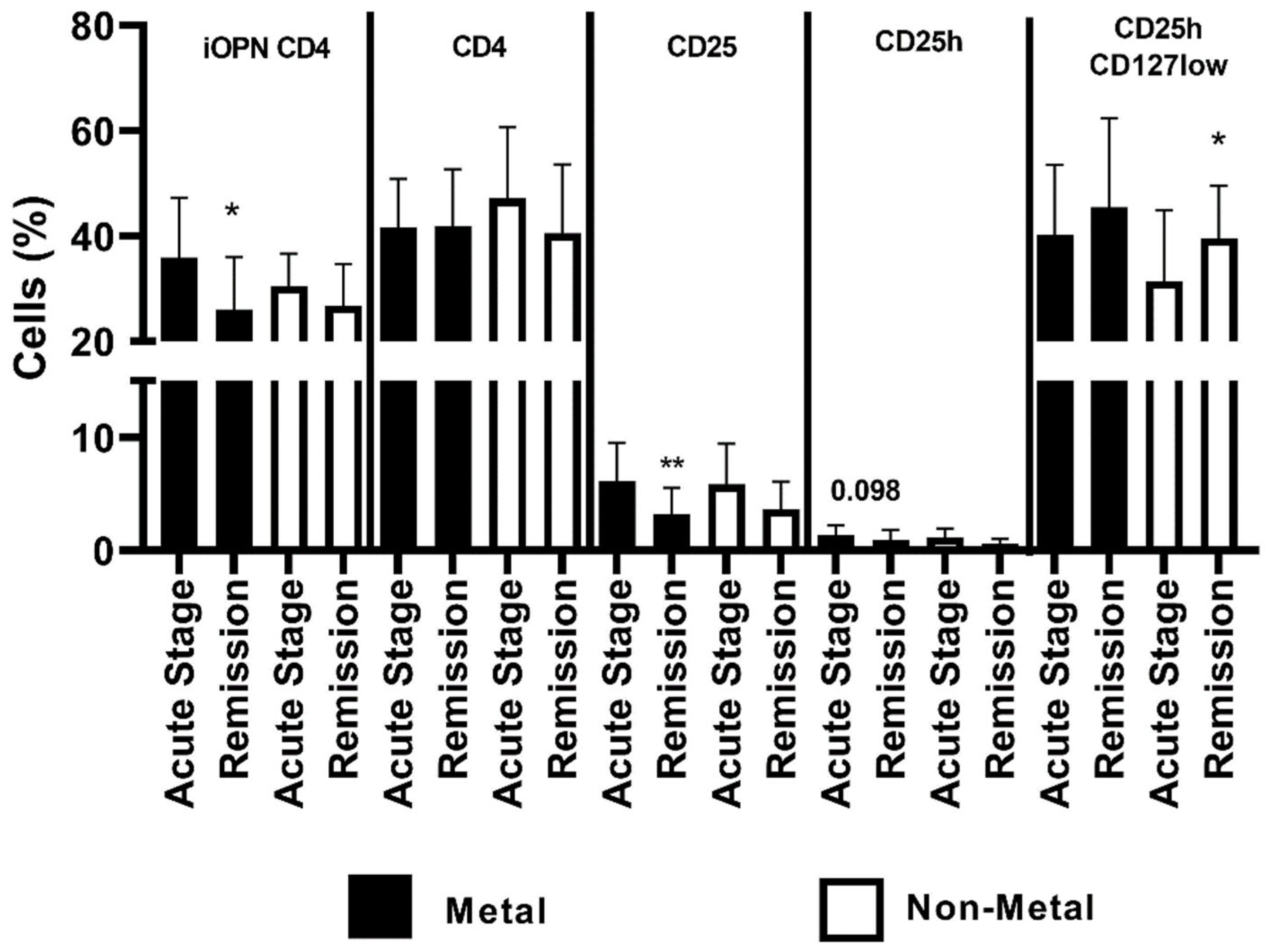
| Parameter | Controls (n = 21) | ACD Patients (Acute Stage) (n = 26) | ACD Patients (Remission) (n = 26) |
|---|---|---|---|
| Age | 45.76 ± 15.15 | 47.5 ± 14.89 NS | - |
| Sex [M/F] | 9/12 | 11/15 NS | - |
| Exacerbation (weeks) | - | 3.7 ± 3.2 (1–12) | - |
| Exacerbation longer than 1 week (Yes/No) | - | 19/7 | - |
| EASI Score | - | 11.6 ± 7.3 (2–29.6) | - |
| EASI subgroup (<15/>15) | - | 17/9 | - |
| Atopic Diseases (Yes/No) | - | 4/22 | - |
| Comorbidities (Yes/No) | - | 8/18 | - |
| Allergies (Metal/Nonmetal) | - | 16/10 | - |
| WBC | 6.95 ± 1.54 | 7.21 ± 1.38 NS | 7.35 ± 1.6 NS |
| Lymphocytes | 1.94 ± 0.56 | 1.69 ± 0.46 NS | 1.68 ± 0.47 NS (p = 0.1049) |
| Lymphocytes (%) | 25.86 ± 6.9 | 24.18 ± 7.02 NS | 24.2 ± 7.1 NS |
| iOPN CD4 vs: | Controls (n = 21) | ACD Patients (Acute Stage) (n = 26) | ACD Patients (Remission) (n = 26) |
|---|---|---|---|
| Age | −0.233 NS | 0.121 NS | 0.008 NS |
| Exacerbation (weeks) | - | −0.157 NS | −0.115 NS |
| EASI Score | - | 0.004 NS | −0.016 NS |
| CD4 | 0.33 NS | 0.174 NS | 0.054 NS |
| CD25 | 0.072 NS | 0.054 NS | 0.229 NS |
| CD25h | −0.029 NS | 0.213 NS | 0.338 NS (p = 0.092) |
| CD25hCD127Iow | 0.026 NS | 0.266 NS | 0.124 NS |
| (A) | ||
| EASI I–iOPN CD4 vs: | ACD Patients (Acute Stage) (n = 17) | ACD Patients (Remission) (n = 17) |
| Age | 0.095 NS | −0.045 NS |
| Exacerbation (weeks) | −0.061 NS | −0.373 NS |
| EASI Score | −0.057 NS | 0.177 NS |
| CD4 | 0.167 NS | −0.107 NS |
| CD25 | 0.000 NS | 0.32 NS |
| CD25h | 0.301 NS | 0.153 NS |
| CD25hCD127Iow | 0.068 NS | 0.352 NS |
| EASI I–iOPN CD4, CD4 T cells with expression of intracellular osteopontin in patients with mild to moderate eczema area and severity index (<15); ACD, allergic contact dermatitis; n, number of patients; NS, non-significant; EASI, Eczema Area and Severity Index; CD4, CD4 positive T lymphocytes; CD25, subset of CD4 positive T lymphocytes; CD25h, CD25 high T lymphocytes; CD25hCD127low, CD25highCD127low T cells—regulatory T cells. Data are presented as R values and followed with p values when p < 0.1. | ||
| (B) | ||
| EASI II–iOPN CD4 vs: | ACD Patients (Acute Stage) (n = 9) | ACD Patients (Remission) (n = 9) |
| Age | 0.282 NS | 0.191 NS |
| Exacerbation (weeks) | −0.155 NS | 0.205 NS |
| EASI Score | −0.102 NS | 0.260 NS |
| CD4 | 0.102 NS | 0.508 (p = 0.136) |
| CD25 | −0.089 NS | −0.005 NS |
| CD25h | −0.023 NS | 0.622 (0.061) |
| CD25hCD127Iow | 0.481 (p = 0.143) | 0.322 NS |
| EASI II–iOPN CD4, CD4 T cells with expression of intracellular osteopontin in patients with severe eczema area and severity index (>15); ACD, allergic contact dermatitis; n, number of patients; NS, non-significant; EASI, Eczema Area and Severity Index; CD4, CD4 positive T lymphocytes; CD25, subset of CD4 positive T lymphocytes; CD25h, CD25 high T lymphocytes; CD25hCD127low, CD25highCD127low T cells—regulatory T cells. Data are presented as R values and followed with p values when p < 0.2. | ||
| (A) | |||||
| Non-Comorbidities (n = 18) Acute Stage | Comorbidities (n = 8) Acute Stage | Non-Comorbidities (n = 18) Remission | Comorbidities (n = 8) Remission | ||
| Age | 45.06 ± 13.77 | 53 ± 16.78 NS | |||
| Exacerbation (weeks) | 4 (1–12) | 2 (1–12) NS | |||
| EASI Score | 8.8 (2–21.9) | 12.8 (5.8–29.6) NS | |||
| CD4 | 42.06 ± 10.77 | 47.63 ± 11.69 | 41.78 ± 11.71 | 40.38 ± 11.76 | |
| CD25 | 5.42 ± 2.26 | 7.38 ± 4.95 (p = 0.18) | 2.9 ± 1.68 *** | 4.44 ± 3.31 * | |
| CD25h | 1.101 ± 0.57 | 1.638 ± 1.16 (p = 0.12) | 0.86 ± 0.45 | 0.71 ± 0.44 | |
| CD25hCD127low | 37.31 ± 15.04 | 35.63 ± 11.56 | 45.44 ± 13.59 (p = 0.073) | 38.11 ± 16.82 | |
| n, number of patients; EASI, Eczema Area and Severity Index; CD4, CD4 positive T lymphocytes; CD25, subset of CD4 positive T lymphocytes; CD25h, CD25 high T lymphocytes; CD25hCD127low, CD25highCD127low T cells—regulatory T cells; NS, non-significant; *, means statistical significance higher the percentage of CD25 with p values < 0.05 between patients with comorbidities in acute stage of ACD than in remission; ***, means statistical significantly higher the percentage of CD25 with p < 0.0001 between patients without comorbidities in acute stage of ACD than in remission. | |||||
| (B) | |||||
| iOPN CD4 vs: | Controls (n = 21) | Non-Comorbidities (n = 18) Acute Stage | Comorbidities (n = 8) Acute Stage | Non-Comorbidities (n = 18) Remission | Comorbidities (n = 8) Remission |
| Age | −0.233 NS | 0.345 NS | −0.452 NS | 0.257 NS | −0.311 NS |
| Exacerbation (weeks) | - | −0.131 NS | −0.393 NS | −0.193 NS | −0.519 NS |
| EASI Score | - | −0.043 NS | 0.091 NS | 0.164 NS | 0.033 NS |
| CD4 | 0.33 NS | 0.266 NS | 0.012 NS | −0.147 NS | 0.807 (0.019) * |
| CD25 | 0.072 NS | −0.132 NS | 0.357 NS | 0.472 (0.042) * | 0.355 NS |
| CD25h | −0.029 NS | 0.11 NS | 0.345 NS | 0.523 (0.0342) * | 0.229 NS |
| CD25hCD127Iow | 0.026 NS | 0.27 NS | 0.429 NS | 0.382 NS | 0.012 NS |
| iOPN CD4, CD4 T lymphocytes with expression of intracellular osteopontin; n, number of patients; EASI, Eczema Area and Severity Index; CD4, CD4 positive T lymphocytes; CD25, subset of CD4 positive T lymphocytes; CD25h, CD25 high T lymphocytes; CD25hCD127low, CD25highCD127low T cells—regulatory T cells; NS, non-significant; *, means statistical significance with p < 0.05 between iOPN CD4 percentage and CD4 in patients with comorbidities during remission and between iOPN CD4 percentage and CD25, CD25h in patients without comorbidities during remission. Data are presented as R values and followed with p values when p < 0.05. | |||||
| (A) | |||||
| Metals (n = 16) Acute Stage | Non-Metals (n = 10) Acute Stage | Metals (n = 16) Remission | Non-Metals (n = 10) Remission | ||
| Age | 48.5 ± 14.92 | 45.9 ± 15.5 NS | |||
| Exacerbation (weeks) | 3.5 (1–12) | 2 (1–8) NS | |||
| EASI Score | 13.25 (2–29.6) | 8.8 (3.2–21.6) | |||
| CD4 | 41.69 ± 9.16 | 47.1 ± 13.59 | 41.81 ± 10.88 | 40.6 ± 13.02 | |
| CD25 | 6.13 ± 3.39 | 5.85 ± 3.61 | 3.19 ± 2.35 ** | 3.66 ± 2.45 (0.098) | |
| CD25h | 1.33 ± 0.88 | 1.17 ± 0.76 | 0.92 ± 0.89 | 0.65 ± 0.37 * | |
| CD25hCD127Iow | 40.16 ± 13.38 | 31.4 ± 13.49 | 45.43 ± 16.98 | 39.6 ± 9.91 | |
| n, number of patients; EASI, Eczema Area and Severity Index; CD4, CD4 positive T lymphocytes; CD25, subset of CD4 positive T lymphocytes; CD25h, CD25 high T lymphocytes; CD25hCD127low, CD25highCD127low T cells—regulatory T cells; NS, non-significant; *, means statistical significance with p value < 0.05 between proportion of CD25h in patients allergic to other allergens during the acute stage than the remission; **, means statistical significance with p value < 0.01 between proportion of CD25 in patients allergic to metals during the acute stage than in remission. | |||||
| (B) | |||||
| iOPN CD4 vs: | Controls (n = 21) | Metals (n = 16) Acute Stage | Non-Metals (n = 10) Acute Stage | Metals (n = 16) Remission | Non-Metals (n = 10) Remission |
| Age | −0.233 NS | −0.057 NS | 0.394 NS | 0.261 NS | −0.276 NS |
| Exacerbation (weeks) | - | −0.465 (p=0.052) | 0.438 NS | −0.346 NS | 0.436 NS |
| EASI Score | - | −0.014 NS | −0.296 NS | 0.165 NS | −0.507 (p = 0.11) |
| CD4 | 0.33 NS | 0.285 NS | −0.072 NS | 0.251 NS | −0.352 NS |
| CD25 | 0.072 NS | 0.192 NS | 0.167 NS | 0.304 NS | −0.025 NS |
| CD25h | −0.029 NS | 0.146 NS | 0.542 (p = 0.81) | 0.633 (0.0062) ** | −0.316 NS |
| CD25hCD127Iow | 0.026 NS | 0.251 NS | −0.046 NS | 0.26 NS | 0.462 (p = 0.13) |
| iOPN CD4, CD4 T lymphocytes with expression of intracellular osteopontin; n, number of patients; EASI, Eczema Area and Severity Index; CD4, CD4 positive T lymphocytes; CD25, subset of CD4 positive T lymphocytes; CD25h, CD25 high T lymphocytes; CD25hCD127low, CD25highCD127low T cells—regulatory T cells; NS, non-significant; **, means statistical significance correlation with p values < 0.01 between iOPN CD4 percentage and CD25h in patients allergic to metals examined during remission. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reduta, T.; Bacharewicz-Szczerbicka, J.; Stasiak-Barmuta, A.; Kaminski, T.W.; Flisiak, I. Osteopontin and Regulatory T Cells in Effector Phase of Allergic Contact Dermatitis. J. Clin. Med. 2023, 12, 1397. https://doi.org/10.3390/jcm12041397
Reduta T, Bacharewicz-Szczerbicka J, Stasiak-Barmuta A, Kaminski TW, Flisiak I. Osteopontin and Regulatory T Cells in Effector Phase of Allergic Contact Dermatitis. Journal of Clinical Medicine. 2023; 12(4):1397. https://doi.org/10.3390/jcm12041397
Chicago/Turabian StyleReduta, Teresa, Joanna Bacharewicz-Szczerbicka, Anna Stasiak-Barmuta, Tomasz W. Kaminski, and Iwona Flisiak. 2023. "Osteopontin and Regulatory T Cells in Effector Phase of Allergic Contact Dermatitis" Journal of Clinical Medicine 12, no. 4: 1397. https://doi.org/10.3390/jcm12041397
APA StyleReduta, T., Bacharewicz-Szczerbicka, J., Stasiak-Barmuta, A., Kaminski, T. W., & Flisiak, I. (2023). Osteopontin and Regulatory T Cells in Effector Phase of Allergic Contact Dermatitis. Journal of Clinical Medicine, 12(4), 1397. https://doi.org/10.3390/jcm12041397







