Risk of Migraine after Traumatic Brain Injury and Effects of Injury Management Levels and Treatment Modalities: A Nationwide Population-Based Cohort Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sourses
2.2. Study Design and Sampled Participants
2.3. Outcome Measurement
2.4. Potential Confounders
2.5. Subgourp Analysis
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
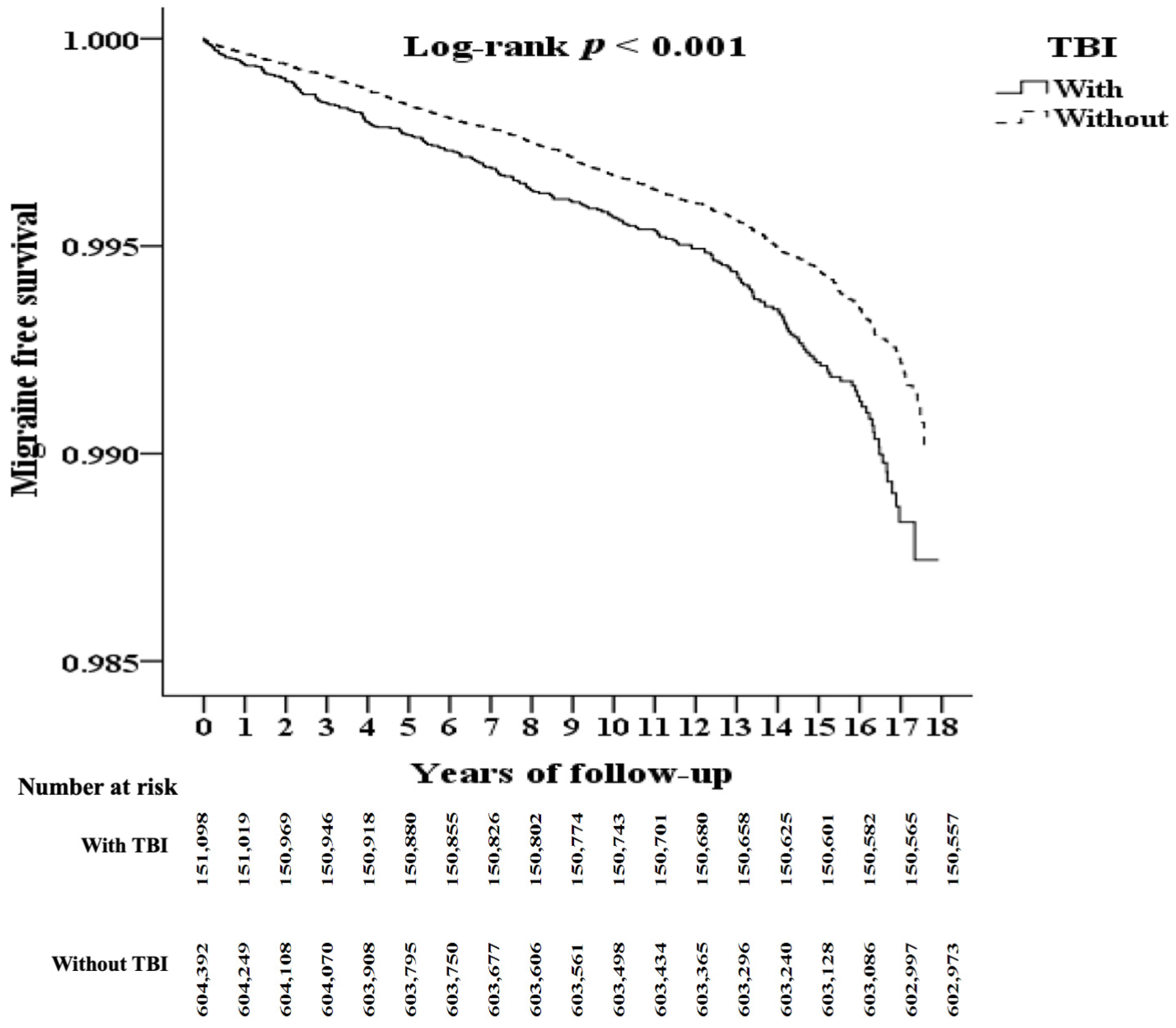
3.2. Kaplan–Meier Model for Assessing the Cumulative Risk of Migraine
3.3. HRs for Migraine in the TBI Cohort
3.4. HRs for Migraine Subtypes in the TBI Cohort
3.5. HRs for TBI Subtypes
3.6. Effects of Treatment Modalities of TBI on Risk of Migraine
4. Discussion
4.1. Pathophysiological Links between Migraine and TBI
4.2. Effects of the Severity of TBI on the Risk of Migraine
4.3. Effects of Treatment Modalities for TBI on the Risk of Migraine
4.4. Strengths and Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | Abbreviation | ICD-9-CM/ICD-10/NHI Code/ATC Code/Definition |
|---|---|---|
| Study population: Traumatic brain injury | TBI | 310.2, 800–804, 850–854, 870–873, 905.0, 907.0, 950.1, 950.3, 950.91, V15.52; S01, S02, S06, T90 |
| Minor cases | OPD | ≧3 outpatient department visits |
| Emergent cases | ER | Emergency room visits |
| Severe cases | ADM | Admission visits |
| Brain surgery | Any of the following | |
| Microvascular decompression | OP04.41, 83001B, 83030B, 83087B | |
| Craniotomy/Cranioplasty | OP01.21–OP01.28, OP02.01–OP02.07, OP02.12, 64002B, 64005B, 65067B, 65068B, 83004B, 83005B, 83011B, 83012B, 83013C, 83015C, 83016B, 83036C, 88037B, 83039B, 83047B, 83077B, 83078B | |
| Ventriculostomy with or without shunting | OP02.2–OP02.4, 83013C, 83051B, 83049B, 83050B, 83052C, 83055B | |
| Removal of subdural/epidural/brain hematoma | OP01.01–OP02.12, 29001C, 29024B, 64143B, 64204B–64206B, 65060B, 83010B, 83013C, 83016B–83019B, 88037B, 83037C, 83038C, 83039B, 83047B, 83056B, 83080B–83082B, 83088B, 84034B | |
| Endarterectomy | OP38.12, 69004B | |
| Bypass surgeries | OP39.28, 69008B, 83063B | |
| Occupational therapy | OT | OP93.83 |
| Simple OT | 43001A, 43002B, 43003C | |
| Moderate OT | 43004A, 43005B, 43006C, 43007A, 43008B, 43009C, 43027C, 43028C | |
| Complicated OT | 43029A, 43030B, 43031, 43032C | |
| Physical therapy | PT | OP91.1–OP93.6 |
| Simple PT | 42001A, 42002B, 42003C, 42004A, 42005B, 42006C | |
| Moderate PT | 42007A, 42008B, 42009C, 42010A, 42011B, 42012C, 42017C, 42018C | |
| Complicated PT | 42013A, 42014B, 42015C, 42019C | |
| Pharmacological treatment | ||
| Antiepileptics | N03A | |
| Osmotic diuresis | B05BC | |
| Minor trauma | Injury severity score (ISS) < 16 | |
| Major trauma | ISS ≥ 16 | |
| Excluding: | ||
| Epidemic vertigo | 078.81; A88.1 | |
| Benign neoplasm of cranial nerves | 225.1; D33.3 | |
| Vertiginous syndromes and other disorders of vestibular system | 386; H81.1–H81.3 | |
| Sudden hearing loss | 388.2; H91.2 | |
| Dizziness and giddiness | 780.4; R42 | |
| Events: Migraine | 346; G43; Diagnosis by otolaryngologist or neurologist and medical visits ≧3 | |
| Migraine with aura | 346.0; G43.1 | |
| Migraine without aura | 346.1; G43.0 | |
| Comorbidities: | ||
| Hypertension | HTN | 401–405; I10–I15 |
| Diabetes mellitus | DM | 250; E10–E14 |
| Depression | 296.2, 296.3, 296.82, 300.4, 311; F32, F33, F34.1 | |
| Congestive heart failure | CHF | 428; I50 |
| Cerebrovascular accident | CVA | 430–438; I60–I69 |
| Chronic obstructive pulmonary disease | COPD | 490–496; J40–J47 |
| Liver disease | 571; K70-K77excluding K70.9 | |
| Alcoholism | 291, 303, 571.3; F10, K70.9 | |
| Chronic kidney disease | CKD | 585-586; N18–N19 |
| Gout | 274; M10 | |
| Osteoporosis | 733.0; M81 | |
| Hyperlipidemia | 272.0–272.4; E78.4, E78.5, E78.8, E78.9 | |
| Autoimmune disease | AID | 710; M32–M35 |
| TBI | Total | With | Without | p Value | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 755,490 | 151,098 | 20.00 | 604,392 | 80.00 | ||
| Migraine | <0.001 | ||||||
| Without | 753,530 | 99.74 | 150,557 | 99.64 | 602,973 | 99.77 | |
| With | 1960 | 0.26 | 541 | 0.36 | 1419 | 0.23 | |
| Sex | 0.999 | ||||||
| Male | 469,605 | 62.16 | 93,921 | 62.16 | 375,684 | 62.16 | |
| Female | 285,885 | 37.84 | 57,177 | 37.84 | 228,708 | 37.84 | |
| Age (years) | 51.26 ± 19.91 | 51.06 ± 19.80 | 51.31 ± 19.94 | <0.001 | |||
| Age group (yrs) | <0.001 | ||||||
| 18–29 | 144,333 | 19.10 | 28,822 | 19.08 | 115,511 | 19.11 | |
| 30–39 | 148,152 | 19.61 | 28,729 | 19.01 | 119,423 | 19.76 | |
| 40–49 | 122,259 | 16.18 | 24,603 | 16.28 | 97,656 | 16.16 | |
| 50–59 | 99,888 | 13.22 | 20,592 | 13.63 | 79,296 | 13.12 | |
| ≧60 | 240,858 | 31.88 | 48,352 | 32.00 | 192,506 | 31.85 | |
| Insured premium (NT$) | <0.001 | ||||||
| <15,840 | 739,707 | 97.91 | 147,919 | 97.90 | 591,788 | 97.91 | |
| 15,841–25,000 | 11,311 | 1.50 | 2471 | 1.64 | 8840 | 1.46 | |
| >25,001 | 4472 | 0.59 | 708 | 0.47 | 3764 | 0.62 | |
| Hypertension | <0.001 | ||||||
| Without | 660,422 | 87.42 | 134,100 | 88.75 | 526,322 | 87.08 | |
| With | 95,068 | 12.58 | 16,998 | 11.25 | 78,070 | 12.92 | |
| Diabetes mellitus | <0.001 | ||||||
| Without | 672,278 | 88.99 | 136,373 | 90.25 | 535,905 | 88.67 | |
| With | 83,212 | 11.01 | 14,725 | 9.75 | 68,487 | 11.33 | |
| Depression | 0.526 | ||||||
| Without | 749,334 | 99.19 | 149,847 | 99.17 | 599,487 | 99.19 | |
| With | 6156 | 0.81 | 1251 | 0.83 | 4905 | 0.81 | |
| Congestive Heart Failure | <0.001 | ||||||
| Without | 732,951 | 97.02 | 147,573 | 97.67 | 585,378 | 96.85 | |
| With | 22,539 | 2.98 | 3525 | 2.33 | 19,014 | 3.15 | |
| Cerebrovascular accident | 0.004 | ||||||
| Without | 715,208 | 94.67 | 142,815 | 94.52 | 572,393 | 94.71 | |
| With | 40,282 | 5.33 | 8283 | 5.48 | 31,999 | 5.29 | |
| Chronic Obstructive Pulmonary Disease | <0.001 | ||||||
| Without | 717,782 | 95.01 | 144,491 | 95.63 | 573,291 | 94.85 | |
| With | 37,708 | 4.99 | 6607 | 4.37 | 31,101 | 5.15 | |
| Liver cirrhosis | <0.001 | ||||||
| Without | 717,492 | 94.97 | 144,204 | 95.44 | 573,288 | 94.85 | |
| With | 37,998 | 5.03 | 6894 | 4.56 | 31,104 | 5.15 | |
| Alcoholism | <0.001 | ||||||
| Without | 749,859 | 99.25 | 149,362 | 98.85 | 600,497 | 99.36 | |
| With | 5631 | 0.75 | 1736 | 1.15 | 3895 | 0.64 | |
| Chronic Kidney Disease | <0.001 | ||||||
| Without | 716,140 | 94.79 | 145,276 | 96.15 | 570,864 | 94.45 | |
| With | 39,350 | 5.21 | 5822 | 3.85 | 33,528 | 5.55 | |
| Osteoporosis | 0.428 | ||||||
| Without | 753,309 | 99.71 | 150,647 | 99.70 | 602,662 | 99.71 | |
| With | 2181 | 0.29 | 451 | 0.30 | 1730 | 0.29 | |
| Hyperlipidemia | <0.001 | ||||||
| Without | 739,645 | 97.90 | 148,439 | 98.24 | 591,206 | 97.82 | |
| With | 15,845 | 2.10 | 2659 | 1.76 | 13,186 | 2.18 | |
| Autoimmune Disease | <0.001 | ||||||
| Without | 753,281 | 99.71 | 150,874 | 99.85 | 602,407 | 99.67 | |
| With | 2209 | 0.29 | 224 | 0.15 | 1985 | 0.33 | |
| Season | <0.001 | ||||||
| Spring | 185,326 | 24.53 | 35,908 | 23.76 | 149,418 | 24.72 | |
| Summer | 193,581 | 25.62 | 38,195 | 25.28 | 155,386 | 25.71 | |
| Autumn | 197,234 | 26.11 | 40,407 | 26.74 | 156,827 | 25.95 | |
| Winter | 179,349 | 23.74 | 36,588 | 24.21 | 142,761 | 23.62 | |
| Location | <0.001 | ||||||
| Northern Taiwan | 296,795 | 39.29 | 43,135 | 28.55 | 253,660 | 41.97 | |
| Central Taiwan | 220,244 | 29.15 | 50,436 | 33.38 | 169,808 | 28.10 | |
| Southern Taiwan | 193,657 | 25.63 | 48,062 | 31.81 | 145,595 | 24.09 | |
| Eastern Taiwan | 41,605 | 5.51 | 8888 | 5.88 | 32,717 | 5.41 | |
| Outlying islands | 3189 | 0.42 | 577 | 0.38 | 2612 | 0.43 | |
| Urbanization level | <0.001 | ||||||
| 1 (The highest) | 242,751 | 32.13 | 38,210 | 25.29 | 204,541 | 33.84 | |
| 2 | 325,348 | 43.06 | 62,274 | 41.21 | 263,074 | 43.53 | |
| 3 | 66,913 | 8.86 | 16,935 | 11.21 | 49,978 | 8.27 | |
| 4 (The lowest) | 120,478 | 15.95 | 33,679 | 22.29 | 86,799 | 14.36 | |
| Variables | Crude HR | 95% CI (Low) | 95% CI (High) | p Value | Adjusted HR | 95% CI (Low) | 95% CI (High) | p Value |
|---|---|---|---|---|---|---|---|---|
| TBI | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.688 | 1.454 | 2.006 | < 0.001 | 1.484 | 1.276 | 1.724 | <0.001 |
| Sex | ||||||||
| Male | 0.407 | 0.356 | 0.466 | <0.001 | 0.393 | 0.342 | 0.452 | <0.001 |
| Female | Reference | Reference | ||||||
| Age group (yrs) | ||||||||
| 18–29 | Reference | Reference | ||||||
| 30–39 | 0.532 | 0.412 | 0.689 | <0.001 | 0.542 | 0.419 | 0.702 | <0.001 |
| 40–49 | 0.596 | 0.460 | 0.773 | <0.001 | 0.692 | 0.531 | 0.900 | <0.001 |
| 50–59 | 0.597 | 0.462 | 0.770 | <0.001 | 0.615 | 0.472 | 0.803 | <0.001 |
| ≧60 | 0.253 | 0.196 | 0.326 | <0.001 | 0.242 | 0.184 | 0.319 | <0.001 |
| Insured premium * (NT$) | ||||||||
| <15,840 | Reference | Reference | ||||||
| 15,841–25,000 | 1.131 | 0.690 | 1.854 | 0.726 | 1.043 | 0.636 | 1.711 | 0.745 |
| >25,001 | 0.235 | 0.034 | 1.673 | 0.268 | 0.232 | 0.033 | 1.652 | 0.291 |
| Hypertension | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.876 | 0.741 | 1.033 | 0.084 | 1.076 | 0.892 | 1.299 | 0.109 |
| Diabetes mellitus | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.727 | 1.603 | 1.882 | <0.001 | 1.247 | 0.984 | 1.547 | 0.063 |
| Depression | ||||||||
| Without | Reference | Reference | ||||||
| With | 6.397 | 4.977 | 8.220 | <0.001 | 5.497 | 5.281 | 6.069 | <0.001 |
| Congestive Heart Failure | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.277 | 0.148 | 0.516 | <0.001 | 0.434 | 0.232 | 0.814 | <0.001 |
| Cerebrovascular accident | ||||||||
| Without | Reference | Reference | ||||||
| With | 2.021 | 1.654 | 2.469 | <0.001 | 2.902 | 2.336 | 3.605 | <0.001 |
| Chronic Obstructive Pulmonary Disease | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.066 | 0.819 | 1.388 | 0.184 | 1.602 | 1.219 | 2.104 | 0.001 |
| Liver cirrhosis | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.801 | 0.580 | 1.110 | 0.486 | 1.059 | 0.759 | 1.477 | 0.577 |
| Alcoholism | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.958 | 0.478 | 1.922 | 0.597 | 1.086 | 0.382 | 1.596 | 0.634 |
| Chronic Kidney Disease | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.264 | 1.159 | 1.443 | <0.001 | 1.408 | 1.242 | 1.695 | <0.001 |
| Osteoporosis | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.507 | 0.127 | 2.031 | 0.811 | 0.607 | 0.152 | 2.441 | 0.862 |
| Hyperlipidemia | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.682 | 1.277 | 2.216 | <0.001 | 1.742 | 1.307 | 2.322 | <0.001 |
| Autoimmune Disease | ||||||||
| Without | Reference | Reference | ||||||
| With | 2.214 | 1.148 | 4.269 | <0.001 | 1.845 | 0.965 | 3.634 | 0.073 |
| Season ** | ||||||||
| Spring | Reference | Reference | ||||||
| Summer | 0.859 | 0.715 | 1.033 | 0.186 | 0.856 | 0.712 | 1.029 | 0.194 |
| Autumn | 0.719 | 0.597 | 0.866 | <0.001 | 0.713 | 0.592 | 0.859 | <0.001 |
| Winter | 0.915 | 0.761 | 1.101 | 0.293 | 0.929 | 0.772 | 1.118 | 0.345 |
| Location | Multicollinearity with urbanization level | |||||||
| Northern Taiwan | Reference | |||||||
| Central Taiwan | 1.563 | 1.330 | 1.837 | <0.001 | ||||
| Southern Taiwan | 1.161 | 0.971 | 1.390 | 0.079 | ||||
| Eastern Taiwan | 1.536 | 1.181 | 1.997 | <0.001 | ||||
| Outlying islands | 3.254 | 1.675 | 6.322 | <0.001 | ||||
| Urbanization level *** | ||||||||
| 1 (The highest) | 0.554 | 0.454 | 0.676 | <0.001 | 0.665 | 0.533 | 0.829 | <0.001 |
| 2 | 0.851 | 0.720 | 1.007 | 0.056 | 0.988 | 0.828 | 1.179 | 0.188 |
| 3 | 0.853 | 0.663 | 1.097 | 0.234 | 0.776 | 0.602 | 0.999 | 0.049 |
| 4 (The lowest) | Reference | Reference | ||||||
| Level of care | ||||||||
| Hospital center | 0.454 | 0.380 | 0.541 | <0.001 | 0.577 | 0.472 | 0.705 | <0.001 |
| Regional hospital | 0.570 | 0.490 | 0.662 | <0.001 | 0.626 | 0.537 | 0.731 | <0.001 |
| District hospital | Reference | Reference | ||||||
| TBI | With | Without (Reference) | With vs. Without (Reference) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stratified | Events | PYs | Rate (Per 105 PYs) | Events | PYs | Rate (Per 105 PYs) | Adjusted HR | 95% CI (Low) | 95% CI (High) | p Value |
| Total | 541 | 1,010,916.25 | 53.52 | 1419 | 4,027,839.97 | 35.23 | 1.484 | 1.276 | 1.724 | <0.001 |
| Sex | ||||||||||
| Male | 199 | 597,601.83 | 33.30 | 554 | 2,457,518.36 | 22.54 | 1.445 | 1.244 | 1.678 | <0.001 |
| Female | 342 | 413,314.42 | 82.75 | 865 | 1,570,321.61 | 55.08 | 1.522 | 1.324 | 1.811 | <0.001 |
| Age group (yrs) | ||||||||||
| 18–29 | 91 | 64,215.20 | 141.71 | 152 | 201,963.33 | 75.26 | 1.833 | 1.578 | 2.133 | <0.001 |
| 30–39 | 97 | 197,296.94 | 49.16 | 290 | 874,603.74 | 33.16 | 1.236 | 1.068 | 1.436 | <0.001 |
| 40–49 | 117 | 157,731.04 | 74.18 | 283 | 634,482.35 | 44.60 | 1.665 | 1.432 | 1.934 | <0.001 |
| 50–59 | 125 | 175,126.11 | 71.38 | 321 | 693,473.11 | 46.29 | 1.516 | 1.304 | 1.760 | <0.001 |
| ≧60 | 111 | 416,546.96 | 26.65 | 373 | 1,623,317.44 | 22.98 | 1.204 | 1.035 | 1.398 | 0.013 |
| Insured premium (NT$) | ||||||||||
| <15,840 | 530 | 989,004.25 | 53.59 | 1393 | 3,944,623.39 | 35.31 | 1.483 | 1.274 | 1.723 | <0.001 |
| 15,841–25,000 | 11 | 17,911.53 | 61.41 | 25 | 62,678.54 | 39.89 | 1.559 | 1.341 | 1.811 | <0.001 |
| >25,001 | 0 | 4000.47 | 0.00 | 1 | 20,538.04 | 4.87 | 0.000 | - | - | 0.994 |
| Hypertension | ||||||||||
| Without | 448 | 825,842.92 | 54.25 | 1133 | 3,143,631.22 | 36.04 | 1.471 | 1.262 | 1.709 | <0.001 |
| With | 93 | 185,073.33 | 50.25 | 286 | 884,208.75 | 32.35 | 1.513 | 1.302 | 1.764 | <0.001 |
| Diabetes mellitus | ||||||||||
| Without | 467 | 863,203.95 | 54.10 | 1230 | 3,339,985.71 | 36.83 | 1.439 | 1.235 | 1.673 | <0.001 |
| With | 74 | 147,712.30 | 50.10 | 189 | 687,854.26 | 27.48 | 1.752 | 1.507 | 2.037 | <0.001 |
| Depression | ||||||||||
| Without | 482 | 997,210.86 | 48.33 | 1308 | 3,979,648.05 | 32.87 | 1.430 | 1.225 | 1.670 | <0.001 |
| With | 59 | 13,705.39 | 430.49 | 111 | 48,191.92 | 230.33 | 1.797 | 1.543 | 2.074 | <0.001 |
| Congestive Heart Failure | ||||||||||
| Without | 537 | 978,539.19 | 54.88 | 1401 | 3,859,770.22 | 36.30 | 1.511 | 1.278 | 1.731 | <0.001 |
| With | 4 | 32,377.06 | 12.35 | 18 | 168,069.75 | 10.71 | 1.258 | 1.079 | 1.455 | <0.001 |
| Cerebrovascular accident | ||||||||||
| Without | 462 | 941,570.69 | 49.07 | 1231 | 3,766,339.26 | 32.68 | 1.456 | 1.252 | 1.694 | <0.001 |
| With | 79 | 69,345.56 | 113.92 | 188 | 261,500.71 | 71.89 | 1.606 | 1.412 | 1.868 | <0.001 |
| Chronic Obstructive Pulmonary Disease | ||||||||||
| Without | 505 | 951,627.52 | 53.07 | 1324 | 3,770,829.55 | 35.11 | 1.448 | 1.232 | 1.700 | <0.001 |
| With | 36 | 59,288.73 | 60.72 | 95 | 257,010.42 | 36.96 | 1.586 | 1.361 | 1.837 | <0.001 |
| Liver cirrhosis | ||||||||||
| Without | 515 | 951,563.23 | 54.12 | 1361 | 3,815,760.64 | 35.67 | 1.475 | 1.241 | 1.655 | <0.001 |
| With | 26 | 59,353.02 | 43.81 | 58 | 212,079.33 | 27.35 | 1.624 | 1.408 | 1.927 | <0.001 |
| Alcoholism | ||||||||||
| Without | 530 | 995,286.01 | 53.25 | 1412 | 3,996,191.15 | 35.33 | 1.469 | 1.251 | 1.646 | <0.001 |
| With | 11 | 15,630.24 | 70.38 | 7 | 31,648.82 | 22.12 | 3.310 | 2.847 | 3.863 | <0.001 |
| Chronic Kidney Disease | ||||||||||
| Without | 519 | 964,562.99 | 53.81 | 1394 | 3,780,041.75 | 36.88 | 1.407 | 1.219 | 1.614 | <0.001 |
| With | 22 | 46,353.26 | 47.46 | 25 | 247,798.22 | 10.09 | 4.368 | 3.760 | 5.067 | <0.001 |
| Osteoporosis | ||||||||||
| Without | 541 | 1,006,506.97 | 53.75 | 1416 | 4,010,101.47 | 35.31 | 1.487 | 1.279 | 1.731 | <0.001 |
| With | 0 | 4409.28 | 0.00 | 3 | 17,738.50 | 16.91 | 0.000 | - | - | 0.972 |
| Hyperlipidemia | ||||||||||
| Without | 509 | 980,866.57 | 51.89 | 1330 | 3,876,852.71 | 34.31 | 1.480 | 1.271 | 1.700 | <0.001 |
| With | 32 | 30,049.68 | 106.49 | 89 | 150,987.26 | 58.95 | 1.727 | 1.484 | 2.012 | <0.001 |
| Autoimmune Disease | ||||||||||
| Without | 538 | 1,008,504.58 | 53.35 | 1403 | 4,007,721.73 | 35.01 | 1.456 | 1.247 | 1.701 | <0.001 |
| With | 3 | 2411.67 | 124.40 | 16 | 20,118.24 | 79.53 | 2.335 | 2.012 | 2.723 | <0.001 |
| Season | ||||||||||
| Spring | 172 | 229,526.25 | 74.94 | 380 | 935,681.28 | 40.61 | 1.802 | 1.551 | 2.093 | <0.001 |
| Summer | 117 | 255,445.33 | 45.80 | 325 | 1,023,297.87 | 31.76 | 1.415 | 1.218 | 1.646 | <0.001 |
| Autumn | 113 | 290,161.74 | 38.94 | 351 | 1,138,255.24 | 30.84 | 1.225 | 1.055 | 1.426 | <0.001 |
| Winter | 139 | 235,782.93 | 58.95 | 363 | 930,605.58 | 39.01 | 1.478 | 1.272 | 1.717 | <0.001 |
| Urbanization level | ||||||||||
| 1 (The highest) | 74 | 243,266.98 | 30.42 | 299 | 1,239,987.58 | 24.11 | 1.212 | 1.042 | 1.409 | 0.007 |
| 2 | 215 | 423,537.37 | 50.76 | 625 | 1,784,778.60 | 35.02 | 1.410 | 1.211 | 1.639 | <0.001 |
| 3 | 56 | 107,878.36 | 51.91 | 128 | 344,857.12 | 37.12 | 1.351 | 1.162 | 1.575 | <0.001 |
| 4 (The lowest) | 196 | 236,233.54 | 82.97 | 367 | 658,216.67 | 55.76 | 1.480 | 1.270 | 1.727 | <0.001 |
| Level of care | ||||||||||
| Hospital center | 89 | 246,378.80 | 36.12 | 374 | 1,365,977.28 | 27.38 | 1.287 | 1.105 | 1.496 | <0.001 |
| Regional hospital | 213 | 481,275.26 | 44.26 | 600 | 1,816,767.20 | 33.03 | 1.313 | 1.130 | 1.529 | <0.001 |
| District hospital | 239 | 283,262.19 | 84.37 | 445 | 845,095.49 | 52.66 | 1.562 | 1.344 | 1.814 | <0.001 |
References
- Timofeev, I.; Santarius, T.; Kolias, A.G.; Hutchinson, P.J. Decompressive craniectomy-operative technique and perioperative care. Adv. Tech. Stand. Neurosurg. 2012, 38, 115–136. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1–18. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Buki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Faul, M.; Coronado, V. Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 3–13. [Google Scholar] [CrossRef]
- Tagliaferri, F.; Compagnone, C.; Korsic, M.; Servadei, F.; Kraus, J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006, 148, 255–268; discussion 268. [Google Scholar] [CrossRef]
- Lele, A.V. Traumatic Brain Injury in Different Age Groups. J. Clin. Med. 2022, 11, 6739. [Google Scholar] [CrossRef]
- Stocchetti, N.; Zanier, E.R. Chronic impact of traumatic brain injury on outcome and quality of life: A narrative review. Crit. Care 2016, 20, 148. [Google Scholar] [CrossRef]
- Garcia, K.; Moore, B.; Kim, G.; Dsurney, J.; Chan, L. The Impact of Affective States on Postconcussive Symptoms in a TBI Population. Mil. Med. 2019, 184, 168–173. [Google Scholar] [CrossRef]
- Gardner, R.C.; Byers, A.L.; Barnes, D.E.; Li, Y.; Boscardin, J.; Yaffe, K. Mild TBI and risk of Parkinson disease: A Chronic Effects of Neurotrauma Consortium Study. Neurology 2018, 90, e1771–e1779. [Google Scholar] [CrossRef]
- Theeler, B.; Lucas, S.; Riechers, R.G., 2nd; Ruff, R.L. Post-traumatic headaches in civilians and military personnel: A comparative, clinical review. Headache 2013, 53, 881–900. [Google Scholar] [CrossRef] [PubMed]
- Rauen, K.; Reichelt, L.; Probst, P.; Schapers, B.; Muller, F.; Jahn, K.; Plesnila, N. Quality of life up to 10 years after traumatic brain injury: A cross-sectional analysis. Health Qual. Life Outcomes 2020, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Mollayeva, T.; Mollayeva, S.; Colantonio, A. The Risk of Sleep Disorder Among Persons with Mild Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2016, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.; Dumkrieger, G.; Chong, C.D.; Ross, K.; Berisha, V.; Schwedt, T.J. Symptoms of Autonomic Dysfunction Among Those With Persistent Posttraumatic Headache Attributed to Mild Traumatic Brain Injury: A Comparison to Migraine and Healthy Controls. Headache 2018, 58, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Rauen, K.; Spani, C.B.; Tartaglia, M.C.; Ferretti, M.T.; Reichelt, L.; Probst, P.; Schapers, B.; Muller, F.; Jahn, K.; Plesnila, N. Quality of life after traumatic brain injury: A cross-sectional analysis uncovers age- and sex-related differences over the adult life span. Geroscience 2021, 43, 263–278. [Google Scholar] [CrossRef]
- Ishii, R.; Schwedt, T.J.; Trivedi, M.; Dumkrieger, G.; Cortez, M.M.; Brennan, K.C.; Digre, K.; Dodick, D.W. Mild traumatic brain injury affects the features of migraine. J. Headache Pain 2021, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- Nampiaparampil, D.E. Prevalence of chronic pain after traumatic brain injury: A systematic review. JAMA 2008, 300, 711–719. [Google Scholar] [CrossRef]
- Dwyer, B. Posttraumatic Headache. Semin. Neurol. 2018, 38, 619–626. [Google Scholar] [CrossRef]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Capi, M.; Pomes, L.M.; Andolina, G.; Curto, M.; Martelletti, P.; Lionetto, L. Persistent Post-Traumatic Headache and Migraine: Pre-Clinical Comparisons. Int. J. Env. Res. Public Health 2020, 17, 2585. [Google Scholar] [CrossRef]
- Marklund, N.; Bellander, B.M.; Godbolt, A.K.; Levin, H.; McCrory, P.; Thelin, E.P. Treatments and rehabilitation in the acute and chronic state of traumatic brain injury. J. Intern. Med. 2019, 285, 608–623. [Google Scholar] [CrossRef] [PubMed]
- Pinggera, D.; Rhomberg, P.; Beer, R.; Thome, C.; Petr, O. Brain Tissue Damage Induced by Multimodal Neuromonitoring In Situ during MRI after Severe Traumatic Brain Injury: Incidence and Clinical Relevance. J. Clin. Med. 2022, 11, 3169. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.R.; Chesnut, R.; Ghajar, J.; Gordon, D.; Hartl, R.; Newell, D.W.; Servadei, F.; Walters, B.C.; Wilberger, J.E.; Surgical Management of Traumatic Brain Injury Author Group. Surgical management of acute subdural hematomas. Neurosurgery 2006, 58, S16–S24; discussion Si–Siv. [Google Scholar] [CrossRef] [PubMed]
- Tapper, J.; Skrifvars, M.B.; Kivisaari, R.; Siironen, J.; Raj, R. Primary decompressive craniectomy is associated with worse neurological outcome in patients with traumatic brain injury requiring acute surgery. Surg. Neurol. Int. 2017, 8, 141. [Google Scholar] [CrossRef]
- Dang, B.; Chen, W.; He, W.; Chen, G. Rehabilitation Treatment and Progress of Traumatic Brain Injury Dysfunction. Neural Plast. 2017, 2017, 1582182. [Google Scholar] [CrossRef]
- Yang, S.; Orlova, Y.; Lipe, A.; Boren, M.; Hincapie-Castillo, J.M.; Park, H.; Chang, C.Y.; Wilson, D.L.; Adkins, L.; Lo-Ciganic, W.H. Trends in the Management of Headache Disorders in US Emergency Departments: Analysis of 2007-2018 National Hospital Ambulatory Medical Care Survey Data. J. Clin. Med. 2022, 11, 1401. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, C.; Carretta, A.; Nicolosi, F.; Morselli, C. Epidemiology of severe traumatic brain injury. J. Neurosurg. Sci. 2018, 62, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Yen, J.C.; Wu, C.C.; Lin, H.Y.; Hsu, M.H. Effects of the COVID-19 Pandemic on Treatment Efficiency for Traumatic Brain Injury in the Emergency Department: A Multicenter Study in Taiwan. J. Clin. Med. 2021, 10, 5314. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S. Posttraumatic Headache: Clinical Characterization and Management. Curr. Pain Headache Rep. 2015, 19, 48. [Google Scholar] [CrossRef]
- Ashina, H.; Iljazi, A.; Amin, F.M.; Ashina, M.; Lipton, R.B.; Schytz, H.W. Interrelations between migraine-like headache and persistent post-traumatic headache attributed to mild traumatic brain injury: A prospective diary study. J. Headache Pain 2020, 21, 134. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Tinawi, S.; Lamoureux, J.; Feyz, M.; de Guise, E. Detecting Migraine in Patients with Mild Traumatic Brain Injury Using Three Different Headache Measures. Behav. Neurol. 2015, 2015, 693925. [Google Scholar] [CrossRef] [PubMed]
- Committee on Medical Aspects of Automotive Safety. Rating the severity of tissue damage. I. The abbreviated scale. JAMA 1971, 215, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.P.; O’Neill, B.; Haddon, W., Jr.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef]
- Bree, D.; Levy, D. Development of CGRP-dependent pain and headache related behaviours in a rat model of concussion: Implications for mechanisms of post-traumatic headache. Cephalalgia 2018, 38, 246–258. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transpl. 2017, 26, 1118–1130. [Google Scholar] [CrossRef]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef]
- Packard, R.C.; Ham, L.P. Pathogenesis of posttraumatic headache and migraine: A common headache pathway? Headache 1997, 37, 142–152. [Google Scholar] [CrossRef]
- Mofatteh, M. Examining the association between traumatic brain injury and headache. J. Integr. Neurosci. 2021, 20, 1079–1094. [Google Scholar] [CrossRef]
- Ruff, R.L.; Blake, K. Pathophysiological links between traumatic brain injury and post-traumatic headaches. F1000Research 2016, 5, 1–11. [Google Scholar] [CrossRef]
- McKee, A.C.; Cantu, R.C.; Nowinski, C.J.; Hedley-Whyte, E.T.; Gavett, B.E.; Budson, A.E.; Santini, V.E.; Lee, H.S.; Kubilus, C.A.; Stern, R.A. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009, 68, 709–735. [Google Scholar] [CrossRef]
- Ling, H.; Hardy, J.; Zetterberg, H. Neurological consequences of traumatic brain injuries in sports. Mol. Cell. Neurosci. 2015, 66, 114–122. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Daneshvar, D.H.; Alvarez, V.E.; Stein, T.D. The neuropathology of sport. Acta Neuropathol. 2014, 127, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Hains, B.C.; Waxman, S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006, 26, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Coffman, C.; Reyes, D.; Hess, M.C.; Giakas, A.M.; Thiam, M.; Sico, J.J.; Seng, E.; Renthal, W.; Rhoades, C.; Cai, G.; et al. Relationship Between Headache Characteristics and a Remote History of TBI in Veterans: A 10-Year Retrospective Chart Review. Neurology 2022, 99, e187–e198. [Google Scholar] [CrossRef]
- Khellaf, A.; Khan, D.Z.; Helmy, A. Recent advances in traumatic brain injury. J. Neurol. 2019, 266, 2878–2889. [Google Scholar] [CrossRef]
- Kristman, V.L.; Borg, J.; Godbolt, A.K.; Salmi, L.R.; Cancelliere, C.; Carroll, L.J.; Holm, L.W.; Nygren-de Boussard, C.; Hartvigsen, J.; Abara, U.; et al. Methodological issues and research recommendations for prognosis after mild traumatic brain injury: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 2014, 95, S265–S277. [Google Scholar] [CrossRef]
- Do, T.P.; Remmers, A.; Schytz, H.W.; Schankin, C.; Nelson, S.E.; Obermann, M.; Hansen, J.M.; Sinclair, A.J.; Gantenbein, A.R.; Schoonman, G.G. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology 2019, 92, 134–144. [Google Scholar] [CrossRef]
- De Oliveira, D.V.; Vieira, R.C.A.; Pipek, L.Z.; de Sousa, R.M.C.; de Souza, C.P.E.; Santana-Santos, E.; Paiva, W.S. Long-Term Outcomes in Severe Traumatic Brain Injury and Associated Factors: A Prospective Cohort Study. J. Clin. Med. 2022, 11, 6466. [Google Scholar] [CrossRef]
- Fife, T.D.; Kalra, D. Persistent vertigo and dizziness after mild traumatic brain injury. Ann. N. Y. Acad. Sci. 2015, 1343, 97–105. [Google Scholar] [CrossRef]
- Walker, W.C.; Seel, R.T.; Curtiss, G.; Warden, D.L. Headache after moderate and severe traumatic brain injury: A longitudinal analysis. Arch. Phys. Med. Rehabil. 2005, 86, 1793–1800. [Google Scholar] [CrossRef]
- Prince, C.; Bruhns, M.E. Evaluation and Treatment of Mild Traumatic Brain Injury: The Role of Neuropsychology. Brain Sci 2017, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chan, R.C.; Deng, Y. Examination of postconcussion-like symptoms in healthy university students: Relationships to subjective and objective neuropsychological function performance. Arch. Clin. Neuropsychol. 2006, 21, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, A.R.; Arnett, P.A. Positive psychology perspective on traumatic brain injury recovery and rehabilitation. Appl. Neuropsychol. Adult 2018, 25, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.W.; Field, M.; Lovell, M.R.; Iverson, G.; Johnston, K.M.; Maroon, J.; Fu, F.H. Relationship between postconcussion headache and neuropsychological test performance in high school athletes. Am. J. Sport. Med. 2003, 31, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, J.P.; Stump, J.E.; Collins, M.W.; Lovell, M.R.; Field, M.; Maroon, J.C. Posttraumatic migraine characteristics in athletes following sports-related concussion. J. Neurosurg. 2005, 102, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Asplund, C.A.; McKeag, D.B.; Olsen, C.H. Sport-related concussion: Factors associated with prolonged return to play. Clin. J. Sport Med. 2004, 14, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Albanese, M. Clinical Management of Migraine. J. Clin. Med. 2022, 11, 5225. [Google Scholar] [CrossRef]
- Valente, M.; Garbo, R.; Filippi, F.; Antonutti, A.; Ceccarini, V.; Tereshko, Y.; Di Lorenzo, C.; Gigli, G.L. Migraine Prevention through Ketogenic Diet: More than Body Mass Composition Changes. J. Clin. Med. 2022, 11, 4946. [Google Scholar] [CrossRef]
- Heslot, C.; Azouvi, P.; Perdrieau, V.; Granger, A.; Lefevre-Dognin, C.; Cogne, M. A Systematic Review of Treatments of Post-Concussion Symptoms. J. Clin. Med. 2022, 11, 6224. [Google Scholar] [CrossRef]
- Lo Castro, F.; Baraldi, C.; Pellesi, L.; Guerzoni, S. Clinical Evidence of Cannabinoids in Migraine: A Narrative Review. J. Clin. Med. 2022, 11, 1479. [Google Scholar] [CrossRef]
- Nissan, G.R.; Kim, R.; Cohen, J.M.; Seminerio, M.J.; Krasenbaum, L.J.; Carr, K.; Martin, V. Reducing the Burden of Migraine: Safety and Efficacy of CGRP Pathway-Targeted Preventive Treatments. J. Clin. Med. 2022, 11, 4359. [Google Scholar] [CrossRef] [PubMed]

| TBI | Total | With | Without | p Value | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 755,490 | 151,098 | 20.00 | 604,392 | 80.00 | ||
| Sex | 0.999 | ||||||
| Male | 469,605 | 62.16 | 93,921 | 62.16 | 375,684 | 62.16 | |
| Female | 285,885 | 37.84 | 57,177 | 37.84 | 228,708 | 37.84 | |
| Age (years) | 44.45 ± 18.76 | 44.43 ± 18.55 | 44.46 ± 18.81 | 0.578 | |||
| Age group (yrs) | 0.999 | ||||||
| 18–29 | 221,875 | 29.37 | 44,375 | 29.37 | 177,500 | 29.37 | |
| 30–39 | 129,805 | 17.18 | 25,961 | 17.18 | 103,844 | 17.18 | |
| 40–49 | 132,020 | 17.47 | 26,404 | 17.47 | 105,616 | 17.47 | |
| 50–59 | 89,195 | 11.81 | 17,839 | 11.81 | 71,356 | 11.81 | |
| ≧60 | 182,595 | 24.17 | 36,519 | 24.17 | 146,076 | 24.17 | |
| Insured premium (NT$) | <0.001 | ||||||
| <15,840 | 739,707 | 97.91 | 147,919 | 97.90 | 591,788 | 97.91 | |
| 15,841–25,000 | 11,311 | 1.50 | 2471 | 1.64 | 8840 | 1.46 | |
| >25,001 | 4472 | 0.59 | 708 | 0.47 | 3764 | 0.62 | |
| Hypertension | 0.656 | ||||||
| Without | 707,968 | 93.71 | 141,556 | 93.68 | 566,412 | 93.72 | |
| With | 47,522 | 6.29 | 9542 | 6.32 | 37,980 | 6.28 | |
| Diabetes mellitus | 0.693 | ||||||
| Without | 717,896 | 95.02 | 143,609 | 95.04 | 574,287 | 95.02 | |
| With | 37,594 | 4.98 | 7489 | 4.96 | 30,105 | 4.98 | |
| Depression | 0.164 | ||||||
| Without | 754,065 | 99.81 | 150,792 | 99.80 | 603,273 | 99.81 | |
| With | 1425 | 0.19 | 306 | 0.20 | 1119 | 0.19 | |
| Congestive Heart Failure | 0.511 | ||||||
| Without | 753,032 | 99.67 | 150,620 | 99.68 | 602,412 | 99.67 | |
| With | 2458 | 0.33 | 478 | 0.32 | 1980 | 0.33 | |
| Cerebrovascular accident | 0.708 | ||||||
| Without | 739,105 | 97.83 | 147,802 | 97.82 | 591,303 | 97.83 | |
| With | 16,385 | 2.17 | 3296 | 2.18 | 13,089 | 2.17 | |
| Chronic Obstructive Pulmonary Disease | 0.547 | ||||||
| Without | 745,876 | 98.73 | 149,199 | 98.74 | 596,677 | 98.72 | |
| With | 9614 | 1.27 | 1899 | 1.26 | 7715 | 1.28 | |
| Liver cirrhosis | 0.538 | ||||||
| Without | 740,388 | 98.32 | 148,119 | 98.34 | 592,269 | 98.32 | |
| With | 12,644 | 1.68 | 2501 | 1.66 | 10,143 | 1.68 | |
| Alcoholism | 0.751 | ||||||
| Without | 749,242 | 99.17 | 149,859 | 99.18 | 599,383 | 99.17 | |
| With | 6248 | 0.83 | 1239 | 0.82 | 5009 | 0.83 | |
| Chronic Kidney Disease | 0.903 | ||||||
| Without | 748,708 | 99.10 | 149,746 | 99.11 | 598,962 | 99.10 | |
| With | 6782 | 0.90 | 1352 | 0.89 | 5430 | 0.90 | |
| Osteoporosis | 0.617 | ||||||
| Without | 754,083 | 99.81 | 150,809 | 99.81 | 603,274 | 99.82 | |
| With | 1407 | 0.19 | 289 | 0.19 | 1118 | 0.18 | |
| Hyperlipidemia | 0.697 | ||||||
| Without | 751,341 | 99.45 | 150,258 | 99.44 | 601,083 | 99.45 | |
| With | 4149 | 0.55 | 840 | 0.56 | 3309 | 0.55 | |
| Autoimmune Disease | 0.527 | ||||||
| Without | 755,298 | 99.97 | 151,056 | 99.97 | 604,242 | 99.98 | |
| With | 192 | 0.03 | 42 | 0.03 | 150 | 0.02 | |
| Season | 0.999 | ||||||
| Spring (Mar–May) | 189,785 | 25.12 | 37,957 | 25.12 | 151,828 | 25.12 | |
| Summer (Jun–Aug) | 188,910 | 25.00 | 37,782 | 25.00 | 151,128 | 25.00 | |
| Autumn (Sep–Nov) | 190,670 | 25.24 | 38,134 | 25.24 | 152,536 | 25.24 | |
| Winter (Dec–Feb) | 186,125 | 24.64 | 37,225 | 24.64 | 148,900 | 24.64 | |
| Location | <0.001 | ||||||
| Northern Taiwan | 295,631 | 39.13 | 40,025 | 26.49 | 255,606 | 42.29 | |
| Central Taiwan | 221,807 | 29.36 | 52,864 | 34.99 | 168,943 | 27.95 | |
| Southern Taiwan | 193,680 | 25.64 | 48,299 | 31.97 | 145,381 | 24.05 | |
| Eastern Taiwan | 41,169 | 5.45 | 9287 | 6.15 | 31,882 | 5.28 | |
| Outlying islands | 3203 | 0.42 | 623 | 0.41 | 2580 | 0.43 | |
| Urbanization level | <0.001 | ||||||
| 1 (The highest) | 247,547 | 32.77 | 32,824 | 21.72 | 214,723 | 35.53 | |
| 2 | 316,113 | 41.84 | 59,273 | 39.23 | 256,840 | 42.50 | |
| 3 | 70,152 | 9.29 | 20,006 | 13.24 | 50,146 | 8.30 | |
| 4 (The lowest) | 121,678 | 16.11 | 38,995 | 25.81 | 82,683 | 13.68 | |
| Level of care | <0.001 | ||||||
| Hospital center | 229,155 | 30.33 | 22,648 | 14.99 | 206,507 | 34.17 | |
| Regional hospital | 251,367 | 33.27 | 47,438 | 31.40 | 203,929 | 33.74 | |
| District hospital | 274,968 | 36.40 | 81,012 | 53.62 | 193,956 | 32.09 | |
| Years of Follow-Up | Years to Migraine | |||||||
|---|---|---|---|---|---|---|---|---|
| TBI | Min | Median | Max | Mean ± SD | Min | Median | Max | Mean ± SD |
| With | 0.01 | 8.63 | 17.86 | 10.75 ± 8.42 | 0.02 | 6.18 | 17.49 | 7.02 ± 4.86 |
| Without | 0.01 | 8.86 | 17.93 | 10.88 ± 8.65 | 0.03 | 6.75 | 17.62 | 7.41 ± 5.03 |
| Overall | 0.01 | 8.79 | 17.93 | 10.85 ± 8.60 | 0.02 | 6.64 | 17.62 | 7.33 ± 5.00 |
| TBI | With vs. Without (Reference) | |||
|---|---|---|---|---|
| Migraine Subgroup | Adjusted HR | 95% CI | 95% CI | p Value |
| Overall | 1.484 | 1.276 | 1.724 | <0.001 |
| Migraine with aura | 1.558 | 1.341 | 1.806 | <0.001 |
| Migraine without aura | 1.464 | 1.260 | 1.709 | <0.001 |
| Diagnosis by otolaryngologist | 1.395 | 1.201 | 1.638 | <0.001 |
| Diagnosis by neurologist | 1.572 | 1.343 | 1.799 | <0.001 |
| TBI Subgroup | Populations | Adjusted HR | 95% CI | p Value | Adjusted HR | 95% CI | p Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Without TBI | 604,392 | Reference | |||||||
| With TBI | 151,098 | 1.484 | 1.276 | 1.724 | <0.001 | ||||
| OPD | 45,330 | 1.251 | 1.081 | 1.459 | <0.001 | Reference | |||
| ER | 54,783 | 1.293 | 1.120 | 1.498 | <0.001 | 1.060 | 0.878 | 1.365 | 0.124 |
| ADM | 50,985 | 1.915 | 1.648 | 2.222 | <0.001 | 1.557 | 1.203 | 1.837 | <0.001 |
| Without brain surgery | 84,456 | 1.495 | 1.311 | 1.739 | <0.001 | Reference * | |||
| Without OT/PT | 43,727 | 1.536 | 1.321 | 1.784 | <0.001 | Reference | |||
| With OT/PT | 40,729 | 1.504 | 1.293 | 1.742 | <0.001 | 0.978 | 0.613 | 1.174 | 0.389 |
| With brain surgery | 66,642 | 1.469 | 1.265 | 1.708 | <0.001 | 0.998 | 0.662 | 1.234 | 0.472 |
| Without OT/PT | 34,492 | 1.489 | 1.281 | 1.730 | <0.001 | 0.965 | 0.604 | 1.136 | 0.427 |
| With OT/PT | 32,150 | 1.377 | 1.186 | 1.605 | <0.001 | 0.893 | 0.588 | 1.025 | 0.433 |
| Without OT/PT | 78,219 | 1.487 | 1.313 | 1.768 | <0.001 | Reference | |||
| With OT/PT | 72,879 | 1.452 | 1.240 | 1.682 | <0.001 | 0.983 | 0.624 | 1.199 | 0.397 |
| ISS < 16 | 103,153 | 1.233 | 1.060 | 1.435 | <0.001 | Reference | |||
| ISS ≥ 16 | 47,945 | 2.023 | 1.742 | 2.359 | <0.001 | 1.670 | 1.325 | 2.011 | <0.001 |
| Without pharmacological treatment | 31,296 | 1.480 | 1.271 | 1.719 | <0.001 | Reference | |||
| With pharmacological treatment | 119,802 | 1.485 | 1.279 | 1.727 | <0.001 | 1.001 | 0.672 | 1.287 | 0.594 |
| Brain Surgery | Total | With | Without | p * | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 151,098 | 100 | 66,642 | 44.11 | 84,456 | 55.89 | |
| OT/PT | 0.945 | ||||||
| Without | 78,219 | 51.77 | 34,492 | 51.76 | 43,727 | 51.77 | |
| With | 72,879 | 48.23 | 32,150 | 48.24 | 40,729 | 48.23 | |
| Brain Surgery | Population | Duration (Months) Mean (SD) | p * | Intensity (Times) Mean (SD) | p * |
|---|---|---|---|---|---|
| With | 32,150 | 11.14 (10.22) | 7.4 (6.7) | ||
| Without | 40,729 | 9.86 (9.51) | 6.7 (6.3) | ||
| Overall | 72,879 | 10.42 (9.85) | <0.001 | 7.0 (6.5) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-H.; Sung, Y.-F.; Chien, W.-C.; Chung, C.-H.; Chen, J.-W. Risk of Migraine after Traumatic Brain Injury and Effects of Injury Management Levels and Treatment Modalities: A Nationwide Population-Based Cohort Study in Taiwan. J. Clin. Med. 2023, 12, 1530. https://doi.org/10.3390/jcm12041530
Chen M-H, Sung Y-F, Chien W-C, Chung C-H, Chen J-W. Risk of Migraine after Traumatic Brain Injury and Effects of Injury Management Levels and Treatment Modalities: A Nationwide Population-Based Cohort Study in Taiwan. Journal of Clinical Medicine. 2023; 12(4):1530. https://doi.org/10.3390/jcm12041530
Chicago/Turabian StyleChen, Mei-Hui, Yueh-Feng Sung, Wu-Chien Chien, Chi-Hsiang Chung, and Jeng-Wen Chen. 2023. "Risk of Migraine after Traumatic Brain Injury and Effects of Injury Management Levels and Treatment Modalities: A Nationwide Population-Based Cohort Study in Taiwan" Journal of Clinical Medicine 12, no. 4: 1530. https://doi.org/10.3390/jcm12041530
APA StyleChen, M.-H., Sung, Y.-F., Chien, W.-C., Chung, C.-H., & Chen, J.-W. (2023). Risk of Migraine after Traumatic Brain Injury and Effects of Injury Management Levels and Treatment Modalities: A Nationwide Population-Based Cohort Study in Taiwan. Journal of Clinical Medicine, 12(4), 1530. https://doi.org/10.3390/jcm12041530






