Retrospective National “Real Life” Experience of the SFCE with the Metronomic MEMMAT and MEMMAT-like Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. The MEMMAT Regimen
- -
- Daily oral thalidomide (3 mg/kg/d);
- -
- Daily oral fenofibrate (90 mg/m2/d);
- -
- Twice daily oral celecoxib (100 to 400 mg 2x/d);
- -
- Alternating 21-day cycles of low-dose oral etoposide (50 mg/m2/d) and cyclophosphamide (2.5 mg/kg/d) [24].
- -
- Intra-venous Bevacizumab (10 mg/kg) every two weeks;
- -
- IVe therapy consisting of alternating etoposide (0.25 mg to 0.5 mg/d) for five consecutive days [28], alternating with liposomal cytarabine (25–50 mg) in combination with oral steroids to prevent chemical meningitis every 3 weeks [29]. When liposomal cytarabine was no longer available, it was switched for standard aqueous aracytine.
2.3. Evaluation
2.3.1. Response to Treatment
2.3.2. Evaluation of Toxicity
2.4. Statistical Methods
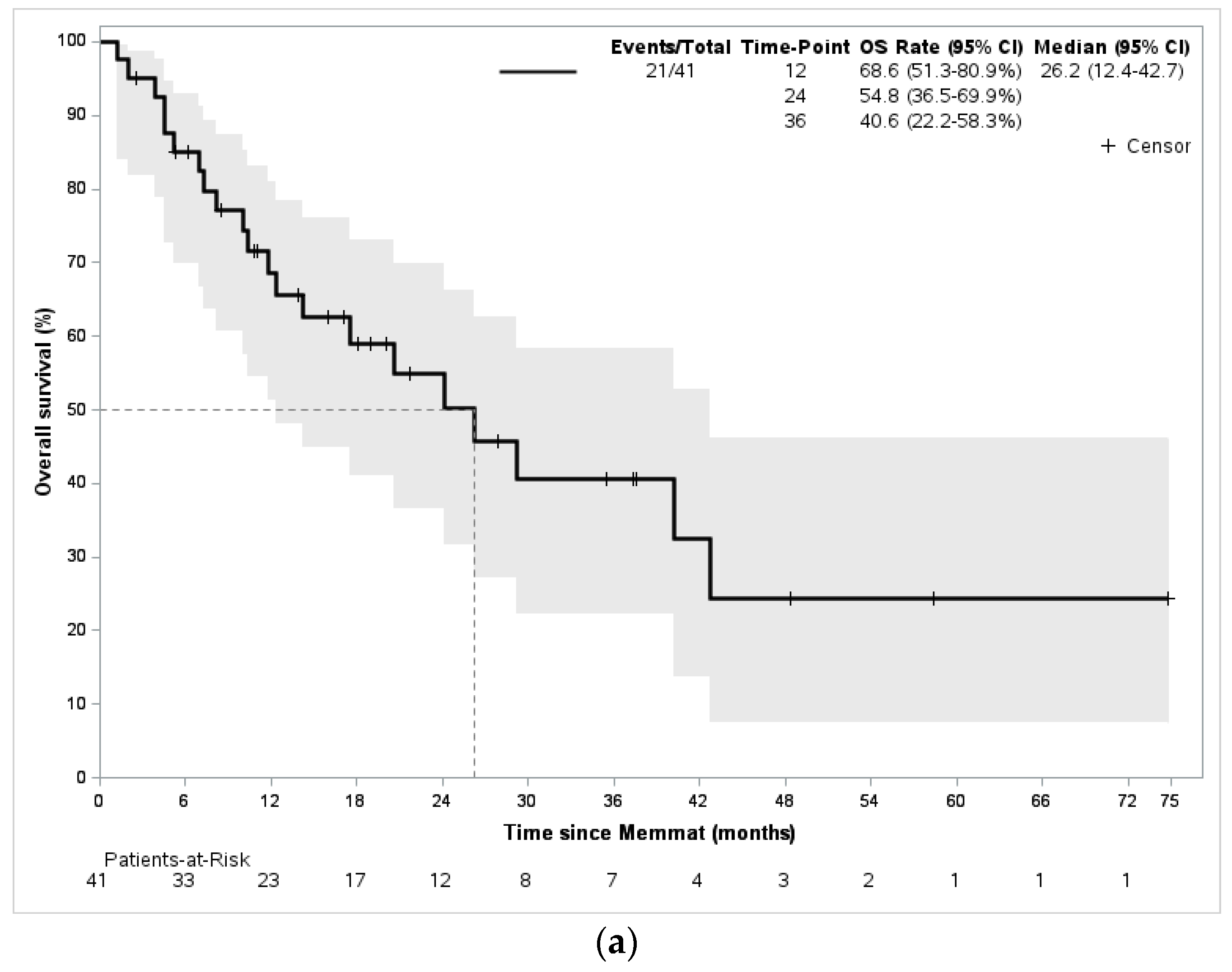
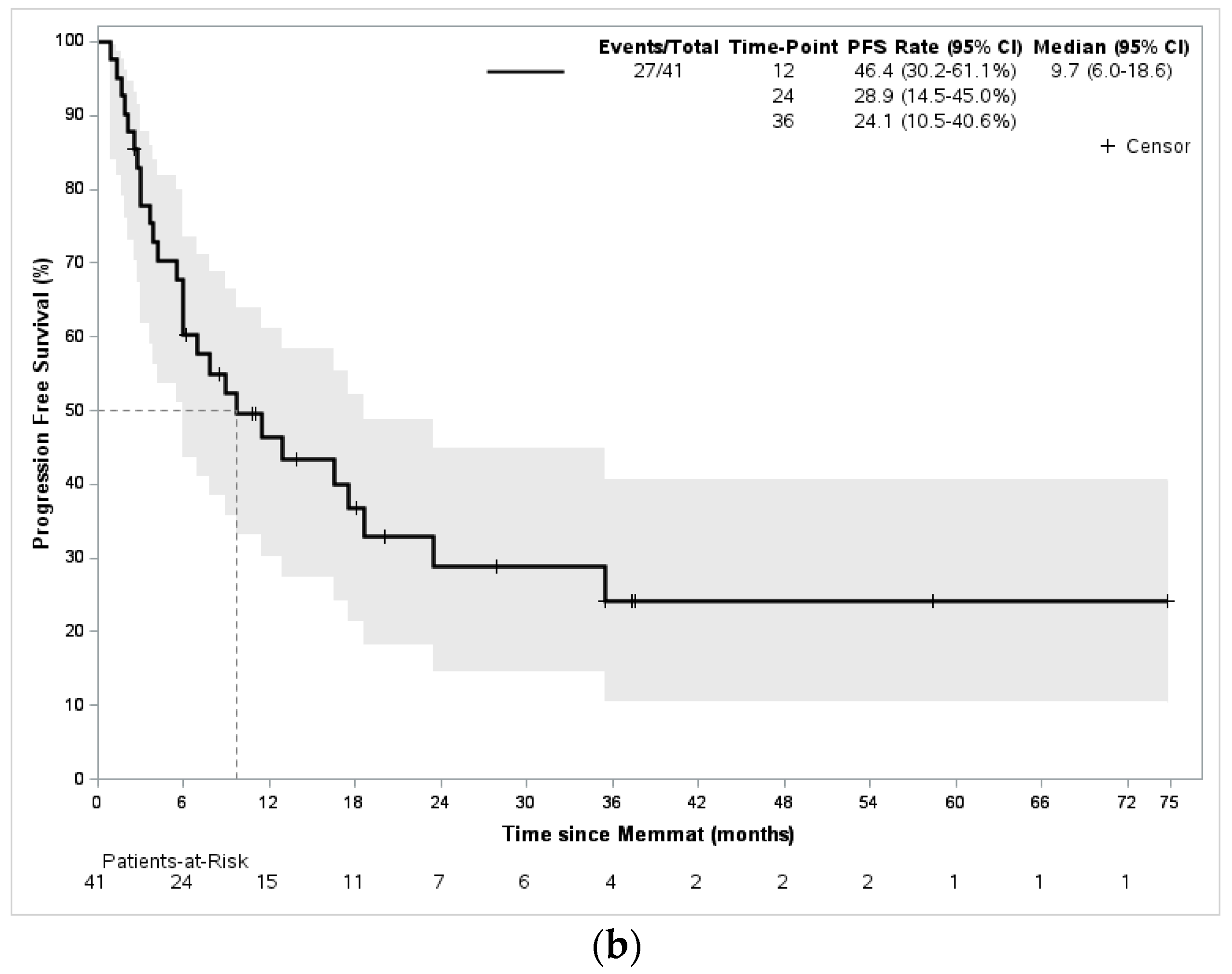
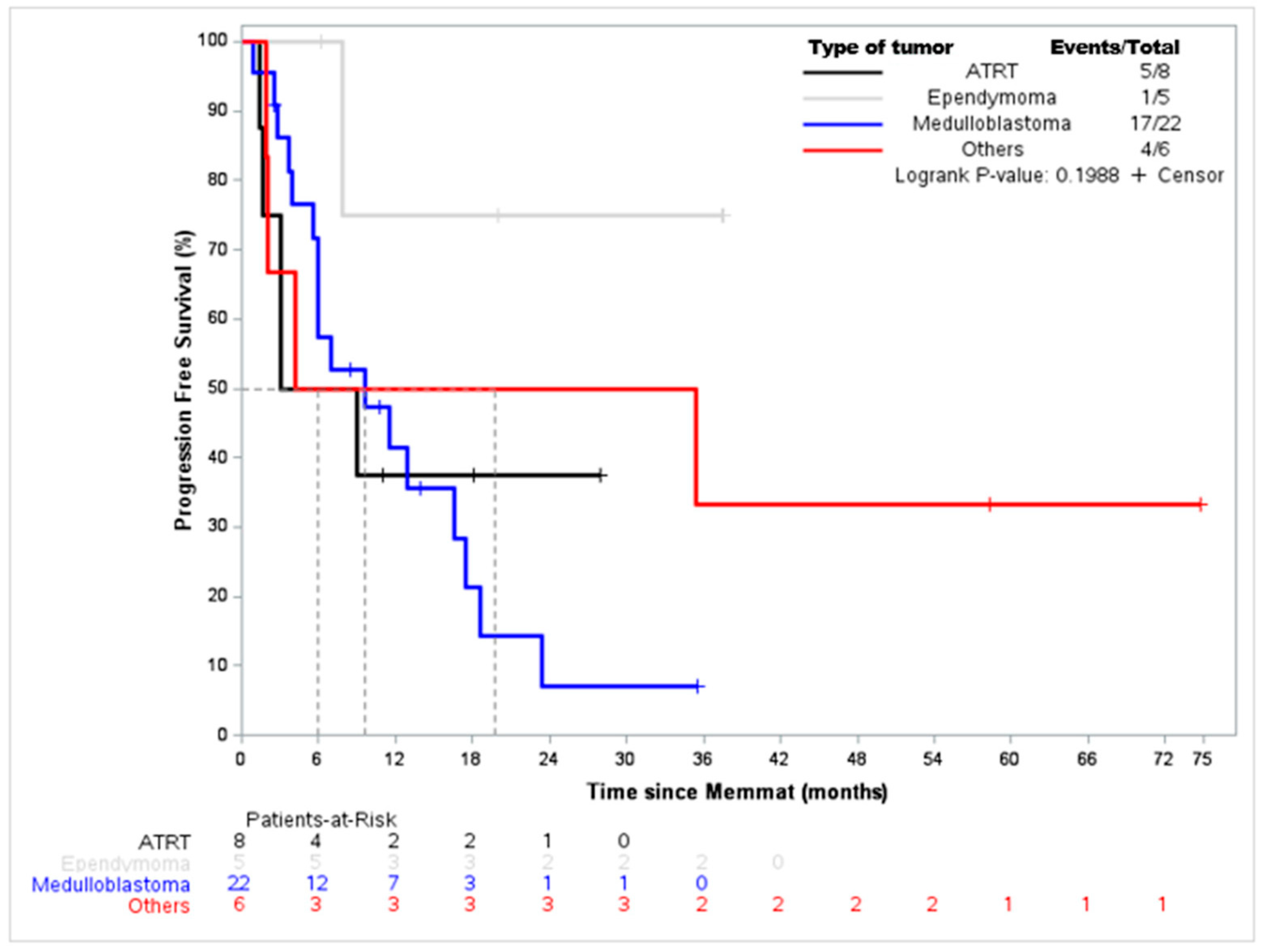
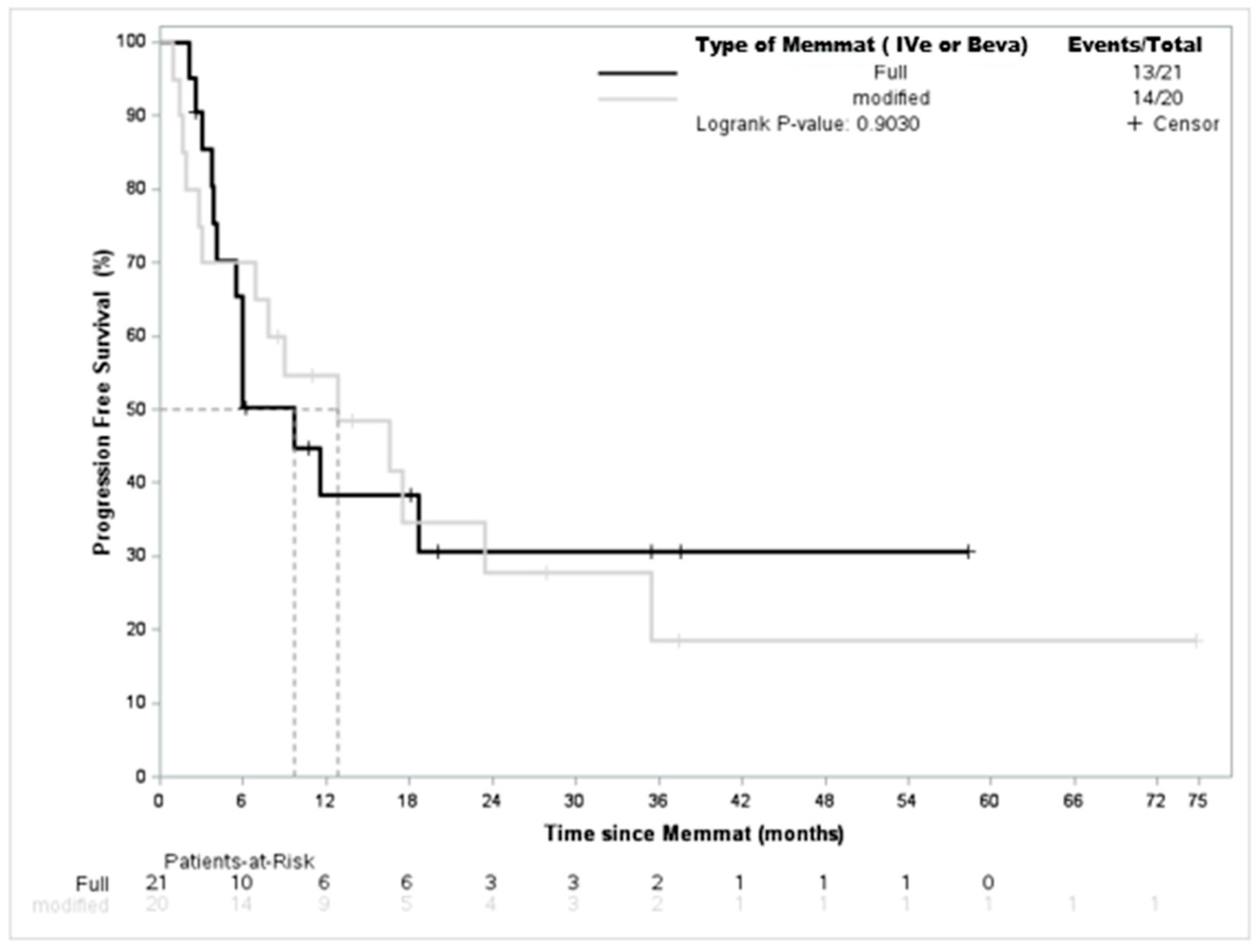
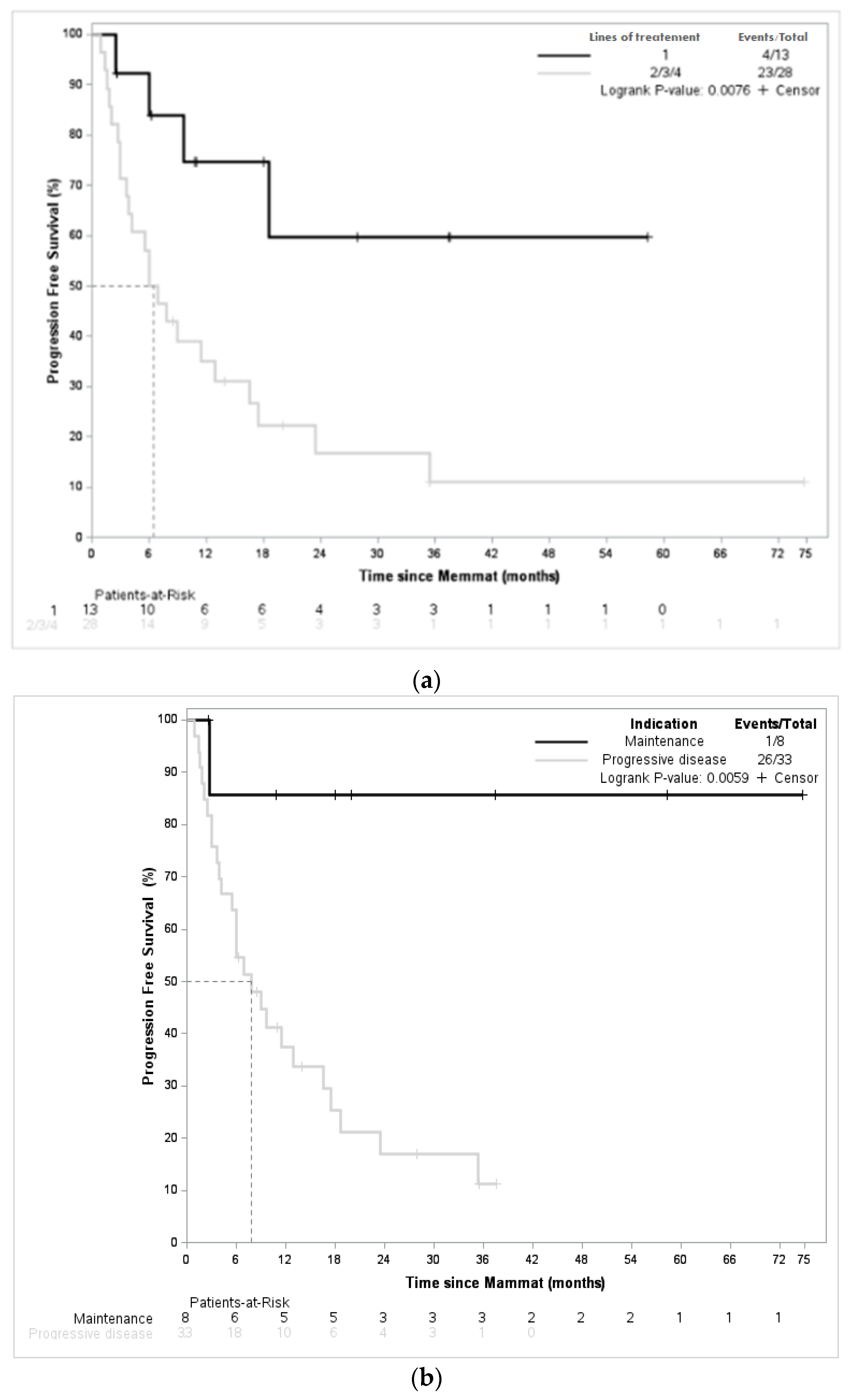
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J.; et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncol. 2015, 16, x1–x36. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood Brain Tumors: Current Management, Biological Insights, and Future Directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F. Brain Tumors in Children. N. Engl. J. Med. 1994, 331, 1500–1507. [Google Scholar] [CrossRef]
- Pritchard-Jones, K.; Kaatsch, P.; Steliarova-Foucher, E.; Stiller, C.A.; Coebergh, J.W.W. Cancer in Children and Adolescents in Europe: Developments over 20 Years and Future Challenges. Eur. J. Cancer 2006, 42, 2183–2190. [Google Scholar] [CrossRef]
- Packer, R.J.; Gajjar, A.; Vezina, G.; Rorke-Adams, L.; Burger, P.C.; Robertson, P.L.; Bayer, L.; LaFond, D.; Donahue, B.R.; Marymont, M.H.; et al. Phase III Study of Craniospinal Radiation Therapy Followed by Adjuvant Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J. Clin. Oncol. 2006, 24, 4202–4208. [Google Scholar] [CrossRef]
- Bailey, S.; André, N.; Gandola, L.; Massimino, M.; Rutkowski, S.; Clifford, S.C. Clinical Trials in High-Risk Medulloblastoma: Evolution of the SIOP-Europe HR-MB Trial. Cancers 2022, 14, 374. [Google Scholar] [CrossRef]
- Banerjee, A.; Jakacki, R.I.; Onar-Thomas, A.; Wu, S.; Nicolaides, T.; Young Poussaint, T.; Fangusaro, J.; Phillips, J.; Perry, A.; Turner, D.; et al. A Phase I Trial of the MEK Inhibitor Selumetinib (AZD6244) in Pediatric Patients with Recurrent or Refractory Low-Grade Glioma: A Pediatric Brain Tumor Consortium (PBTC) Study. Neuro-Oncol. 2017, 19, 1135–1144. [Google Scholar] [CrossRef]
- Fangusaro, J.; Onar-Thomas, A.; Poussaint, T.Y.; Wu, S.; Ligon, A.H.; Lindeman, N.; Campagne, O.; Banerjee, A.; Gururangan, S.; Kilburn, L.B.; et al. A Phase II Trial of Selumetinib in Children with Recurrent Optic Pathway and Hypothalamic Low-Grade Glioma without NF1: A Pediatric Brain Tumor Consortium Study. Neuro-Oncol. 2021, 23, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Robinson, G.W.; Gauvain, K.; Basu, E.M.; Macy, M.E.; Maese, L.; Whipple, N.S.; Sabnis, A.J.; Foster, J.H.; Shusterman, S.; et al. Entrectinib in Children and Young Adults with Solid or Primary CNS Tumors Harboring NTRK, ROS1 or ALK Aberrations (STARTRK-NG). Neuro-Oncol. 2022, 24, 1776–1789. [Google Scholar] [CrossRef]
- Tauziède-Espariat, A.; Dangouloff-Ros, V.; Figarella-Branger, D.; Uro-Coste, E.; Nicaise, Y.; André, N.; Scavarda, D.; Testud, B.; Girard, N.; Rousseau, A.; et al. Clinicopathological and Molecular Characterization of Three Cases Classified by DNA-Methylation Profiling as “Glioneuronal Tumors, NOS, Subtype A”. Acta Neuropathol. 2022, 144, 1179–1183. [Google Scholar] [CrossRef]
- André, N.; Carré, M.; Pasquier, E. Metronomics: Towards Personalized Chemotherapy? Nat. Rev. Clin. Oncol. 2014, 11, 413–431. [Google Scholar] [CrossRef]
- Kerbel, R.S.; Kamen, B.A. The Anti-Angiogenic Basis of Metronomic Chemotherapy. Nat. Rev. Cancer 2004, 4, 423–436. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Lee, H.-T.; Chen, C.-M.; Shen, C.-C.; Ma, H.-I. Celecoxib Suppresses the Phosphorylation of STAT3 Protein and Can Enhance the Radiosensitivity of Medulloblastoma-Derived Cancer Stem-Like Cells. Int. J. Mol. Sci. 2014, 15, 11013–11029. [Google Scholar] [CrossRef] [PubMed]
- Nars, M.S.; Kaneno, R. Immunomodulatory Effects of Low Dose Chemotherapy and Perspectives of Its Combination with Immunotherapy. Int. J. Cancer 2013, 132, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-B.; Yi, S.-Y.; Ruan, J.; Zhao, L.; Nan, K.-J. New Insights into Metronomic Chemotherapy-Induced Immunoregulation. Cancer Lett. 2014, 354, 220–226. [Google Scholar] [CrossRef]
- André, N.; Tsai, K.; Carré, M.; Pasquier, E. Metronomic Chemotherapy: Direct Targeting of Cancer Cells after All? Trends Cancer 2017, 3, 319–325. [Google Scholar] [CrossRef]
- Kieran, M.W.; Turner, C.D.; Rubin, J.B.; Chi, S.N.; Zimmerman, M.A.; Chordas, C.; Klement, G.; Laforme, A.; Gordon, A.; Thomas, A.; et al. A Feasibility Trial of Antiangiogenic (Metronomic) Chemotherapy in Pediatric Patients with Recurrent or Progressive Cancer. J. Pediatr. Hematol. Oncol. 2005, 27, 573–581. [Google Scholar] [CrossRef]
- Zapletalova, D.; André, N.; Deak, L.; Kyr, M.; Bajciova, V.; Mudry, P.; Dubska, L.; Demlova, R.; Pavelka, Z.; Zitterbart, K.; et al. Metronomic Chemotherapy with the COMBAT Regimen in Advanced Pediatric Malignancies: A Multicenter Experience. Oncology 2012, 82, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Fousseyni, T.; Diawara, M.; Pasquier, E.; Andre, N. Children Treated With Metronomic Chemotherapy in a Low-Income Country: METRO-MALI-0. J. Pediatr. Hematol. Oncol. 2011, 33, 31–34. [Google Scholar] [CrossRef]
- André, N.; Abed, S.; Orbach, D.; Alla, C.A.; Padovani, L.; Pasquier, E.; Gentet, J.C.; Verschuur, A. Pilot Study of a Pediatric Metronomic 4-Drug Regimen. Oncotarget 2011, 2, 960–965. [Google Scholar] [CrossRef] [PubMed]
- André, N.; Orbach, D.; Pasquier, E. Metronomic Maintenance for High-Risk Pediatric Malignancies: One Size Will Not Fit All. Trends Cancer 2020, 6, 819–828. [Google Scholar] [CrossRef]
- André, N.; Banavali, S.; Snihur, Y.; Pasquier, E. Has the Time Come for Metronomics in Low-Income and Middle-Income Countries? Lancet Oncol. 2013, 14, e239–e248. [Google Scholar] [CrossRef]
- Revon-Rivière, G.; Banavali, S.; Heississen, L.; Gomez Garcia, W.; Abdolkarimi, B.; Vaithilingum, M.; Li, C.-K.; Leung, P.C.; Malik, P.; Pasquier, E.; et al. Metronomic Chemotherapy for Children in Low- and Middle-Income Countries: Survey of Current Practices and Opinions of Pediatric Oncologists. J. Glob. Oncol. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Robison, N.J.; Campigotto, F.; Chi, S.N.; Manley, P.E.; Turner, C.D.; Zimmerman, M.A.; Chordas, C.A.; Werger, A.M.; Allen, J.C.; Goldman, S.; et al. A Phase II Trial of a Multi-Agent Oral Antiangiogenic (Metronomic) Regimen in Children with Recurrent or Progressive Cancer. Pediatr. Blood Cancer 2014, 61, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Kieran, M.W.; Sterba, J.; Shaked, Y.; Baruchel, S.; Oberlin, O.; Kivivuori, M.S.; Peyrl, A.; Diawarra, M.; Casanova, M.; et al. Moving Forward with Metronomic Chemotherapy: Meeting Report of the 2nd International Workshop on Metronomic and Anti-Angiogenic Chemotherapy in Paediatric Oncology. Transl. Oncol. 2011, 4, 203–211. [Google Scholar] [CrossRef]
- Peyrl, A.; Chocholous, M.; Kieran, M.W.; Azizi, A.A.; Prucker, C.; Czech, T.; Dieckmann, K.; Schmook, M.-T.; Haberler, C.; Leiss, U.; et al. Antiangiogenic Metronomic Therapy for Children with Recurrent Embryonal Brain Tumors. Pediatr. Blood Cancer 2012, 59, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Klement, G.; Baruchel, S.; Rak, J.; Man, S.; Clark, K.; Hicklin, D.J.; Bohlen, P.; Kerbel, R.S. Continuous Low-Dose Therapy with Vinblastine and VEGF Receptor-2 Antibody Induces Sustained Tumor Regression without Overt Toxicity. J. Clin. Investig. 2000, 105, R15–R24. [Google Scholar] [CrossRef]
- Fleischhack, G.; Reif, S.; Hasan, C.; Jaehde, U.; Hettmer, S.; Bode, U. Feasibility of Intraventricular Administration of Etoposide in Patients with Metastatic Brain Tumours. Br. J. Cancer 2001, 84, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Peyrl, A.; Sauermann, R.; Chocholous, M.; Azizi, A.A.; Jäger, W.; Höferl, M.; Slavc, I. Pharmacokinetics and Toxicity of Intrathecal Liposomal Cytarabine in Children and Adolescents Following Age-Adapted Dosing. Clin. Pharm. 2014, 53, 165–173. [Google Scholar] [CrossRef]
- Warren, K.E.; Vezina, G.; Poussaint, T.Y.; Warmuth-Metz, M.; Chamberlain, M.C.; Packer, R.J.; Brandes, A.A.; Reiss, M.; Goldman, S.; Fisher, M.J.; et al. Response Assessment in Medulloblastoma and Leptomeningeal Seeding Tumors: Recommendations from the Response Assessment in Pediatric Neuro-Oncology Committee. Neuro-Oncol. 2018, 20, 13–23. [Google Scholar] [CrossRef]
- Slavc, I.; Mayr, L.; Stepien, N.; Gojo, J.; Aliotti Lippolis, M.; Azizi, A.A.; Chocholous, M.; Baumgartner, A.; Hedrich, C.S.; Holm, S.; et al. Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a “MEMMAT-like” Metronomic Antiangiogenic Approach. Cancers 2022, 14, 5128. [Google Scholar] [CrossRef]
- Pasquier, E.; Kavallaris, M.; André, N. Metronomic Chemotherapy: New Rationale for New Directions. Nat. Rev. Clin. Oncol. 2010, 7, 455–465. [Google Scholar] [CrossRef]
- Li, L.; Patel, M.; Nguyen, H.S.; Doan, N.; Sharma, A.; Maiman, D. Primary Atypical Teratoid/Rhabdoid Tumor of the Spine in an Adult Patient. Surg. Neurol. Int. 2016, 7, 27. [Google Scholar] [CrossRef]
- Gotti, G.; Biassoni, V.; Schiavello, E.; Spreafico, F.; Antonelli, M.; Calareso, G.; Pecori, E.; Gandola, L.; Massimino, M. A Case of Relapsing Spinal Atypical Teratoid/Rhabdoid Tumor (AT/RT) Responding to Vinorelbine, Cyclophosphamide, and Celecoxib. Childs Nerv. Syst. 2015, 31, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Steinbügl, M.; Nemes, K.; Gruhle, M.; Johann, P.; Gil-da-Costa, M.J.; Ebinger, M.; Sehested, A.; Hauser, P.; Reinhard, H.; Hettmer, S.; et al. ATRT-09. Outcome and Therapeutic Interventions in Relapsed and Refractory ATRT—The EU-RHAB Perspective. Neuro-Oncol. 2022, 24, i4. [Google Scholar] [CrossRef]
- Berland, M.; Padovani, L.; Rome, A.; Pech-Gourg, G.; Figarella-Branger, D.; André, N. Sustained Complete Response to Metronomic Chemotherapy in a Child with Refractory Atypical Teratoid Rhabdoid Tumor: A Case Report. Front. Pharmacol. 2017, 8, 792. [Google Scholar] [CrossRef] [PubMed]
- Porkholm, M.; Toiviainen-Salo, S.; Seuri, R.; Lönnqvist, T.; Vepsäläinen, K.; Saarinen-Pihkala, U.M.; Pentikäinen, V.; Kivivuori, S.-M. Metronomic Therapy Can Increase Quality of Life during Paediatric Palliative Cancer Care, but Careful Patient Selection Is Essential. Acta Paediatr. 2016, 105, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, S.C.; Doz, F.; Kool, M.; Dufour, C.; Vassal, G.; Milde, T.; et al. Risk Stratification of Childhood Medulloblastoma in the Molecular Era: The Current Consensus. Acta Neuropathol. 2016, 131, 821–831. [Google Scholar] [CrossRef]
| Patient Characteristics | Frequency | |
|---|---|---|
| N = 41 | ||
| Age (years) | ||
| Median age (min; max) | 14 (1; 19) | |
| Gender | ||
| Female | 20 | (48.8%) |
| Male | 21 | (51.2%) |
| Type of malignancy | ||
| Medulloblastoma | 22 | (53.7%) |
| Atypical Teratoid Rhabdoid Tumor | 8 | (19.5%) |
| Ependymoma | 5 | (12.2%) |
| Others | 6 | (14.6%) |
| Number of previous lines of treatment | ||
| 1 | 13 | (31.7%) |
| 2 | 17 | (41.5%) |
| 3 | 5 | (12.2%) |
| 4 | 6 | (14.6%) |
| Type of treatment previously received | ||
| Chemotherapy | 39 | (95.1%) |
| Surgery Radiotherapy Immunotherapy Targeted therapies | 40 39 0 3 | (97.6%) (95.1%) (0%) (7.3%) |
| MEMMAT received | ||
| Full-type | 16 | (39.0%) |
| Modified-type | 25 | (61.0%) |
| Drugs not administrated in modified MEMMAT | ||
| Bevacizumab | 10 | (24.4%) |
| Intrathecal injections Thalidomide | 14 13 | (34.1%) (31.7%) |
| Toxicity (Grade 3–4) | Number of Patients N = 41 |
|---|---|
| Hematological toxicity | |
| -Neutropenia | 20 (49%) |
| -Thrombopenia -Anemia | 4 (10%) 2 (5%) |
| Neurological disorder | |
| -Neuropathy | 7 (17%) |
| -Status epilepticus | 2 (4%) |
| -Hemiparesis | 1 (2%) |
| -Cerebellitis | 1 (2%) |
| Hemorrhagic disorder | |
| -Macroscopic hematuria | 1 (2%) |
| -Rectal bleeding | 1 (2%) |
| Intraventricular reservoir disorder | 3 (7%) |
| Infections | 2 (5%) |
| Others | |
| -Mucositis -Rhinitis | 2 (5%) 2 (5%) |
| -Thyroiditis | 1 (2%) |
| Diagnosis | Tumor Status Prior to MEMMAT | nb Previous Lines of Treatment | Type of Treatment Previously Received | Time to Progression Prior to MEMMAT (days) | Time to Progression with MEMMAT (days) | |
|---|---|---|---|---|---|---|
| Patient #1, 7 y-o | Anaplastic grade 3 | local disease | 1 | Surgery, chemotherapy, radiation therapy | 0 | 0 |
| Patient #2, 21 y-o | Grade 2 | local disease | 3 | Surgery, chemotherapy, radiation therapy | 122 | 0 |
| Patient #3, 1 y-o | Anaplastic grade 3 | Metastatic disease, CSF positive | 4 | Surgery, chemotherapy, radiation therapy | 240 | 34 |
| Patient #4, 9 y-o | Anaplastic grade 3 | Metastatic disease | 1 | Surgery, radiation therapy | 452 | 0 |
| Patient #5, 4 y-o | No data | Metastatic disease | 1 | Surgery, chemotherapy, radiation therapy | 55 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winnicki, C.; Leblond, P.; Bourdeaut, F.; Pagnier, A.; Paluenzela, G.; Chastagner, P.; Duhil-De Benaze, G.; Min, V.; Sudour-Bonnange, H.; Piette, C.; et al. Retrospective National “Real Life” Experience of the SFCE with the Metronomic MEMMAT and MEMMAT-like Protocol. J. Clin. Med. 2023, 12, 1415. https://doi.org/10.3390/jcm12041415
Winnicki C, Leblond P, Bourdeaut F, Pagnier A, Paluenzela G, Chastagner P, Duhil-De Benaze G, Min V, Sudour-Bonnange H, Piette C, et al. Retrospective National “Real Life” Experience of the SFCE with the Metronomic MEMMAT and MEMMAT-like Protocol. Journal of Clinical Medicine. 2023; 12(4):1415. https://doi.org/10.3390/jcm12041415
Chicago/Turabian StyleWinnicki, Camille, Pierre Leblond, Franck Bourdeaut, Anne Pagnier, Gilles Paluenzela, Pascal Chastagner, Gwenaelle Duhil-De Benaze, Victoria Min, Hélène Sudour-Bonnange, Catherine Piette, and et al. 2023. "Retrospective National “Real Life” Experience of the SFCE with the Metronomic MEMMAT and MEMMAT-like Protocol" Journal of Clinical Medicine 12, no. 4: 1415. https://doi.org/10.3390/jcm12041415
APA StyleWinnicki, C., Leblond, P., Bourdeaut, F., Pagnier, A., Paluenzela, G., Chastagner, P., Duhil-De Benaze, G., Min, V., Sudour-Bonnange, H., Piette, C., Entz-Werle, N., Chabaud, S., & André, N. (2023). Retrospective National “Real Life” Experience of the SFCE with the Metronomic MEMMAT and MEMMAT-like Protocol. Journal of Clinical Medicine, 12(4), 1415. https://doi.org/10.3390/jcm12041415






