Radiation Recall Pneumonitis: The Open Challenge in Differential Diagnosis of Pneumonia Induced by Oncological Treatments
Abstract
:1. Introduction
2. Mechanisms of Lung Radiation Damage
3. Diagnostic Management
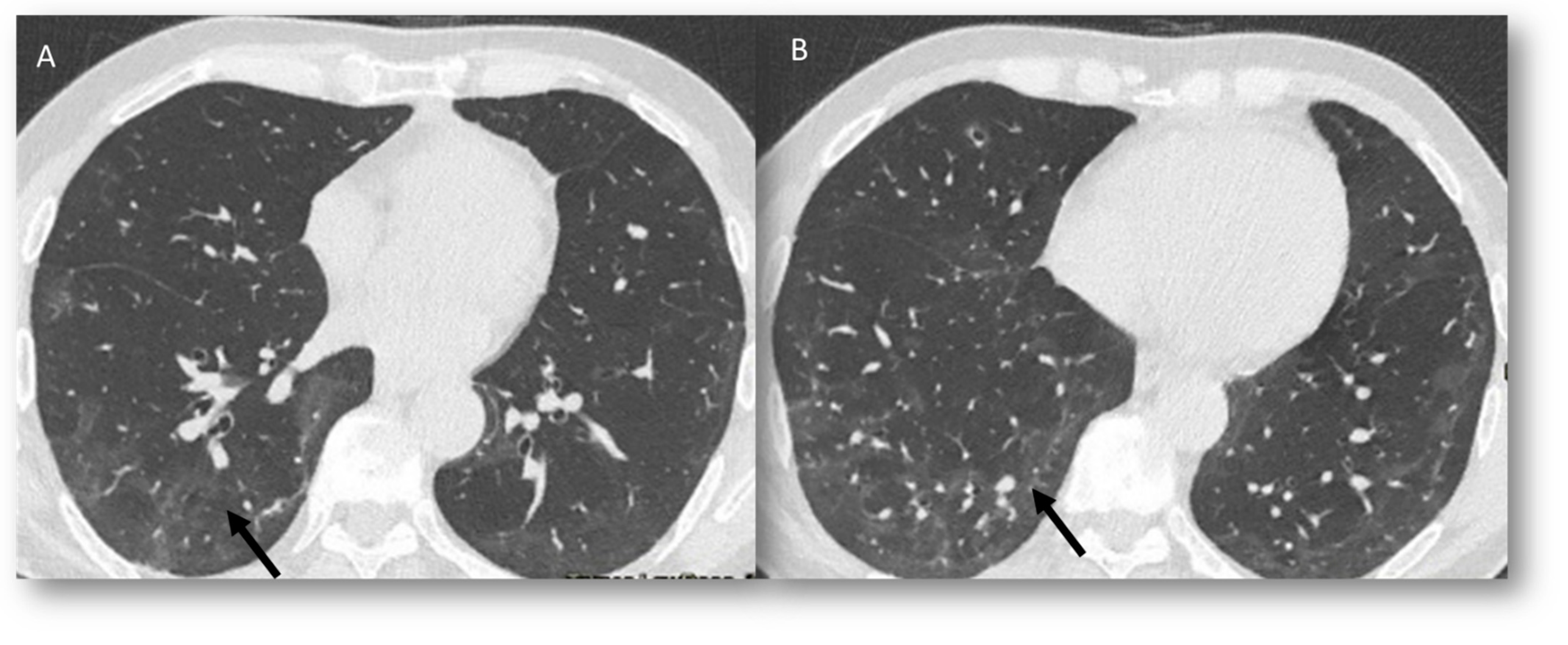
3.1. Radiological and Clinical Setting
Radiation Recall Pneumonia
3.2. Immune-Related Pneumonia
3.3. Radiation Pneumonia
3.4. COVID-19 Pneumonitis and COVID-19 Vaccine Radiation Recall
3.5. Progression Disease: Pulmonary Lymphangitis Carcinomatosa
3.6. Machine Learning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Fleming, C.; Rimner, A.; Foster, A.; Woo, K.M.; Zhang, Z.; Wu, A.J. Palliative efficacy and local control of conventional radiotherapy for lung metastases. Ann. Palliat. Med. 2017, 6 (Suppl. S1), S21–S27. [Google Scholar] [CrossRef]
- Ottaiano, A.; Petito, A.; Santorsola, M.; Gigantino, V.; Capuozzo, M.; Fontanella, D.; Di Franco, R.; Borzillo, V.; Buonopane, S.; Ravo, V.; et al. Prospective Evaluation of Radiotherapy-Induced Immunologic and Genetic Effects in Colorectal Cancer Oligo-Metastatic Patients with Lung-Limited Disease: The PRELUDE-1 Study. Cancers 2021, 13, 4236. [Google Scholar] [CrossRef]
- Cellini, F.; Di Franco, R.; Manfrida, S.; Borzillo, V.; Maranzano, E.; Pergolizzi, S.; Morganti, A.G.; Fusco, V.; Deodato, F.; Santarelli, M.; et al. Palliative radiotherapy indications during the COVID-19 pandemic and in future complex logistic settings: The NORMALITY model. Radiol. Med. 2021, 126, 1619–1656. [Google Scholar] [CrossRef]
- Merlotti, A.; Bruni, A.; Borghetti, P.; Ramella, S.; Scotti, V.; Trovò, M.; Chiari, R.; Lohr, F.; Ricardi, U.; Bria, E.; et al. Sequential chemo-hypofractionated RT versus concurrent standard CRT for locally advanced NSCLC: GRADE recommendation by the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Radiol. Med. 2021, 126, 1117–1128. [Google Scholar] [CrossRef]
- Ottaiano, A.; de Vera d’Aragona, R.P.; Trotta, A.M.; Santorsola, M.; Napolitano, M.; Scognamiglio, G.; Tatangelo, F.; Grieco, P.; Zappavigna, S.; Granata, V.; et al. Characterization of KRAS Mutational Regression in Oligometastatic Patients. Front. Immunol. 2022, 13, 898561. [Google Scholar] [CrossRef]
- Cozzolino, I.; Ronchi, A.; Messina, G.; Montella, M.; Morgillo, F.; Vicidomini, G.; Tirino, V.; Grimaldi, A.; Marino, F.Z.; Santini, M.; et al. Adequacy of Cytologic Samples by Ultrasound-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology of Peripheral Pulmonary Nodules for Morphologic Diagnosis and Molecular Evaluations: Comparison with Computed Tomography–Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology. Arch. Pathol. Lab. Med. 2020, 144, 361–369. [Google Scholar] [CrossRef]
- Meattini, I.; Palumbo, I.; Becherini, C.; Borghesi, S.; Cucciarelli, F.; Dicuonzo, S.; Fiorentino, A.; Spoto, R.; Poortmans, P.; Aristei, C.; et al. The Italian Association for Radiotherapy and Clinical Oncology (AIRO) position statements for postoperative breast cancer radiation therapy volume, dose, and fractionation. Radiol. Med. 2022, 127, 1407–1411. [Google Scholar] [CrossRef]
- Mega, S.; Fiore, M.; Carpenito, M.; Novembre, M.L.; Miele, M.; Trodella, L.E.; Grigioni, F.; Ippolito, E.; Ramella, S. Early GLS changes detection after chemoradiation in locally advanced non-small cell lung cancer (NSCLC). Radiol. Med. 2022, 127, 1355–1363. [Google Scholar] [CrossRef]
- Nardone, V.; Boldrini, L.; Salvestrini, V.; Greco, C.; Petrianni, G.M.; Desideri, I.; De Felice, F. Are you planning to be a radiation oncologist? A survey by the young group of the Italian Association of Radiotherapy and Clinical Oncology (yAIRO). Radiol. Med. 2022. [Google Scholar] [CrossRef]
- Montella, M.; Ciani, G.; Granata, V.; Fusco, R.; Grassi, F.; Ronchi, A.; Cozzolino, I.; Franco, R.; Zito Marino, F.; Urraro, F.; et al. Preliminary Experience of Liquid Biopsy in Lung Cancer Compared to Conventional Assessment: Light and Shadows. J. Pers. Med. 2022, 12, 1896. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Costa, M.; Picone, C.; Cozzi, D.; Moroni, C.; La Casella, G.V.; Montanino, A.; Monti, R.; Mazzoni, F.; et al. Preliminary Report on Computed Tomography Radiomics Features as Biomarkers to Immunotherapy Selection in Lung Adenocarcinoma Patients. Cancers 2021, 13, 3992. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Miele, V.; Larici, A.R.; Sverzellati, N.; Cappabianca, S.; Brunese, L.; Maggialetti, N.; Borghesi, A.; Fusco, R.; et al. Structured Reporting of Lung Cancer Staging: A Consensus Proposal. Diagnostics 2021, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Mazzei, M.A.; Meglio, N.D.; Roscio, D.D.; Moroni, C.; Monti, R.; Cappabianca, C.; Picone, C.; Neri, E.; et al. Quantitative imaging decision support (QIDSTM) tool consistency evaluation and radiomic analysis by means of 594 metrics in lung carcinoma on chest CT scan. Cancer Control 2021, 28, 1073274820985786. [Google Scholar] [CrossRef]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran WJJr Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Di Noia, V.; D’Aveni, A.; D’Argento, E.; Rossi, S.; Ghirardelli, P.; Bortolotti, L.; Vavassori, V.; Bria, E.; Ceresoli, G.L. Treating disease progression with osimertinib in EGFR-mutated non-small-cell lung cancer: Novel targeted agents and combination strategies. ESMO Open 2021, 6, 100280. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, Z.; Wang, X.; Ai, H.; Yang, C.; Luo, Y.; Jiang, X. Radiomics for prediction of response to EGFR-TKI based on metastasis/brain parenchyma (M/BP)-interface. Radiol. Med. 2022, 127, 1342–1354. [Google Scholar] [CrossRef]
- Vicini, S.; Bortolotto, C.; Rengo, M.; Ballerini, D.; Bellini, D.; Carbone, I.; Preda, L.; Laghi, A.; Coppola, F.; Faggioni, L. A narrative review on current imaging applications of artificial intelligence and radiomics in oncology: Focus on the three most common cancers. Radiol. Med. 2022, 127, 819–836. [Google Scholar] [CrossRef]
- Grasso, R.F.; Bernetti, C.; Pacella, G.; Altomare, C.; Castiello, G.; Andresciani, F.; Sarli, M.; Zobel, B.B.; Faiella, E. A comparative analysis of thermal ablation techniques in the treatment of primary and secondary lung tumors: A single-center experience. Radiol. Med. 2022, 127, 714–724. [Google Scholar] [CrossRef]
- Vinod, S.K.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25 (Suppl. S2), 61–71. [Google Scholar] [CrossRef] [PubMed]
- Macchia, G.; Casà, C.; Ferioli, M.; Lancellotta, V.; Pezzulla, D.; Pappalardi, B.; Laliscia, C.; Ippolito, E.; Di Muzio, J.; Huscher, A.; et al. Observational multicenter Italian study on vulvar cancer adjuvant radiotherapy (OLDLADY 1.2): A cooperation among AIRO Gyn, MITO and MaNGO groups. Radiol Med. 2022, 127, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Ganesh, T.; Munshi, A.; Manikandan, A.; Roy, S.; Krishnankutty, S.; Chitral, L.; Sathiya, J.; Pradhan, A.; Mohanti, B.K. Rotational positional error-corrected linear set-up margin calculation technique for lung stereotactic body radiotherapy in a dual imaging environment of 4-D cone beam CT and ExacTrac stereoscopic imaging. Radiol. Med. 2021, 126, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, Y.; Jingu, K.; Yamamoto, T.; Matsushita, H.; Umezawa, R.; Ishikawa, Y.; Takahashi, N.; Takeda, K.; Tasaka, S.; Kadoya, N. Differences in patterns of recurrence of squamous cell carcinoma and adenocarcinoma after radiotherapy for stage III non-small cell lung cancer. Jpn. J. Radiol. 2021, 39, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Iorio, G.C.; Salvestrini, V.; Borghetti, P.; De Felice, F.; Greco, C.; Nardone, V.; Fiorentino, A.; Gregucci, F.; Desideri, I. The impact of modern radiotherapy on radiation-induced late sequelae: Focus on early-stage mediastinal classical Hodgkin Lymphoma. A critical review by the Young Group of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Crit. Rev. Oncol. Hematol. 2021, 161, 103326. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, P.; Branz, J.; Volpi, G.; Pancera, S.; Buraschi, R.; Bianchi, L.N.C.; Bonù, M.L.; Greco, D.; Facheris, G.; Tomasi, C.; et al. Home-based pulmonary rehabilitation in patients undergoing (chemo)radiation therapy for unresectable lung cancer: A prospective explorative study. Radiol. Med. 2022, 127, 1322–1332. [Google Scholar] [CrossRef]
- Ho, M.C.; Chung, Y.S.; Lin, Y.C.; Hung, M.S.; Fang, Y.H. Combination Use of First-Line Afatinib and Proton-Pump Inhibitors Reduces Overall Survival Among Patients with EGFFR Mutant Lung Cancer. OncoTargets Ther. 2022, 15, 1573–1582. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Zhang, L.; Fang, W.; Zhang, H. Combination Treatment with Iodine 125 Seeds Implant and Systemic Therapy vs. Systemic Therapy Alone for Non-small Cell Lung Cancer: A Systematic Review and Meta-analysis. J. Coll. Physicians Surg. Pak. 2023, 33, 84–91. [Google Scholar] [CrossRef]
- Jung, H.A.; Park, S.; Lee, S.H.; Ahn, J.S.; Ahn, M.J.; Sun, J.M. The Role of Brain Radiotherapy Before First-Line Afatinib Therapy, Compared to Gefitinib or Erlotinib, in Patients with EGFR-Mutant Non-Small Cell Lung Cancer. Cancer Res. Treat. 2022. [Google Scholar] [CrossRef]
- Arrieta, O.; Rincón, D.; Garza, C.; Michel, R.; Astorga-Ramos, M.; Barrera, L.; la Garza, J. High Frequency of Radiation Pneumonitis in Patients with Locally Advanced Non-small Cell Lung Cancer Treated with Concurrent Radiotherapy and Gemcitabine after Induction with Gemcitabine and Carboplatin. J. Thorac. Oncol. 2009, 4, 845–852. [Google Scholar] [CrossRef]
- Gong, C.; Xiao, Q.; Li, Y.; Gu, Y.; Zhang, J.; Wang, L.; Cao, J.; Tao, Z.; Zhao, Y.; Xie, Y.; et al. Everolimus-Related Pneumonitis in Patients with Metastatic Breast Cancer: Incidence, Radiographic Patterns, and Relevance to Clinical Outcome. Oncologist 2020, 26, e580–e587. [Google Scholar] [CrossRef] [PubMed]
- Taboada, R.G.; Riechelmann, R.P.; Mauro, C.; Barros, M.; Hubner, R.A.; McNamara, M.G.; Lamarca, A.; Valle, J.W. Everolimus-Induced Pneumonitis in Patients with Neuroendocrine Neoplasms: Real-World Study on Risk Factors and Outcomes. Oncologist 2022, 27, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Simonetti, I.; Fusco, R.; Setola, S.V.; Izzo, F.; Scarpato, L.; Vanella, V.; Festino, L.; Simeone, E.; Ascierto, P.A.; et al. Management of cutaneous melanoma: Radiologists challenging and risk assessment. Radiol. Med. 2022, 127, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Giuranno, L.; Ient, J.; De Ruysscher, D.; Vooijs, M.A. Radiation-Induced Lung Injury (RILI). Front. Oncol. 2019, 9, 877. [Google Scholar] [CrossRef]
- Rahi, M.S.; Parekh, J.; Pednekar, P.; Parmar, G.; Abraham, S.; Nasir, S.; Subramaniyam, R.; Jeyashanmugaraja, G.P.; Gunasekaran, K. Radiation-Induced Lung Injury-Current Perspectives and Management. Clin. Pract. 2021, 11, 410–429. [Google Scholar] [CrossRef]
- United States Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) v5.0; NIH, National Institutes of Health: Bethesda, MD, USA, 2017. [Google Scholar]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Zito Marino, F.; Rossi, G.; Montella, M.; Botti, G.; De Cecio, R.; Morabito, A.; La Manna, C.; Ronchi, A.; Micheli, M.; Salatiello, G.; et al. Heterogeneity of PD-L1 Expression in Lung Mixed Adenocarcinomas and adenosquamous Carcinomas. Am. J. Surg. Pathol. 2020, 44, 378–386. [Google Scholar] [CrossRef]
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.T.; Hanania, N.A.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef]
- Schwarte, S.; Wagner, K.; Karstens, J.H. Radiation Recall Pneumonitis Induced by Gemcitabine. Strahlenther. Onkol. 2007, 183, 215–217. [Google Scholar] [CrossRef]
- Ding, X.; Ji, W.; Li, J.; Zhang, X.; Wang, L. Radiation recall pneumonitis induced by chemotherapy after thoracic radiotherapy for lung cancer. Radiat. Oncol. 2011, 6, 24. [Google Scholar] [CrossRef]
- Awad, R.; Nott, L. RRP induced by erlotinib after palliative thoracic radiotherapy for lung cancer: Case report and literature review. Asia Pac. J. Clin. Oncol. 2016, 12, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Borja, M.; Parrot, A.; Sroussi, D.; Rivin del Campo, E.; Fallet, V.; Cadranel, J. Dramatic RRP Induced by Osimertinib after Palliative Thoracic Radiotherapy for Lung Cancer. J. Thorac. Oncol. 2019, 14, e224–e226. [Google Scholar] [CrossRef] [PubMed]
- Riviere, P.; Sumner, W.; Cornell, M.; Sandhu, A.; Murphy, J.D.; Hattangadi-Gluth, J.; Bruggeman, A.; Kim, S.S.; Randall, J.M.; Sharabi, A.B. RRP After Treatment with Checkpoint Blockade Immunotherapy: A Case Series and Review of Literature. Front. Oncol. 2021, 11, 662954. [Google Scholar] [CrossRef]
- Barcellini, A.; Dusi, V.; Mirandola, A.; Ronchi, S.; Riva, G.; Dal Mas, F.; Massaro, M.; Vitolo, V.; Ciocca, M.; Rordorf, R.; et al. The impact of particle radiotherapy on the functioning of cardiac implantable electronic devices: A systematic review of in vitro and in vivo studies according to PICO criteria. Radiol. Med. 2022, 127, 1046–1058. [Google Scholar] [CrossRef]
- Lazzari, G.; Giua, R.; Verdolino, E.; Solazzo, A.P.; Benevento, I.; Montagna, A.; Castaldo, G.; Rago, L.; Silvano, G. Radiation Recall Pneumonitis COVID-19 Infection Induced After Adjuvant Breast Cancer Radiotherapy a Known Phenomenon in a Unknown Pandemic Disease, A Case Report. Chest 2022, 161, A129. [Google Scholar] [CrossRef]
- Shinada, K.; Murakami, S.; Yoshida, D.; Saito, H. RRP after COVID-19 vaccination. Thorac. Cancer 2022, 13, 144–145. [Google Scholar] [CrossRef] [PubMed]
- D’Angio, G.J.; Farber, S.; Maddock, C.L. Potentiation of x-ray effects by actinomycin D. Radiology 1959, 73, 175–177. [Google Scholar] [CrossRef]
- Voong, K.R.; Hazell, S.Z.; Fu, W.; Hu, C.; Lin, C.T.; Ding, K.; Suresh, K.; Hayman, J.; Hales, R.K.; Alfaifi, S.; et al. Relationship Between Prior Radiotherapy and Checkpoint-Inhibitor Pneumonitis in Patients with Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2019, 20, e470–e479. [Google Scholar] [CrossRef]
- Extermann, M.; Vogt, N.; Forni, M.; Dayer, P. Radiation recall in a patient with breast cancer treated for tuberculosis. Eur. J. Clin. Pharmacol. 1995, 48, 77–78. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Petrillo, A. Introduction to Special Issue of Radiology and Imaging of Cancer. Cancers 2020, 12, 2665. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Galdiero, R.; Setola, S.V.; Palaia, R.; Belli, A.; Silvestro, L.; Cozzi, D.; Brunese, L.; et al. Pancreatic cancer detection and characterization: State of the art and radiomics. Eur. Rev. Med. Pharm. Sci. 2021, 25, 3684–3699. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Castelguidone, E.L.D.; Camera, L.; Tafuto, S.; Avallone, A.; Belli, A.; Incollingo, P.; Palaia, R.; et al. The multidisciplinary team for gastroenteropancreatic neuroendocrine tumours: The radiologist’s challenge. Radiol. Oncol. 2019, 53, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Piccirillo, M.; Pradella, S.; Giordano, M.; Cappabianca, S.; Brunese, L.; et al. Abbreviated MRI Protocol for the Assessment of Ablated Area in HCC Patients. Int. J. Environ. Res. Public Health 2021, 18, 3598. [Google Scholar] [CrossRef] [PubMed]
- Sansone, M.; Marrone, S.; Di Salvio, G.; Belfiore, M.P.; Gatta, G.; Fusco, R.; Vanore, L.; Zuiani, C.; Grassi, F.; Vietri, M.T.; et al. Comparison between two packages for pectoral muscle removal on mammographic images. Radiol. Med. 2022, 127, 848–856. [Google Scholar] [CrossRef]
- Mirabile, A.; Lucarelli, N.M.; Sollazzo, E.P.; Stabile Ianora, A.A.; Sardaro, A.; Mirabile, G.; Lorusso, F.; Racanelli, V.; Maggialetti, N.; Scardapane, A. CT pulmonary angiography appropriateness in a single emergency department: Does the use of re-vised Geneva score matter? Radiol. Med. 2021, 126, 1544–1552. [Google Scholar] [CrossRef]
- Karaboue, M.A.A.; Ferrara, M.; Bertozzi, G.; Berritto, D.; Volonnino, G.; La Russa, R.; Lacasella, G.V. To vaccinate or not: Literacy against hesitancy: Vaccination hesitancy. Med. Hist. 2022, 6, e2022014. [Google Scholar]
- Karaboue, M.A.A.; Milone, V.; La Casella, G.V.; Ferrara, M.; Delogu, G.; Di Fazio, N.; Volonnino, G. What will our children do when we are gone? Italian legislature does not tackle the worries of parents of disabled children. Reflections on disability. Med. Hist. 2022, 6, e2022013. [Google Scholar]
- De Luca, L.; Veneziano, F.A.; Karaboue, M. Late Presenters with ST-Elevation Myocardial Infarction: A Call to Action. J. Clin. Med. 2022, 11, 5169. [Google Scholar] [CrossRef]
- Giaconi, C.; Manetti, A.C.; Turco, S.; Coppola, M.; Forni, D.; Marra, D.; La Russa, R.; Karaboue, M.; Maiese, A.; Papi, L.; et al. Post-mortem computer tomography in ten cases of death while diving: A retrospective evaluation. Radiol. Med. 2022, 127, 318–329. [Google Scholar] [CrossRef]
- Raspini, M.; Cavalcanti, R.; Clementini, M.; Karaboue, M.; Sforza, N.M.; Cairo, F. Periodontitis and italians (2016-2020): Need for clinical guidelines to perform effective therapy. Dent. Cadmos 2021, 89, 346–356. [Google Scholar] [CrossRef]
- Bernetti, A.; La Russa, R.; de Sire, A.; Agostini, F.; De Simone, S.; Farì, G.; Lacasella, G.V.; Santilli, G.; De Trane, S.; Karaboue, M.; et al. Cervical Spine Manipulations: Role of Diagnostic Procedures, Effectiveness, and Safety from a Rehabilitation and Forensic Medicine Perspective: A Systematic Review. Diagnostics 2022, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Iannetti, G.; Miele, V.; Grassi, R.; Karaboue, M.; Cesarano, E.; Vimercati, F.; Calliada, F. Addendum to the sonographic medical act. J. Ultrasound 2021, 24, 229–230. [Google Scholar] [CrossRef]
- Fiorini, F.; Granata, A.; Battaglia, Y.; Karaboue, M.A.A. Talking about medicine through mass media. G. Ital. Nefrol. Organo Uff. Soc. Ital. Nefrol. 2019, 36. [Google Scholar]
- Sansone, M.; Grassi, R.; Belfiore, M.P.; Gatta, G.; Grassi, F.; Pinto, F.; La Casella, G.V.; Fusco, R.; Cappabianca, S.; Granata, V.; et al. Radiomic features of breast parenchyma: Assessing differences between FOR PROCESSING and FOR PRESENTATION digital mammography. Insights Imaging 2021, 12, 147. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Simonetti, I.; Dell’Aversana, F.; Grassi, F.; Bruno, F.; Belli, A.; et al. Complications Risk Assessment and Imaging Findings of Thermal Ablation Treatment in Liver Cancers: What the Radiologist Should Expect. J. Clin. Med. 2022, 11, 2766. [Google Scholar] [CrossRef]
- De Muzio, F.; Cutolo, C.; Dell’Aversana, F.; Grassi, F.; Ravo, L.; Ferrante, M.; Danti, G.; Flammia, F.; Simonetti, I.; Palumbo, P.; et al. Complications after Thermal Ablation of Hepatocellular Carcinoma and Liver Metastases: Imaging Findings. Diagnostics 2022, 12, 1151. [Google Scholar] [CrossRef]
- Acanfora, C.; Grassi, E.; Giacobbe, G.; Ferrante, M.; Granata, V.; Barile, A.; Cappabianca, S. Post-Procedural Follow-Up of the Interventional Radiology’s Management of Osteoid Osteomas and Osteoblastomas. J. Clin. Med. 2022, 11, 1987. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Belli, A.; Palaia, R.; Carrafiello, G.; Miele, V.; Grassi, R.; Petrillo, A.; Izzo, F. Local ablation of pancreatic tumors: State of the art and future perspectives. World J. Gastroenterol. 2021, 27, 3413–3428. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Palaia, R.; Belli, A.; Miele, V.; Brunese, L.; Grassi, R.; Petrillo, A.; et al. Assessment of Ablation Therapy in Pancreatic Cancer: The Radiologist’s Challenge. Front. Oncol. 2020, 10, 560952. [Google Scholar] [CrossRef]
- De Robertis, R.; Geraci, L.; Tomaiuolo, L.; Bortoli, L.; Beleù, A.; Malleo, G.; D’Onofrio, M. Liver metastases in pancreatic ductal adenocarcinoma: A predictive model based on CT texture analysis. Radiol Med. 2022, 127, 1079–1084. [Google Scholar] [CrossRef]
- Granata, V.; Faggioni, L.; Grassi, R.; Fusco, R.; Reginelli, A.; Rega, D.; Maggialetti, N.; Buccicardi, D.; Frittoli, B.; Rengo, M.; et al. Structured reporting of computed tomography in the staging of colon cancer: A Delphi consensus proposal. Radiol. Med. 2022, 127, 21–29. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Grassi, F.; Belli, A.; Silvestro, L.; Ottaiano, A.; et al. Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of liver mucinous colorectal metastases. Radiol. Med. 2022, 127, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Neri, E.; Granata, V.; Montemezzi, S.; Belli, P.; Bernardi, D.; Brancato, B.; Caumo, F.; Calabrese, M.; Coppola, F.; Cossu, E.; et al. Structured reporting of x-ray mammography in the first diagnosis of breast cancer: A Delphi consensus proposal. Radiol. Med. 2022, 127, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Simonetti, I.; Picone, C.; Simeone, E.; Festino, L.; Vanella, V.; Vitale, M.G.; Montanino, A.; et al. Immunotherapy Assessment: A New Paradigm for Radiologists. Diagnostics 2023, 13, 302. [Google Scholar] [CrossRef]
- Yuan, J.; Gnjatic, S.; Li, H.; Powel, S.; Gallardo, H.F.; Ritter, E.; Ku, G.Y.; Jungbluth, A.A.; Segal, N.H.; Rasalan, T.S.; et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc. Natl. Acad. Sci. USA 2008, 105, 20410–20415. [Google Scholar] [CrossRef]
- Khunger, M.; Rakshit, S.; Pasupuleti, V.; Hernandez, A.V.; Mazzone, P.; Stevenson, J.; Pennell, N.A.; Velcheti, V. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017, 152, 271–281. [Google Scholar] [CrossRef]
- Cappabianca, S.; Granata, V.; Di Grezia, G.; Mandato, Y.; Reginelli, A.; Di Mizio, V.; Grassi, R.; Rotondo, A. The role of nasoenteric intubation in the MR study of patients with Crohn’s disease: Our experience and literature review. Radiol. Med. 2011, 116, 389–406. [Google Scholar] [CrossRef]
- Somma, F.; Negro, A.; D’Agostino, V.; Piscitelli, V.; Pace, G.; Tortora, M.; Tortora, F.; Gatta, G.; Caranci, F. COVID-19 and low back pain: Previous infections lengthen recovery time after intradiscal ozone therapy in patients with herniated lumbar disc. Radiol Med. 2022, 127, 673–680. [Google Scholar] [CrossRef]
- Gerasia, R.; Mamone, G.; Amato, S.; Cucchiara, A.; Gallo, G.S.; Tafaro, C.; Fiorello, G.; Caruso, C.; Miraglia, R. COVID-19 safety measures at the Radiology Unit of a Transplant Institute: The non-COVID-19 patient’s confidence with safety procedures. Radiol. Med. 2022, 127, 426–432. [Google Scholar] [CrossRef]
- Caliandro, M.; Fabiana, G.; Surgo, A.; Carbonara, R.; Ciliberti, M.P.; Bonaparte, I.; Caputo, S.; Fiorentino, A. Impact on mental health of the COVID-19 pandemic in a radiation oncology department. Radiol Med. 2022, 127, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Lancellotta, V.; Fanetti, G.; Monari, F.; Mangoni, M.; Mazzarotto, R.; Tagliaferri, L.; Gobitti, C.; Lodi, R.E.; Talomo, S.; Turturici, I.; et al. Stereotactic radiotherapy (SRT) for differentiated thyroid cancer (DTC) oligometastases: An AIRO (Italian association of radiotherapy and clinical oncology) systematic review. Radiol. Med. 2022, 127, 681–689. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.J.; Foster, R. Radiation recall reactions: An oncologic enigma. Crit. Rev. Oncol. 2021, 168, 103527. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Catalano, O.; Avallone, A.; Palaia, R.; Botti, G.; Tatangelo, F.; Granata, F.; Cascella, M.; Izzo, F.; et al. Diagnostic accuracy of magnetic resonance, computed tomography and contrast enhanced ultrasound in radiological multimodality assessment of peribiliary liver metastases. PLoS ONE 2017, 12, e0179951. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Catalano, O.; Avallone, A.; Leongito, M.; Izzo, F.; Petrillo, A. Peribiliary liver metastases MR findings. Med. Oncol. 2017, 34, 124. [Google Scholar] [CrossRef]
- Li, N.; Wakim, J.; Koethe, Y.; Huber, T.; Schenning, R.; Gade, T.P.; Hunt, S.J.; Park, B.J. Multicenter assessment of augmented reality registration methods for image-guided interventions. Radiol. Med. 2022, 127, 857–865. [Google Scholar] [CrossRef]
- Caruso, D.; Polici, M.; Rinzivillo, M.; Zerunian, M.; Nacci, I.; Marasco, M.; Magi, L.; Tarallo, M.; Gargiulo, S.; Iannicelli, E.; et al. CT-based radiomics for prediction of therapeutic response to Everolimus in metastatic neuroendocrine tumors. Radiol. Med. 2022, 127, 691–701. [Google Scholar] [CrossRef]
- Granata, V.; Ianniello, S.; Fusco, R.; Urraro, F.; Pupo., D.; Magliocchetti, S.; Albarello, F.; Campioni, P.; Cristofaro, M.; Di Stefano, F.; et al. Quantitative Analysis of Residual COVID-19 Lung CT Features: Consistency among Two Commercial Software. J. Pers. Med. 2021, 11, 1103. [Google Scholar] [CrossRef]
- Grassi, R.; Belfiore, M.P.; Montanelli, A.; Patelli, G.; Urraro, F.; Giacobbe, G.; Fusco, R.; Granata, V.; Petrillo, A.; Sacco, P.; et al. COVID-19 pneumonia: Computer-aided quantification of healthy lung parenchyma, emphysema, ground glass and consolidation on chest computed tomography (CT). Radiol. Med. 2021, 126, 553–560. [Google Scholar] [CrossRef]
- Fusco, R.; Simonetti, I.; Ianniello, S.; Villanacci, A.; Grassi, F.; Dell’Aversana, F.; Grassi, R.; Cozzi, D.; Bicci, E.; Palumbo, P.; et al. Pulmonary Lymphangitis Poses a Major Challenge for Radiologists in an Oncological Setting during the COVID-19 Pandemic. J. Pers. Med. 2022, 12, 624. [Google Scholar] [CrossRef]
- Dalpiaz, G.; Gamberini, L.; Carnevale, A.; Spadaro, S.; Mazzoli, C.A.; Piciucchi, S.; Allegri, D.; Capozzi, C.; Neziri, E.; Bartolucci, M.; et al. Clinical implications of microvascular CT scan signs in COVID-19 patients requiring invasive mechanical ventilation. Radiol. Med. 2022, 127, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Grassi, R.; Feragalli, B.; Belfiore, M.P.; Montanelli, A.; Patelli, G.; La Porta, M.; Urraro, F.; Fusco, R.; Granata, V.; et al. Coronavirus Disease 2019 (COVID-19) in Italy: Double Reading of Chest CT Examination. Biology 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Grassi, R.; Fusco, R.; Belfiore, M.P.; Montanelli, A.; Patelli, G.; Urraro, F.; Petrillo, A.; Granata, V.; Sacco, P.; Mazzei, M.A.; et al. Coronavirus disease 2019 (COVID-19) in Italy: Features on chest computed tomography using a structured report system. Sci. Rep. 2020, 10, 17236. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Villanacci, A.; Magliocchetti, S.; Urraro, F.; Tetaj, N.; Marchioni, L.; Albarello, F.; Campioni, P.; Cristofaro, M.; et al. Imaging Severity COVID-19 Assessment in Vaccinated and Unvaccinated Patients: Comparison of the Different Variants in a High Volume Italian Reference Center. J. Pers. Med. 2022, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Scotti, G.M.; Ippolito, D.; Morelli, M.J.; Vignale, D.; Gandola, D.; Sironi, S.; De Cobelli, F.; Ferrante, L.; Spessot, M.; et al. Chest CT in the emergency department for suspected COVID-19 pneumonia. Radiol. Med. 2021, 126, 498–502. [Google Scholar] [CrossRef]
- Bronstein, Y.; Ng, C.S.; Hwu, P.; Hwu, W.J. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am. J. Roentgenol. 2011, 197, W992–W1000. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. COVID-19, intradiscal ozone therapy and back pain: A correspondence. Radiol Med. 2022, 127, 1179. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Gaibazzi, N.; Tuttolomondo, D.; Fusco, S.; La Mura, V.; Peyvandi, F.; Aliberti, S.; Blasi, F.; Cozzi, D.; Carrafiello, G.; et al. Deep vein thrombosis in COVID-19 patients in general wards: Prevalence and association with clinical and laboratory variables. Radiol. Med. 2021, 126, 722–728. [Google Scholar] [CrossRef]
- Cardobi, N.; Benetti, G.; Cardano, G.; Arena, C.; Micheletto, C.; Cavedon, C.; Montemezzi, S. CT radiomic models to distinguish COVID-19 pneumonia from other interstitial pneumonias. Radiol. Med. 2021, 126, 1037–1043. [Google Scholar] [CrossRef]
- Özel, M.; Aslan, A.; Araç, S. Use of the COVID-19 Reporting and Data System (CO-RADS) classification and chest computed tomography involvement score (CT-IS) in COVID-19 pneumonia. Radiol. Med. 2021, 126, 679–687. [Google Scholar] [CrossRef]
- Masselli, G.; Almberger, M.; Tortora, A.; Capoccia, L.; Dolciami, M.; D’Aprile, M.R.; Valentini, C.; Avventurieri, G.; Bracci, S.; Ricci, P. Role of CT angiography in detecting acute pulmonary embolism associated with COVID-19 pneumonia. Radiol. Med. 2021, 126, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.S.; Lin, K.T. A meta-analysis of the diagnostic test accuracy of CT-based radiomics for the prediction of COVID-19 severity. Radiol. Med. 2022, 127, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Cereser, L.; Girometti, R.; Da Re, J.; Marchesini, F.; Como, G.; Zuiani, C. Inter-reader agreement of high-resolution computed tomography findings in patients with COVID-19 pneumonia: A multi-reader study. Radiol. Med. 2021, 126, 577–584. [Google Scholar] [CrossRef]
- Shaw, B.; Daskareh, M.; Gholamrezanezhad, A. The lingering manifestations of COVID-19 during and after convalescence: Update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19). Radiol. Med. 2021, 126, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Polici, M.; Zerunian, M.; Pucciarelli, F.; Polidori, T.; Guido, G.; Rucci, C.; Bracci, B.; Muscogiuri, E.; De Dominicis, C.; et al. Quantitative Chest CT analysis in discriminating COVID-19 from non-COVID-19 patients. Radiol. Med. 2021, 126, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Galdiero, R.; Picone, C.; Izzo, F.; D’Aniello, R.; Miele, V.; Grassi, R.; Grassi, R.; et al. Lymphadenopathy after BNT162b2 Covid-19 Vaccine: Preliminary Ultrasound Findings. Biology 2021, 10, 214. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Vallone, P.; Setola, S.V.; Picone, C.; Grassi, F.; Patrone, R.; Belli, A.; Izzo, F.; Petrillo, A. Not only lymphadenopathy: Case of chest lymphangitis assessed with MRI after COVID 19 vaccine. Infect. Agent Cancer 2022, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Li, M.; Yu, J. Radiation recall pneumonitis induced by PD-1/ PD-L1 blockades: Mechanisms and therapeutic implications. BMC Med. 2020, 18, 275. [Google Scholar] [CrossRef]
- Kalisz, K.R.; Ramaiya, N.H.; Laukamp, K.R.; Gupta, A. Immune Checkpoint Inhibitor Therapy-related Pneumonitis: Patterns and Management. Radiographics 2019, 39, 1923–1937. [Google Scholar] [CrossRef]
- Cousin, F.; Desir, C.; Ben Mustapha, S.; Mievis, C.; Coucke, P.; Hustinx, R. Incidence, risk factors, and CT characteristics of radiation recall pneumonitis induced by immune checkpoint inhibitor in lung cancer. Radiother. Oncol. 2021, 157, 47–55. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Zhang, T.; Zhou, Z.; Deng, L.; Wang, X.; Wang, W.; Liu, W.; Tang, W.; Wang, Z.; et al. Comprehensive Pneumonitis Profile of Thoracic Radiotherapy Followed by Immune Checkpoint Inhibitor and Risk Factors for Radiation Recall Pneumonitis in Lung Cancer. Front. Immunol. 2022, 13, 918787. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connel, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, M.; Cadranel, J.; Lusque, A.; Meyer, N.; Gounant, V.; Moro-Sibilot, D.; Michot, J.M.; Raimbourg, J.; Girard, N.; Guisier, F.; et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur. Respir. J. 2017, 50, 1700050. [Google Scholar] [CrossRef] [PubMed]
- Fragkou, P.; Souli, M.; Theochari, M.; Kontopoulou, C.; Loukides, S.; Koumarianou, A. A Case of Organizing Pneumonia (OP) Associated with Pembrolizumab. Drug Target Insights 2016, 10, 9–12. [Google Scholar] [CrossRef]
- Nishino, M.; Hatabu, H.; Hodi, F.S.; Ramaiya, N.H. Drug-Related Pneumonitis in the Era of Precision Cancer Therapy. JCO Precis. Oncol. 2017, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.C.; Berkowitz, E.A. Lung CT: Part 2—The interstitial pneumonias: Clinical, histologic, and CT manifestations. AJR Am. J. Roentgenol. 2012, 199, W464–W476. [Google Scholar] [CrossRef]
- Defraene, G.; Van Elmpt, W.; Crijns, W.; Slagmolen, P.; De Ruysscher, D. CT characteristics allow identification of patient-specific susceptibility for radiation-induced lung damage. Radiother. Oncol. 2015, 117, 29–35. [Google Scholar] [CrossRef]
- Petit, S.F.; Van Elmpt, W.J.C.; Oberije, C.J.G.; Vegt, E.; Dingemans, A.M.C.; Lambin, P.; Dekker, A.L.A.J.; De Ruysscher, D. [18F] fluorodeoxyglucose uptake patterns in lung before radiotherapy identify areas more susceptible to radiation-induced lung toxicity in non-small-cell lung cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 698–705. [Google Scholar] [CrossRef]
- Medhora, M.; Haworth, S.; Liu, Y.; Narayanan, J.; Gao, F.; Zhao, M.; Audi, S.; Jacobs, E.R.; Fish, B.L.; Clough, A.V. Biomarkers for Radiation Pneumonitis Using Noninvasive Molecular Imaging. J. Nucl. Med. 2016, 57, 1296–1301. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Nishino, M.; Severgnini, M.; Manos, M.; Mak, R.H.; Hodi, F.S. Pneumonitis resulting from radiation and immune checkpoint blockade illustrates characteristic clinical, radiologic and circu- lating biomarker features. J. Immunother. Cancer 2019, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Pan, Y.; Huang, W.; Huang, K.; Cui, Y.; Hong, W.; Wang, L.; Ni, D.; Tan, P. Differentiation between immune checkpoint inhibitor-related and radiation pneumonitis in lung cancer by CT radiomics and machine learning. Med. Phys. 2022, 49, 1547–1558. [Google Scholar] [CrossRef]
- Available online: https://covid19.who.int (accessed on 23 January 2023).
- Neri, E.; Miele, V.; Coppola, F.; Grassi, R. Use of CT and artificial intelligence in suspected or COVID-19 positive patients: Statement of the Italian Society of Medical and Interventional Radiology. Radiol. Med. 2020, 125, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Grassi, R.; Granata, V.; Setola, S.V.; Grassi, F.; Cozzi, D.; Pecori, B.; Izzo, F.; Petrillo, A. Artificial Intelligence and COVID-19 Using Chest CT Scan and Chest X-ray Images: Machine Learning and Deep Learning Approaches for Diagnosis and Treatment. J. Pers. Med. 2021, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Francolini, G.; Desideri, I.; Stocchi, G.; Ciccone, L.P.; Salvestrini, V.; Garlatti, P.; Aquilano, M.; Greto, D.; Bonomo, P.; Meattini, I.; et al. Impact of COVID-19 on workload burden of a complex radiotherapy facility. Radiol. Med. 2021, 126, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Palásti, P.; Veréb, D.; Bozsik, B.; Palkó, A.; Kincses, Z.T. The sensitivity and specificity of chest CT in the diagnosis of COVID-19. Eur. Radiol. 2021, 31, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Gabelloni, M.; Faggioni, L.; Cioni, D.; Mendola, V.; Falaschi, Z.; Coppola, S.; Corradi, F.; Isirdi, A.; Brandi, N.; Coppola, F.; et al. Extracorporeal membrane oxygenation (ECMO) in COVID-19 patients: A pocket guide for radiologists. Radiol. Med. 2022, 127, 369–382. [Google Scholar] [CrossRef]
- Masci, G.M.; Iafrate, F.; Ciccarelli, F.; Pambianchi, G.; Panebianco, V.; Pasculli, P.; Ciardi, M.R.; Mastroianni, C.M.; Ricci, P.; Catalano, C.; et al. Tocilizumab effects in COVID-19 pneumonia: Role of CT texture analysis in quantitative assessment of response to therapy. Radiol. Med. 2021, 126, 1170–1180. [Google Scholar] [CrossRef]
- Bianchi, A.; Mazzoni, L.N.; Busoni, S.; Pinna, N.; Albanesi, M.; Cavigli, E.; Cozzi, D.; Poggesi, A.; Miele, V.; Fainardi, E.; et al. Assessment of cerebrovascular disease with computed tomography in COVID-19 patients: Correlation of a novel specific visual score with increased mortality risk. Radiol. Med. 2021, 126, 570–576. [Google Scholar] [CrossRef]
- De Felice, F.; D’Angelo, E.; Ingargiola, R.; Iacovelli, N.A.; Alterio, D.; Franco, P.; Bonomo, P.; Merlotti, A.; Bacigalupo, A.; Maddalo, M.; et al. A snapshot on radiotherapy for head and neck cancer patients during the COVID-19 pandemic: A survey of the Italian Association of Radiotherapy and Clinical Oncology (AIRO) head and neck working group. Radiol. Med. 2021, 126, 343–347. [Google Scholar] [CrossRef]
- Pecoraro, M.; Cipollari, S.; Marchitelli, L.; Messina, E.; Del Monte, M.; Galea, N.; Ciardi, M.R.; Francone, M.; Catalano, C.; Panebianco, V. Cross-sectional analysis of follow-up chest MRI and chest CT scans in patients previously affected by COVID-19. Radiol. Med. 2021, 126, 1273–1281. [Google Scholar] [CrossRef]
- Agostini, A.; Borgheresi, A.; Carotti, M.; Ottaviani, L.; Badaloni, M.; Floridi, C.; Giovagnoni, A. Third-generation iterative reconstruction on a dual-source, high-pitch, low-dose chest CT protocol with tin filter for spectral shaping at 100 kV: A study on a small series of COVID-19 patients. Radiol. Med. 2021, 126, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Guido, G.; Polidori, T.; Rucci, C.; Bracci, B.; Tremamunno, G.; Laghi, A. Diagnostic performance of CT lung severity score and quantitative chest CT for stratification of COVID-19 patients. Radiol. Med. 2022, 127, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Novelli, F.; Pinelli, V.; Chiaffi, L.; Carletti, A.M.; Sivori, M.; Giannoni, U.; Chiesa, F.; Celi, A. Prognostic significance of peripheral consolidations at chest x-ray in severe COVID-19 pneumonia. Radiol. Med. 2022, 127, 602–608. [Google Scholar] [CrossRef]
- Borghesi, A.; Golemi, S.; Scrimieri, A.; Nicosia, C.M.C.; Zigliani, A.; Farina, D.; Maroldi, R. Chest X-ray versus chest computed tomography for outcome prediction in hospitalized patients with COVID-19. Radiol. Med. 2022, 127, 305–308. [Google Scholar] [CrossRef]
- Cartocci, G.; Colaiacomo, M.C.; Lanciotti, S.; Andreoli, C.; De Cicco, M.L.; Brachetti, G.; Pugliese, S.; Capoccia, L.; Tortora, A.; Scala, A.; et al. Correction to: Chest CT for early detection and management of coronavirus disease (COVID-19): A report of 314 patients admitted to Emergency Department with suspected pneumonia. Radiol. Med. 2021, 126, 642. [Google Scholar] [CrossRef] [PubMed]
- Cappabianca, S.; Fusco, R.; de Lisio, A.; Paura, C.; Clemente, A.; Gagliardi, G.; Lombardi, G.; Giacobbe, G.; Russo, G.M.; Belfiore, M.P.; et al. Correction to: Clinical and laboratory data, radiological structured report findings and quantitative evaluation of lung involvement on baseline chest CT in COVID-19 patients to predict prognosis. Radiol. Med. 2021, 126, 643. [Google Scholar] [CrossRef]
- Salman, R.; Sammer, M.B.; Serrallach, B.L.; Sangi-Haghpeykar, H.; Annapragada, A.V.; Paul Guillerman, R. Lower skeletal muscle mass on CT body composition analysis is associated with adverse clinical course and outcome in children with COVID-19. Radiol. Med. 2022, 127, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Barra, S.; Guarnieri, A.; di Monale, E.; Bastia, M.B.; Marcenaro, M.; Tornari, E.; Belgioia, L.; Magrini, S.M.; Ricardi, U.; Corvò, R. Short fractionation radiotherapy for early prostate cancer in the time of COVID-19: Long-term excellent outcomes from a multicenter Italian trial suggest a larger adoption in clinical practice. Radiol. Med. 2021, 126, 142–146. [Google Scholar] [CrossRef]
- Palmisano, A.; Vignale, D.; Boccia, E.; Nonis, A.; Gnasso, C.; Leone, R.; Montagna, M.; Nicoletti, V.; Bianchi, A.G.; Brusamolino, S.; et al. AI-SCoRE (artificial intelligence-SARS CoV2 risk evaluation): A fast, objective and fully automated platform to predict the outcome in COVID-19 patients. Radiol. Med. 2022, 127, 960–972. [Google Scholar] [CrossRef]
- Filograna, L.; Manenti, G.; Ampanozi, G.; Calcagni, A.; Ryan, C.P.; Floris, R.; Thali, M.J. Potentials of post-mortem CT investigations during SARS-COV-2 pandemic: A narrative review. Radiol. Med. 2022, 127, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Rawashdeh, M.A.; Saade, C. Radiation dose reduction considerations and imaging patterns of ground glass opacities in coronavirus: Risk of over exposure in computed tomography. Radiol. Med. 2021, 126, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, E.; Manganaro, L.; Guiducci, E.; Ciaglia, S.; Dolciami, M.; Spagnoli, A.; Alessandri, F.; Angeloni, A.; Vestri, A.; Catalano, C.; et al. Association of serum Krebs von den Lungen-6 and chest CT as potential prognostic factors in severe acute respiratory syndrome SARS-CoV-2: A preliminary experience. Radiol. Med. 2022, 127, 725–732. [Google Scholar] [CrossRef]
- Rizzo, S.; Catanese, C.; Puligheddu, C.; Epistolio, S.; Ramelli, G.; Frattini, M.; Pereira Mestre, R.; Nadarajah, N.; Rezzonico, E.; Magoga, F.; et al. CT evaluation of lung infiltrates in the two months preceding the Coronavirus disease 19 pandemic in Canton Ticino (Switzerland): Were there suspicious cases before the official first case? Radiol. Med. 2022, 127, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Giandola, T.; Maino, C.; Pecorelli, A.; Capodaglio, C.; Ragusi, M.; Porta, M.; Gandola, D.; Masetto, A.; Drago, S.; et al. Acute pulmonary embolism in hospitalized patients with SARS-CoV-2-related pneumonia: Multicentric experience from Italian endemic area. Radiol. Med. 2021, 126, 669–678. [Google Scholar] [CrossRef]
- Moroni, C.; Cozzi, D.; Albanesi, M.; Cavigli, E.; Bindi, A.; Luvarà, S.; Busoni, S.; Mazzoni, L.N.; Grifoni, S.; Nazerian, P.; et al. Chest X-ray in the emergency department during COVID-19 pandemic descending phase in Italy: Correlation with patients’ outcome. Radiol Med. 2021, 126, 661–668. [Google Scholar] [CrossRef]
- Nicosia, L.; Mazzola, R.; Vitale, C.; Cuccia, F.; Figlia, V.; Giaj-Levra, N.; Ricchetti, F.; Rigo, M.; Ruggeri, R.; Cavalleri, S.; et al. Postoperative moderately hypofractionated radiotherapy in prostate cancer: A mono-institutional propensity-score-matching analysis between adjuvant and early-salvage radiotherapy. Radiol. Med. 2022, 127, 560–570. [Google Scholar] [CrossRef]
- Sugimoto, H.; Sugimoto, K.; Inoue, H.; Tanaka, R.; Nakata, K.; Okino, T.; Kinoshita, Y.; Kajimoto, K. Pulmonary lymphangitic carcinomatosis secondary to ureteral cancer. Respir. Med. Case Rep. 2021, 32, 101348. [Google Scholar] [CrossRef]
- Klimek, M. Pulmonary lymphangitis carcinomatosis: Systematic review and meta-analysis of case reports, 1970–2018. Postgrad. Med. 2019, 131, 309–318. [Google Scholar] [CrossRef]
- Leone, A.; Criscuolo, M.; Gullì, C.; Petrosino, A.; Bianco, N.C.; Colosimo, C. Systemic mastocytosis revisited with an emphasis on skeletal manifestations. Radiol. Med. 2021, 126, 585–598. [Google Scholar] [CrossRef]
- Chalayer, E.; Tavernier-Tardy, E.; Clavreul, G.; Bay, J.-O.; Cornillon, J. Carcinomatosis lymphangitis and pleurisy after allo-SCT in two patients with secondary leukemia after breast cancer. Bone Marrow Transplant. 2012, 47, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Quigley, D.; Donnell, R.O.; McDonnell, C. Pulmonary lymphangitis sarcomatosis: A rare cause of severe progressive dyspnoea. BMJ Case Rep. 2022, 15, e246128. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.D.S.; Bonamigo, R.R.; Viapiana, G.L.; Cartell, A. Signet ring cells in carcinomatous lymphangitis due to gastric adenocarcinoma. An. Bras. Dermatol. 2020, 95, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Zieda, A.; Sbardella, S.; Patel, M.; Smith, R. Diagnostic Bias in the COVID-19 Pandemic: A Series of Short Cases. Eur. J. Case Rep. Intern. Med. 2021, 8, 2575. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, A.; Móré, D.; Erdmann, S.; Kintzelé, L.; Fischer, R.M.; Vogel, M.N.; Mangold, D.L.; von Stackelberg, O.; Schnitzler, P.; Zim-mermann, S.; et al. COVID-19 pneumonia and its lookalikes: How radiologists perform in differentiating atypical pneumonias. Eur. J. Radiol. 2021, 144, 110002. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Sverzellati, N.; Polverosi, R.; Balbi, M.; Baratella, E.; Busso, M.; Calandriello, L.; Cortese, G.; Farchione, A.; Iezzi, R.; et al. Impact of the COVID-19 pandemic on the selection of chest imaging modalities and reporting systems: A survey of Italian radiologists. Radiol. Med. 2021, 126, 1258–1272. [Google Scholar] [CrossRef]
- Fushimi, Y.; Yoshida, K.; Okawa, M.; Maki, T.; Nakajima, S.; Sakata, A.; Okuchi, S.; Hinoda, T.; Kanagaki, M.; Nakamoto, Y. Vessel wall MR imaging in neuroradiology. Radiol. Med. 2022, 30, 1032–1045. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Campi, C.; Bianca, B.; Bortolotto, C.; Buccicardi, D.; Francesca, C.; Prost, R.; Rengo, M.; Faggioni, L. Blockchain in radiology research and clinical practice: Current trends and future directions. Radiol. Med. 2022, 127, 391–397. [Google Scholar] [CrossRef]
- Fusco, R.; Setola, S.V.; Raiano, N.; Granata, V.; Cerciello, V.; Pecori, B.; Petrillo, A. Analysis of a monocentric computed tomography dosimetric database using a radiation dose index monitoring software: Dose levels and alerts before and after the implementation of the adaptive statistical iterative reconstruction on CT images. Radiol. Med. 2022, 127, 733–742. [Google Scholar] [CrossRef]
- Tunali, I.; Gillies, R.J.; Schabath, M.B. Application of radiomics and artifcial intelligence for lung cancer precision medicine. Cold Spring Harb. Perspect. Med. 2021, 11, a039537. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Salati, S.; Petrillo, A.; Di Bernardo, E.; Grassi, R.; Palaia, R.; Danti, G.; La Porta, M.; Cadossi, M.; et al. A Systematic Review about Imaging and Histopathological Findings for Detecting and Evaluating Electroporation Based Treatments Response. Int. J. Environ. Res. Public Health 2021, 18, 5592. [Google Scholar] [CrossRef]
- Chiti, G.; Grazzini, G.; Flammia, F.; Matteuzzi, B.; Tortoli, P.; Bettarini, S.; Pasqualini, E.; Granata, V.; Busoni, S.; Messserini, L.; et al. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A radiomic model to predict tumor grade. Radiol. Med. 2022, 127, 928–938. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Ottaiano, A.; Nasti, G.; La Porta, M.; Danti, G.; Cappabianca, S.; et al. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: How radiologist should assess MR features. Radiol. Med. 2021, 126, 1584–1600. [Google Scholar] [CrossRef]
- Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma—Part I: Classification, diagnosis and staging. Dig. Liver Dis. 2020, 52, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Med. 2022, 127, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Esposito, M.; Gianneramo, C.; Di Cola, I.; De Berardinis, A.; Martinese, A.; Nkamtse Tochap, G.; Conforti, A.; Masciocchi, C.; Cipriani, P.; et al. Nail and enthesis assessment in patients with psoriatic disease by high frequency ultrasonography: Findings from a single-centre cross-sectional study. Radiol. Med. 2022, 127, 1400–1406. [Google Scholar] [CrossRef]
- Salaffi, F.; Carotti, M.; Di Matteo, A.; Ceccarelli, L.; Farah, S.; Villota-Eraso, C.; Di Carlo, M.; Giovagnoni, A. Ultrasound and magnetic resonance imaging as diagnostic tools for sarcopenia in immune-mediated rheumatic diseases (IMRDs). Radiol. Med. 2022, 127, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Baldassarre, S.; Cerimele, F.; Pepi, L.; Marconi, E.; Ercolani, P.; Floridi, C.; Argalia, G.; Goteri, G.; Giovagnoni, A. 2D shear wave elastography in evaluation of prognostic factors in breast cancer. Radiol. Med. 2022, 127, 1221–1227. [Google Scholar] [CrossRef]
- Bartolotta, T.V.; Orlando, A.A.M.; Dimarco, M.; Zarcaro, C.; Ferraro, F.; Cirino, A.; Matranga, D.; Vieni, S.; Cabibi, D. Diagnostic performance of 2D-shear wave elastography in the diagnosis of breast cancer: A clinical appraisal of cutoff values. Radiol. Med. 2022, 127, 1209–1220. [Google Scholar] [CrossRef]
- Fresilli, D.; Di Leo, N.; Martinelli, O.; Di Marzo, L.; Pacini, P.; Dolcetti, V.; Del Gaudio, G.; Canni, F.; Ricci, L.I.; De Vito, C.; et al. 3D-Arterial analysis software and CEUS in the assessment of severity and vulnerability of carotid atherosclerotic plaque: A comparison with CTA and histopathology. Radiol. Med. 2022, 127, 1254–1269. [Google Scholar] [CrossRef]
- Bruno, F.; Marrelli, A.; Tommasino, E.; Martinese, G.; Gagliardi, A.; Pertici, L.; Pagliei, V.; Palumbo, P.; Arrigoni, F.; Di Cesare, E.; et al. Advanced MRI imaging of nerve roots in lumbar radiculopathy due to discoradicular conflict: DWI, DTI, and T2 mapping with clinical and neurophysiological correlations. Radiol. Med. 2022, 127, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Pizzini, F.B.; Conti, E.; Bianchetti, A.; Splendiani, A.; Fusco, D.; Caranci, F.; Bozzao, A.; Landi, F.; Gandolfo, N.; Farina, L.; et al. Radiological assessment of dementia: The Italian inter-society consensus for a practical and clinically oriented guide to image acquisition, evaluation, and reporting. Radiol. Med. 2022, 127, 998–1022. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.S.; Balbaa, M.F.; Gallazzi, M.B.; Eid, M.E.; Kotb, H.T.; Shafei, M.E.; Ierardi, A.M.; Daolio, P.A.; Barile, A.; Carrafiello, G. Role of percutaneous CT-guided radiofrequency ablation in treatment of intra-articular, in close contact with cartilage and extra-articular osteoid osteomas: Comparative analysis and new classification system. Radiol. Med. 2022, 127, 1142–1150. [Google Scholar] [CrossRef]
- Song, W.; Chen, Q.; Guo, D.; Jiang, C. Preoperative estimation of the survival of patients with unresectable hepatocellular carcinoma achieving complete response after conventional transcatheter arterial chemoembolization: Assessments of clinical and LI-RADS MR features. Radiol. Med. 2022, 127, 939–949. [Google Scholar] [CrossRef]
- Kang, Y.J.; Cho, J.H.; Hwang, S.H. Diagnostic value of various criteria for deep lobe involvement in radiologic studies with parotid mass: A systematic review and meta-analysis. Radiol. Med. 2022, 127, 1124–1133. [Google Scholar] [CrossRef]
- Sayeed, S.; Faiz, B.Y.; Aslam, S.; Masood, L.; Saeed, R. CT Chest Severity Score for COVID 19 Pneumonia: A Quantitative Imaging Tool for Severity Assessment of Disease. J. Coll. Physicians Surg. Pak. 2021, 30, 388–392. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Grazzini, G.; Pradella, S.; Borgheresi, A.; Bruno, A.; Palumbo, P.; Bruno, F.; Grassi, R.; Giovagnoni, A.; et al. Radiomics in medical imaging: Pitfalls and challenges in clinical management. Jpn. J. Radiol. 2022, 40, 919–929. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Barretta, M.L.; Picone, C.; Avallone, A.; Belli, A.; Patrone, R.; Ferrante, M.; Cozzi, D.; Grassi, R.; et al. Radiomics in hepatic metastasis by colorectal cancer. Infect. Agent Cancer 2021, 16, 39. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Ottaiano, A.; Nasti, G.; Grassi, R.; Pilone, V.; et al. EOB-MR Based Radiomics Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1239. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; De Muzio, F.; Dell’ Aversana, F.; Cutolo, C.; Faggioni, L.; Miele, V.; Izzo, F.; Petrillo, A. CT-Based Radiomics Analysis to Predict Histopathological Outcomes Following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Mattace Raso, M.; Gabelloni, M.; Avallone, A.; Ottaiano, A.; Tatangelo, F.; Brunese, M.C.; et al. Radiomics and Machine Learning Analysis Based on Magnetic Resonance Imaging in the Assessment of Colorectal Liver Metastases Growth Pattern. Diagnostics 2022, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Avallone, A.; De Stefano, A.; Ottaiano, A.; Sbordone, C.; Brunese, L.; Izzo, F.; Petrillo, A. Radiomics-Derived Data by Contrast Enhanced Magnetic Resonance in RAS Mutations Detection in Colorectal Liver Metastases. Cancers 2021, 13, 453. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’ Aversana, F.; Ottaiano, A.; Avallone, A.; Nasti, G.; Grassi, F.; et al. Contrast MR-Based Radiomics and Machine Learning Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases: A Preliminary Study. Cancers 2022, 14, 1110. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.M.; Ippolito, E.; Quattrocchi, C.C.; Greco, C.; Mallio, C.A.; Santo, B.; D’Alessio, P.; Crucitti, P.; Fiore, M.; Zobel, B.B.; et al. Radiation-Induced Pneumonitis in the Era of the COVID-19 Pandemic: Artificial Intelligence for Differential Diagnosis. Cancers 2021, 13, 1960. [Google Scholar] [CrossRef] [PubMed]

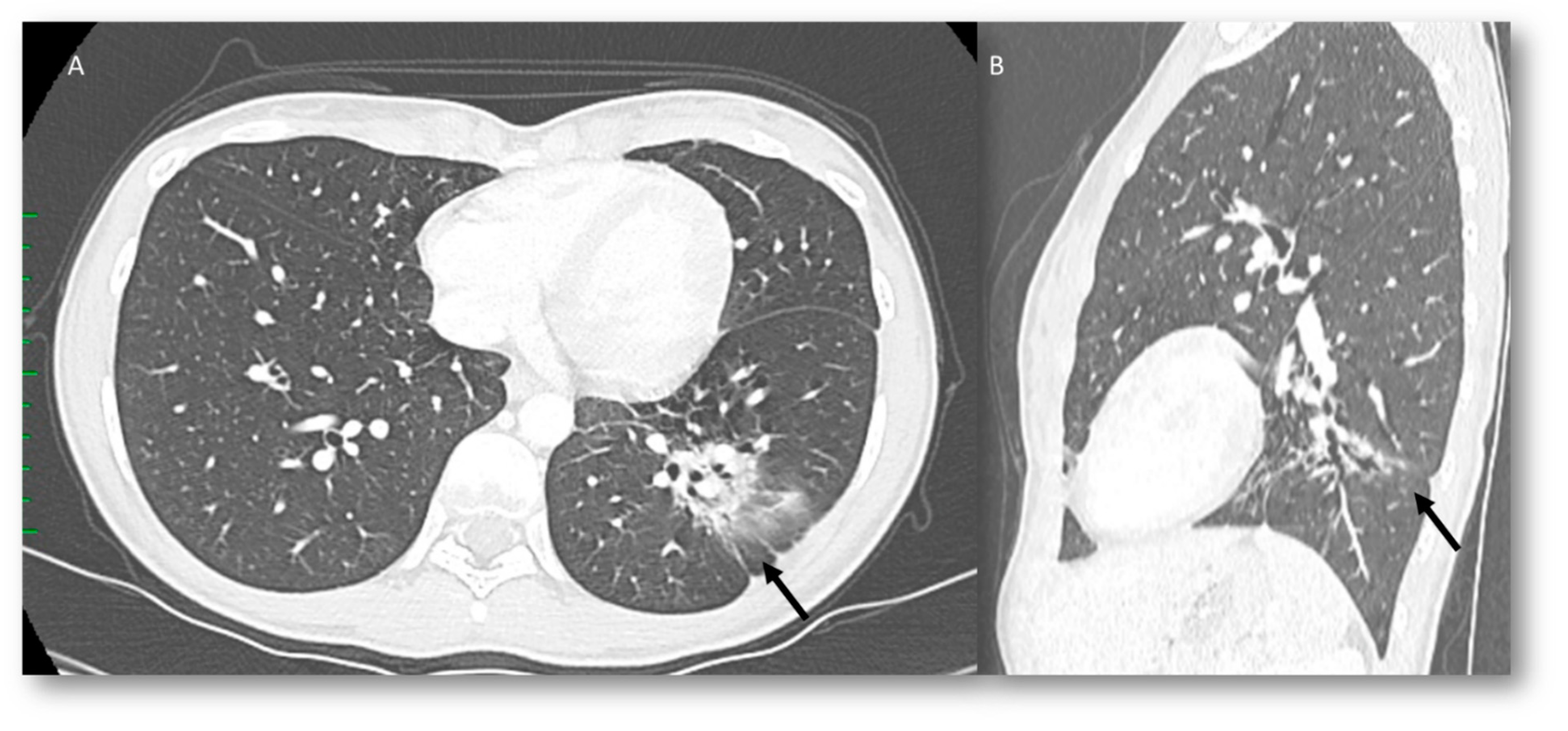
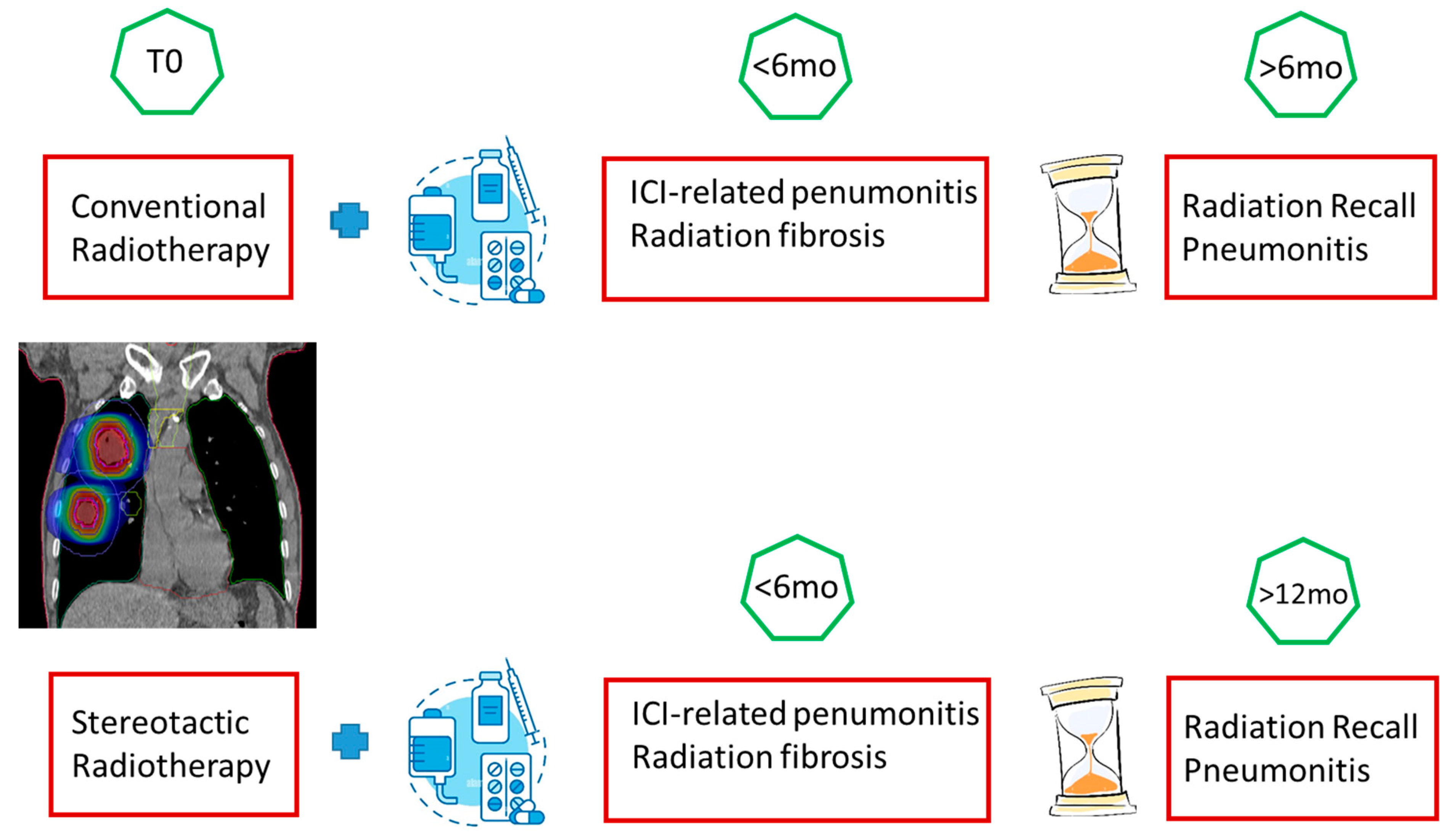

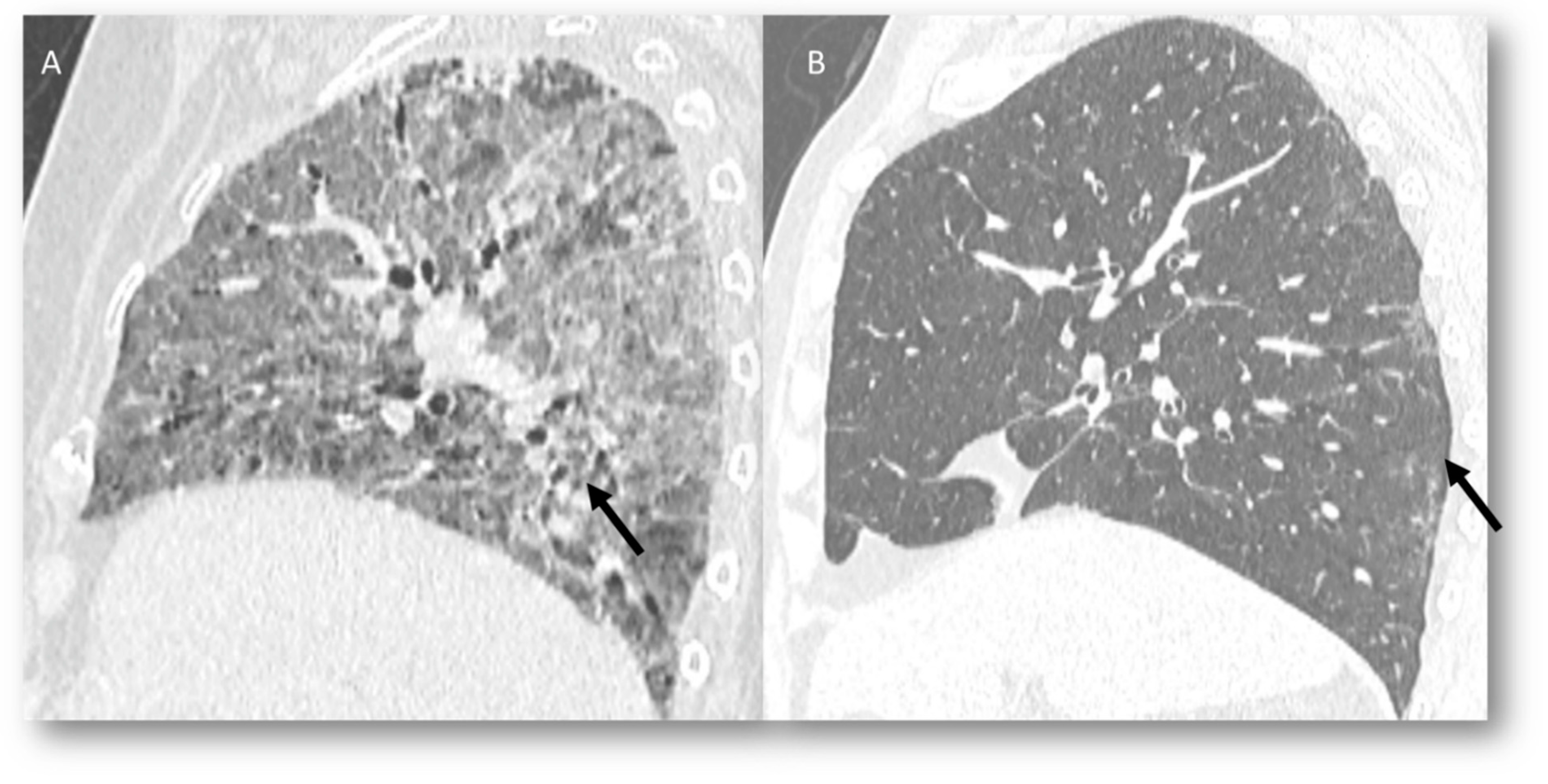
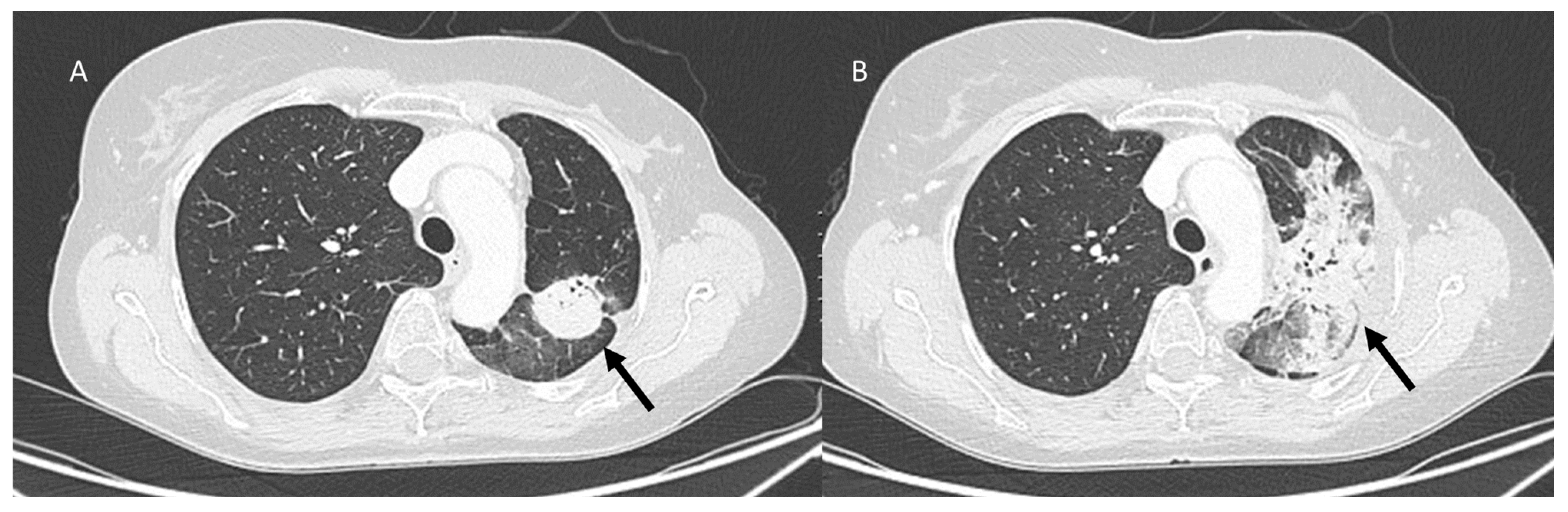


| Type of Pneumonia | Lung Involvement | CT-Patter | Mechanisms of Lung Radiation Damage |
|---|---|---|---|
| RRP | Target area | Ground-glass opacities and consolidative opacities. | Unknown (A non-immune fixed drug reaction-like condition, dysregulated release of reactive oxygen species, abnormalities of tissue vasculature and impaired DNA repair). |
| RP | Target area | Ground-glass opacities and consolidative opacities. | Direct damage to the DNA and indirect damage through the production of reactive oxygen species (ROS), causing changes in vascularity and capillary permeability, activation of the inflammatory response and alteration of immunological response |
| ICI-related pneumonitis | Diffuse (related to the phase of disease) | Ground-glass and reticular opacities; consolidative opacities; interlobular septal thickening; “crazy-paving” pattern | Autoimmune |
| COVID-19 pneumonia | Diffuse (related to the phase of disease) | Ground-glass opacities; crazy-paving pattern; consolidative opacities; interlobular septal thickening (according to the phase of disease) | Unknown, supposed cytokine storms |
| Pulmonary lymphangitis carcinomatosa | Diffuse (related to the phase of disease) | Irregularly interlobular septal thickening; smooth (early stage) or nodular thickening (late development); ground-glass opacities; pleural effusions. | Tumor spread through lymphatic vessels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, F.; Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Gabelloni, M.; Borgheresi, A.; Danti, G.; Picone, C.; Giovagnoni, A.; et al. Radiation Recall Pneumonitis: The Open Challenge in Differential Diagnosis of Pneumonia Induced by Oncological Treatments. J. Clin. Med. 2023, 12, 1442. https://doi.org/10.3390/jcm12041442
Grassi F, Granata V, Fusco R, De Muzio F, Cutolo C, Gabelloni M, Borgheresi A, Danti G, Picone C, Giovagnoni A, et al. Radiation Recall Pneumonitis: The Open Challenge in Differential Diagnosis of Pneumonia Induced by Oncological Treatments. Journal of Clinical Medicine. 2023; 12(4):1442. https://doi.org/10.3390/jcm12041442
Chicago/Turabian StyleGrassi, Francesca, Vincenza Granata, Roberta Fusco, Federica De Muzio, Carmen Cutolo, Michela Gabelloni, Alessandra Borgheresi, Ginevra Danti, Carmine Picone, Andrea Giovagnoni, and et al. 2023. "Radiation Recall Pneumonitis: The Open Challenge in Differential Diagnosis of Pneumonia Induced by Oncological Treatments" Journal of Clinical Medicine 12, no. 4: 1442. https://doi.org/10.3390/jcm12041442
APA StyleGrassi, F., Granata, V., Fusco, R., De Muzio, F., Cutolo, C., Gabelloni, M., Borgheresi, A., Danti, G., Picone, C., Giovagnoni, A., Miele, V., Gandolfo, N., Barile, A., Nardone, V., & Grassi, R. (2023). Radiation Recall Pneumonitis: The Open Challenge in Differential Diagnosis of Pneumonia Induced by Oncological Treatments. Journal of Clinical Medicine, 12(4), 1442. https://doi.org/10.3390/jcm12041442






