Serum Intact Fibroblast Growth Factor 23 Levels Are Negatively Associated with Bone Mineral Density in Chronic Hemodialysis Patients
Abstract
1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Clinical and Laboratory Data Collection
2.3. Bone Mineral Densitometry
2.4. Statistical Analyses
3. Results
3.1. Study Population Characteristics
3.2. Determinants of BMD of Lumbar Spine in Patients on CHD
3.3. Determinants of BMD of Femoral Neck in Patients on CHD
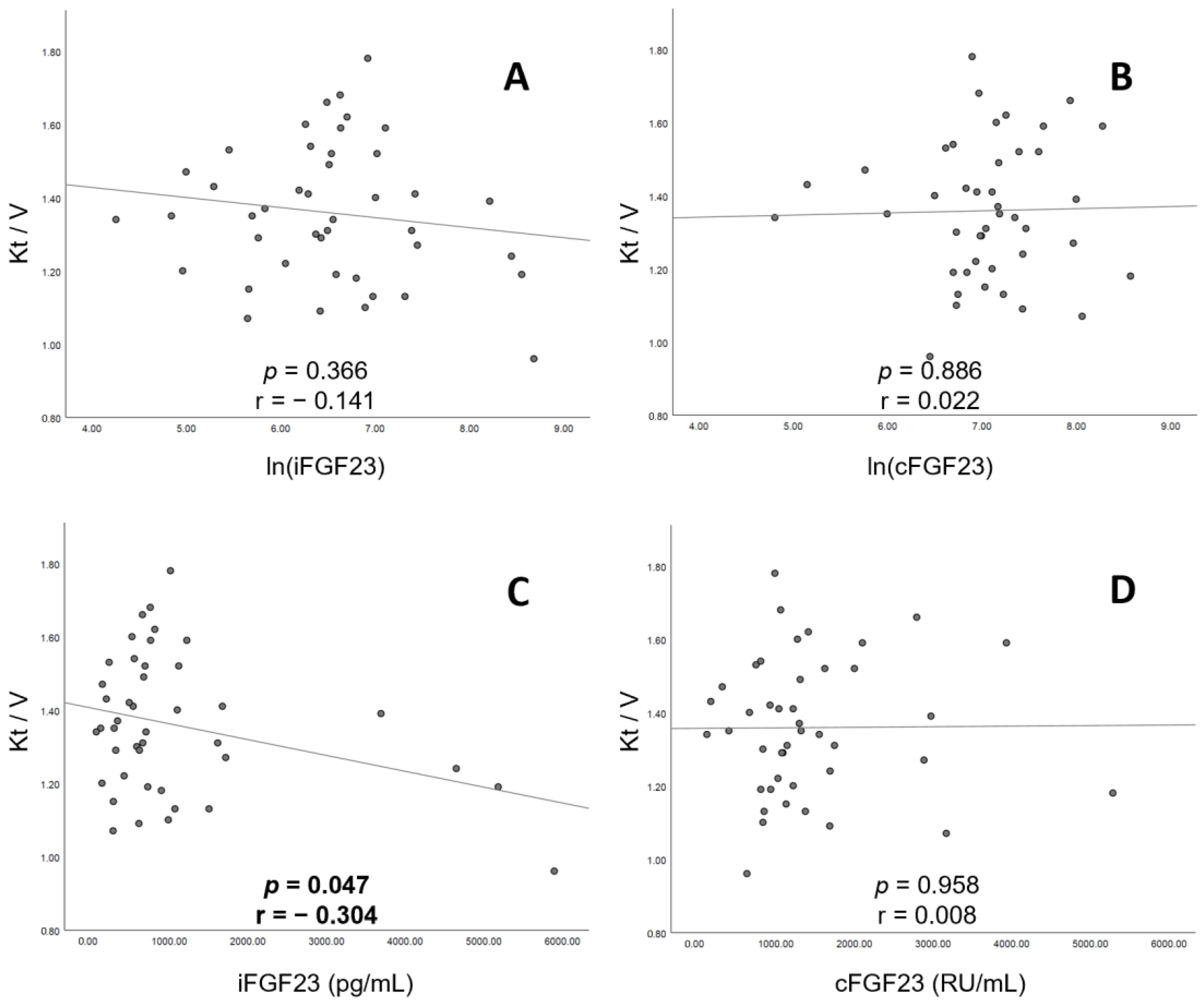
3.4. Correction between Urea Kinetic (Kt/V) and Serum FGF23 in Patients on CHD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.T.; Ng, H.Y.; Chiu, T.T.; Li, L.C.; Pei, S.N.; Kuo, W.H.; Lee, C.T. Association of bone-derived biomarkers with vascular calcification in chronic hemodialysis patients. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 452, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Sugatani, T.; Agapova, O.; Fang, Y. The chronic kidney disease—Mineral bone disorder (CKD-MBD): Advances in pathophysiology. Bone 2017, 100, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.C.; Winkelmayer, W.C. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef]
- Ramli, F.F.; Chin, K.Y. A Review of the Potential Application of Osteocyte-Related Biomarkers, Fibroblast Growth Factor-23, Sclerostin, and Dickkopf-1 in Predicting Osteoporosis and Fractures. Diagnostics 2020, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef]
- Bouquegneau, A.; Evenepoel, P.; Paquot, F.; Malaise, O.; Cavalier, E.; Delanaye, P. Sclerostin within the chronic kidney disease spectrum. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 502, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Figurek, A.; Rroji, M.; Spasovski, G. Sclerostin: A new biomarker of CKD-MBD. Int. Urol. Nephrol. 2020, 52, 107–113. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Verhulst, A.; Babler, A.; D’Haese, P.C.; Evenepoel, P.; Kaesler, N. Sclerostin in chronic kidney disease-mineral bone disorder think first before you block it! Nephrol. Dial. Transplant. 2019, 34, 408–414. [Google Scholar] [CrossRef]
- Grabner, A.; Mazzaferro, S.; Cianciolo, G.; Krick, S.; Capelli, I.; Rotondi, S.; Ronco, C.; La Manna, G.; Faul, C. Fibroblast Growth Factor 23: Mineral Metabolism and Beyond. Contrib. Nephrol. 2017, 190, 83–95. [Google Scholar] [CrossRef]
- Guo, Y.C.; Yuan, Q. Fibroblast growth factor 23 and bone mineralisation. Int. J. Oral. Sci. 2015, 7, 8–13. [Google Scholar] [CrossRef]
- Courbebaisse, M.; Lanske, B. Biology of Fibroblast Growth Factor 23: From Physiology to Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a031260. [Google Scholar] [CrossRef]
- Stubbs, J.R.; He, N.; Idiculla, A.; Gillihan, R.; Liu, S.; David, V.; Hong, Y.; Quarles, L.D. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J. Bone Min. Res. 2012, 27, 38–46. [Google Scholar] [CrossRef]
- Shimada, T.; Urakawa, I.; Isakova, T.; Yamazaki, Y.; Epstein, M.; Wesseling-Perry, K.; Wolf, M.; Salusky, I.B.; Jüppner, H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J. Clin. Endocrinol. Metab. 2010, 95, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; White, K.E. Coupling fibroblast growth factor 23 production and cleavage: Iron deficiency, rickets, and kidney disease. Curr. Opin. Nephrol. Hypertens. 2014, 23, 411–419. [Google Scholar] [CrossRef]
- Baralic, M.; Brkovic, V.; Stojanov, V.; Stankovic, S.; Lalic, N.; Duric, P.; Dukanovic, L.; Kasikovic, M.; Petrovic, M.; Petrovic, M.; et al. Dual Roles of the Mineral Metabolism Disorders Biomarkers in Prevalent Hemodilysis Patients: In Renal Bone Disease and in Vascular Calcification. J. Med. Biochem. 2019, 38, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Martin-Nunez, E.; Hernandez-Carballo, C.; Ferri, C.; Tagua, V.G.; Delgado-Molinos, A.; Lopez-Castillo, A.; Rodriguez-Ramos, S.; Cerro-Lopez, P.; Lopez-Tarruella, V.C.; et al. Fibroblast growth factor 23 expression in human calcified vascular tissues. Aging 2019, 11, 7899–7913. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Leu, J.G.; Fang, Y.W.; Liou, H.H. High Fibroblast Growth Factor 23 Levels Associated with Low Hemoglobin Levels in Patients with Chronic Kidney Disease Stages 3 and 4. Medicine 2016, 95, e3049. [Google Scholar] [CrossRef]
- Mehta, R.; Cai, X.; Hodakowski, A.; Lee, J.; Leonard, M.; Ricardo, A.; Chen, J.; Hamm, L.; Sondheimer, J.; Dobre, M.; et al. Fibroblast Growth Factor 23 and Anemia in the Chronic Renal Insufficiency Cohort Study. Clin. J. Am. Soc. Nephrol. 2017, 12, 1795–1803. [Google Scholar] [CrossRef]
- Xue, C.; Yang, B.; Zhou, C.; Dai, B.; Liu, Y.; Mao, Z.; Yu, S.; Mei, C. Fibroblast Growth Factor 23 Predicts All-Cause Mortality in a Dose-Response Fashion in Pre-Dialysis Patients with Chronic Kidney Disease. Am. J. Nephrol. 2017, 45, 149–159. [Google Scholar] [CrossRef]
- Isakova, T.; Xie, H.; Yang, W.; Xie, D.; Anderson, A.H.; Scialla, J.; Wahl, P.; Gutierrez, O.M.; Steigerwalt, S.; He, J.; et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011, 305, 2432–2439. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Nam, K.H.; Jhee, J.H.; Yun, H.R.; Park, J.T.; Han, S.H.; Chung, W.; Oh, K.H.; Park, S.K.; et al. The effect of interactions between proteinuria, activity of fibroblast growth factor 23 and serum phosphate on renal progression in patients with chronic kidney disease: A result from the KoreaN cohort study for Outcome in patients with Chronic Kidney Disease study. Nephrol. Dial. Transplant. 2020, 35, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Goretti Pinedo, M.; Alon, U.S. Erratum to: Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2017, 32, 1999. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.; Debnam, E.S.; Unwin, R.J. Phosphate homeostasis and the renal-gastrointestinal axis. Am. J. Physiol. Renal. Physiol. 2010, 299, F285–F296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Chen, P. Higher Fibroblast Growth Factor 23 Levels Are Causally Associated with Lower Bone Mineral Density of Heel and Femoral Neck: Evidence from Two-Sample Mendelian Randomization Analysis. Front. Public Health 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Jovanovich, A.; Buzkova, P.; Chonchol, M.; Robbins, J.; Fink, H.A.; de Boer, I.H.; Kestenbaum, B.; Katz, R.; Carbone, L.; Lee, J.; et al. Fibroblast growth factor 23, bone mineral density, and risk of hip fracture among older adults: The cardiovascular health study. J. Clin. Endocrinol. Metab. 2013, 98, 3323–3331. [Google Scholar] [CrossRef] [PubMed]
- Stransky, M.; Rysava, L. Nutrition as prevention and treatment of osteoporosis. Physiol. Res. 2009, 58 (Suppl 1), S7–S11. [Google Scholar] [CrossRef]
- Naylor, K.L.; Garg, A.X.; Zou, G.; Langsetmo, L.; Leslie, W.D.; Fraser, L.A.; Adachi, J.D.; Morin, S.; Goltzman, D.; Lentle, B.; et al. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin. J. Am. Soc. Nephrol. 2015, 10, 646–653. [Google Scholar] [CrossRef]
- Malluche, H.H.; Davenport, D.L.; Cantor, T.; Monier-Faugere, M.C. Bone mineral density and serum biochemical predictors of bone loss in patients with CKD on dialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1254–1262. [Google Scholar] [CrossRef]
- Wu, Q.; Xiao, D.M.; Fan, W.F.; Ye, X.W.; Niu, J.Y.; Gu, Y. Effect of serum fibroblast growth factor-23, matrix Gla protein and Fetuin-A in predicting osteoporosis in maintenance hemodialysis patients. Ther. Apher. Dial. 2014, 18, 427–433. [Google Scholar] [CrossRef]
- Bouksila, M.; Mrad, M.; Kaabachi, W.; Kalai, E.; Smaoui, W.; Rekik, S.; Krir, A.; Issaoui, N.; Hamzaoui, K.; Sahli, H.; et al. Correlation of Fgf23 and Balp with Bone Mineral Density in Hemodialysis Patients. J. Med. Biochem. 2019, 38, 418–426. [Google Scholar] [CrossRef]
- Slouma, M.; Sahli, H.; Bahlous, A.; Laadhar, L.; Smaoui, W.; Rekik, S.; Gharsallah, I.; Sallami, M.; Moussa, F.B.; Elleuch, M.; et al. Mineral bone disorder and osteoporosis in hemodialysis patients. Adv. Rheumatol. 2020, 60, 15. [Google Scholar] [CrossRef] [PubMed]
- Urena Torres, P.; Friedlander, G.; de Vernejoul, M.C.; Silve, C.; Prie, D. Bone mass does not correlate with the serum fibroblast growth factor 23 in hemodialysis patients. Kidney Int. 2008, 73, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jeong, K.H.; Moon, J.Y.; Lee, S.H.; Ihm, C.G.; Rhee, S.Y.; Woo, J.T.; Oh, I.H.; Lee, T.W. The relationship between circulating fibroblast growth factor 23 and bone metabolism factors in Korean hemodialysis patients. Clin. Exp. Nephrol. 2010, 14, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, Y.; Zheng, Y.; Zhou, Z.; Li, Z. Correlation of serum levels of fibroblast growth factor 23 and Klotho protein levels with bone mineral density in maintenance hemodialysis patients. Eur. J. Med. Res. 2018, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Bouma-de Krijger, A.; Vervloet, M.G. Fibroblast growth factor 23: Are we ready to use it in clinical practice? J. Nephrol. 2020, 33, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Cai, M.M.; McMahon, L.P.; Holt, S.G. Biological variability of plasma intact and C-terminal FGF23 measurements. J. Clin. Endocrinol. Metab. 2012, 97, 3357–3365. [Google Scholar] [CrossRef] [PubMed]
- Keselman, H.J.; Algina, J.; Kowalchuk, R.K. The analysis of repeated measures designs: A review. Br. J. Math. Stat. Psychol. 2001, 54, 1–20. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Marchais, S.J.; Guérin, A.P.; Boutouyrie, P.; Métivier, F.; de Vernejoul, M.C. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J. Am. Soc. Nephrol. 2008, 19, 1827–1835. [Google Scholar] [CrossRef]
- Hyder, J.A.; Allison, M.A.; Wong, N.; Papa, A.; Lang, T.F.; Sirlin, C.; Gapstur, S.M.; Ouyang, P.; Carr, J.J.; Criqui, M.H. Association of coronary artery and aortic calcium with lumbar bone density: The MESA Abdominal Aortic Calcium Study. Am. J. Epidemiol. 2009, 169, 186–194. [Google Scholar] [CrossRef]
- Iseri, K.; Dai, L.; Chen, Z.; Qureshi, A.R.; Brismar, T.B.; Stenvinkel, P.; Lindholm, B. Bone mineral density and mortality in end-stage renal disease patients. Clin. Kidney J. 2020, 13, 307–321. [Google Scholar] [CrossRef]
- Vervloet, M.G.; van Ittersum, F.J.; Büttler, R.M.; Heijboer, A.C.; Blankenstein, M.A.; ter Wee, P.M. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin. J. Am. Soc. Nephrol. 2011, 6, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Luo, X.; Huang, W.; Zhang, J.; Peng, C. Fibroblast growth factor 23 and risk of all-cause mortality and cardiovascular events: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2014, 174, 824–828. [Google Scholar] [CrossRef] [PubMed]
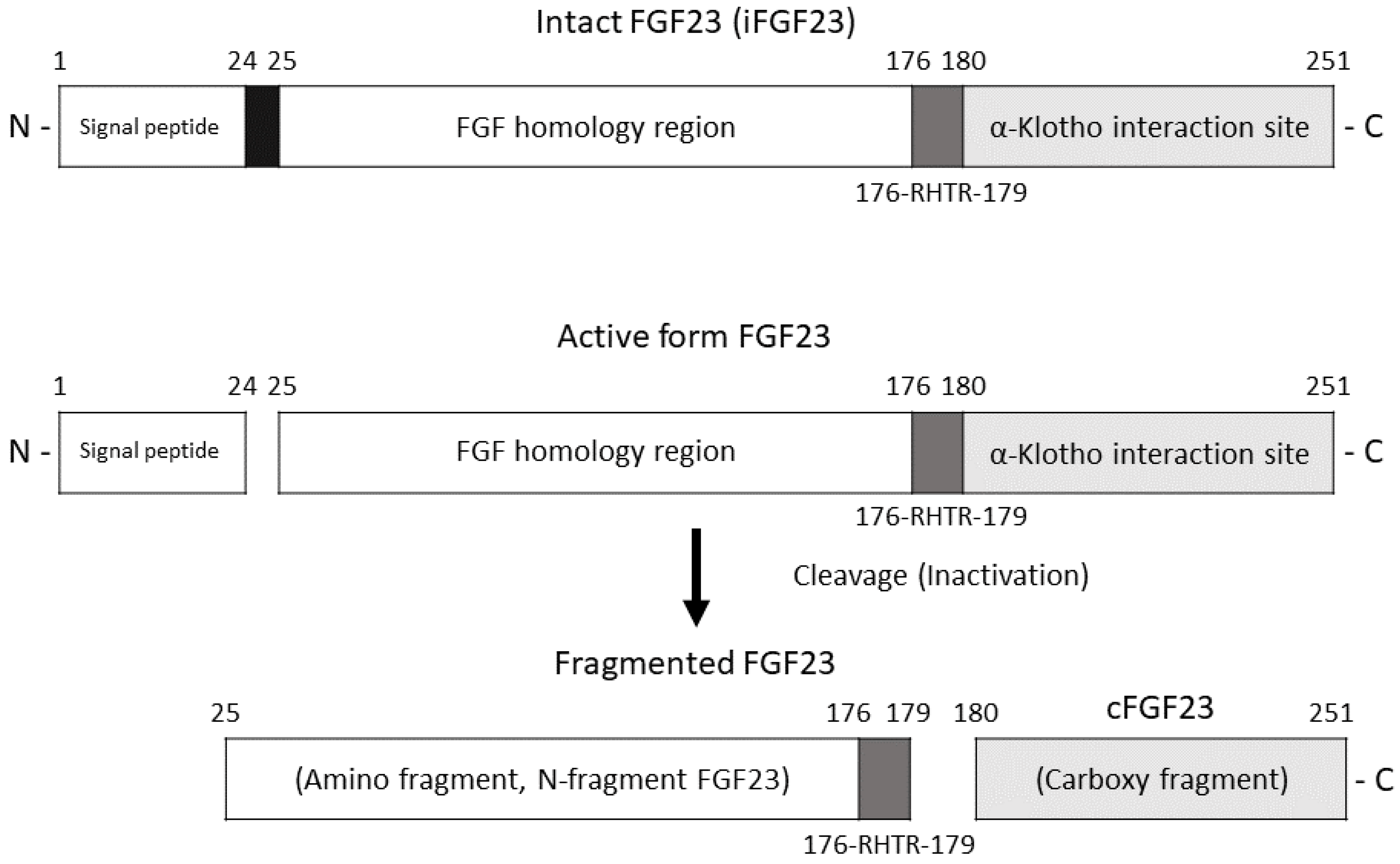
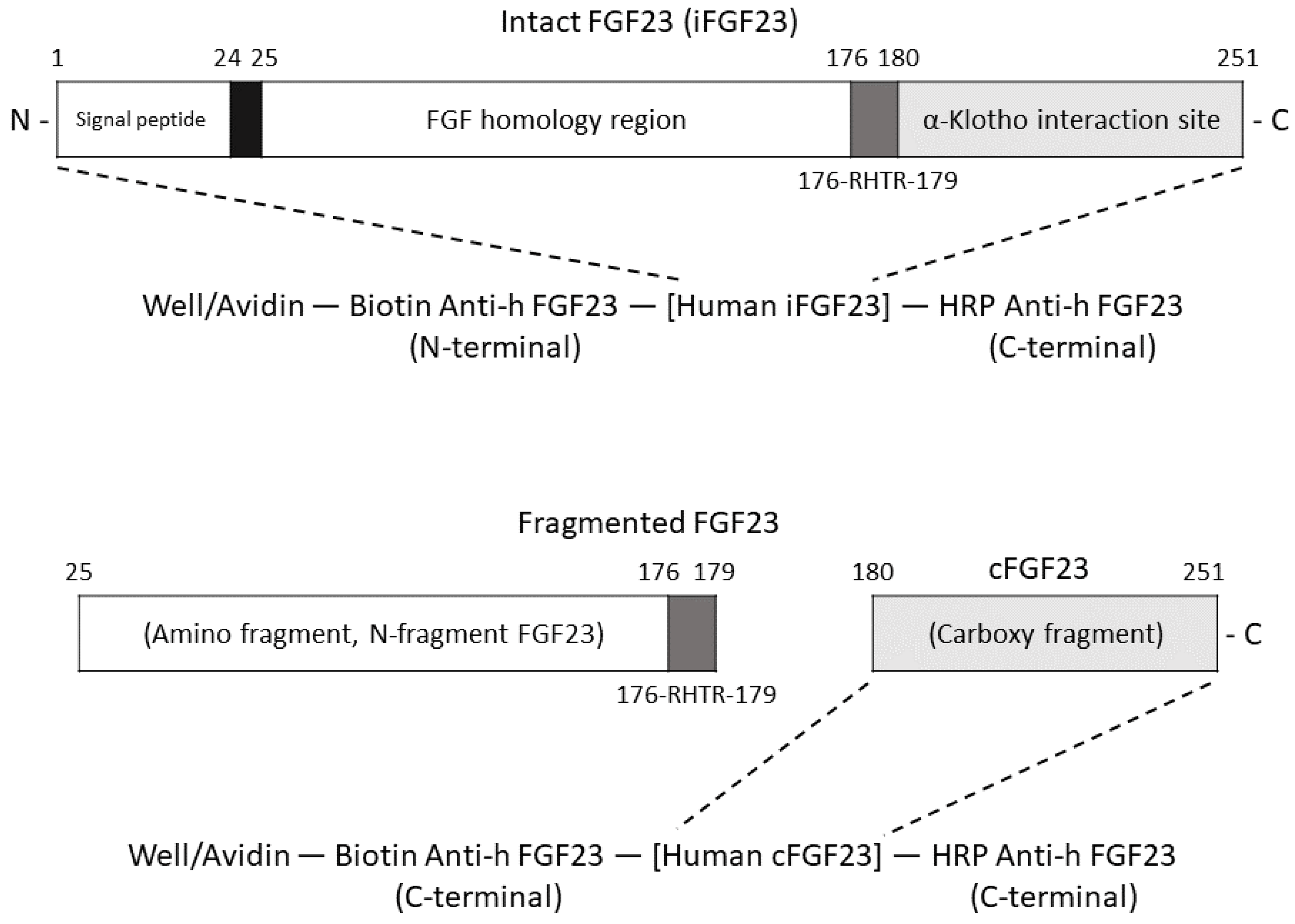
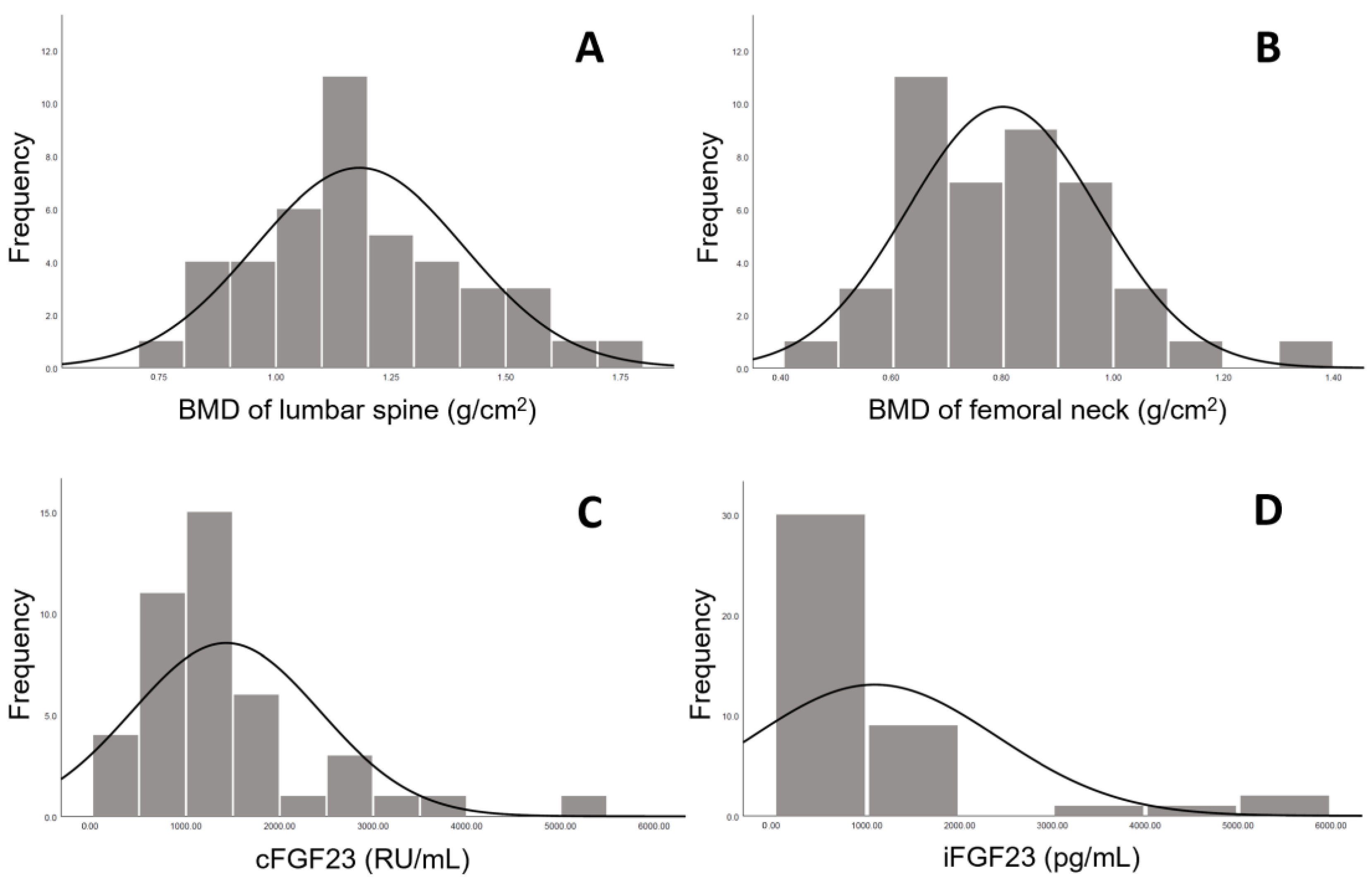
| Characteristic | Value (n = 43) |
|---|---|
| Age (years) | 59.4 ± 12.3 |
| Male (N (%)) | 28 (65.1) |
| Duration of dialysis (year) | 5.4 (2.4, 11.1) |
| Diabetes mellitus (N (%)) | 23 (53.5) |
| Cardiovascular disease (N (%)) | 16 (27) |
| Body mass index (kg/m2) | 23.1 ± 3.4 |
| iFGF23 (pg/mL) | 673 (341, 1100) |
| cFGF23 (RU/mL) | 1141 (835, 1684) |
| DDK1 (pmol/L) | 3.34 (1.64, 6.41) |
| Sclerostin (pmol/L) | 167 ± 71 |
| α-Klotho (pg/mL) | 102 (75, 161) |
| 1,25(OH)2D (pmol/L) | 5.44 (2.35, 10.67) |
| Blood nitrogen (mg/dL) | 75.5 ± 16.3 |
| Creatinine (mg/dL) | 10.71 ± 2.0 |
| Sodium (meq/L) | 138 ± 3 |
| Potassium (meq/L) | 4.6 ± 0.6 |
| Calcium (mg/dL) | 9.3 ± 1.3 |
| Phosphate (mg/dL) | 5.1 ± 1.2 |
| Albumin (g/dL) | 4.1 ± 0.3 |
| Triglyceride (mg/dL) | 106 (69, 186) |
| Cholesterol level (mg/dL) | 166 ± 38 |
| Kt/V | 1.36 ± 0.19 |
| Hemoglobin (g/dL) | 10.5 ±1.0 |
| iPTH (pg/mL) | 214 (89, 426) |
| Alk-P (U/L) | 68 (49, 89) |
| Transferrin saturation (%) | 32.6 ± 12 |
| Cardiothoracic ratio (%) | 47.3 ± 5.5 |
| Lumbar spine BMD (L1–L4) (g/cm3) | 1.18 ± 0.23 |
| Femoral neck BMD (bilateral) (g/cm3) | 0.80 ± 0.17 |
| Parameter | BMD of Lumbar Spine | BMD of Femoral Neck | ||
|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | |
| Age (per 10 years) | 0.01 (−0.05, 0.07) | 0.794 | −0.05 (−0.09, −0.01) | 0.030 |
| Male (vs. female) | 0.06 (−0.09, 0.20) | 0.431 | 0.03 (−0.09, 0.14) | 0.644 |
| Duration of HD (per 10 years) | −0.03 (−0.14, 0.07) | 0.524 | −0.05 (−0.13, 0.03) | 0.208 |
| Diabetes mellitus (vs. none) | 0.09 (−0.05, 0.23) | 0.204 | 0.02 (−0.09, 0.13) | 0.723 |
| CVD (vs. none) | 0.03 (−0.12, 0.17) | 0.726 | 0.01 (−0.11, 0.12) | 0.904 |
| Body mass index (per 1 kg/m2) | 0.03 (0.01, 0.04) | 0.016 | 0.02 (0.01, 0.04) | 0.010 |
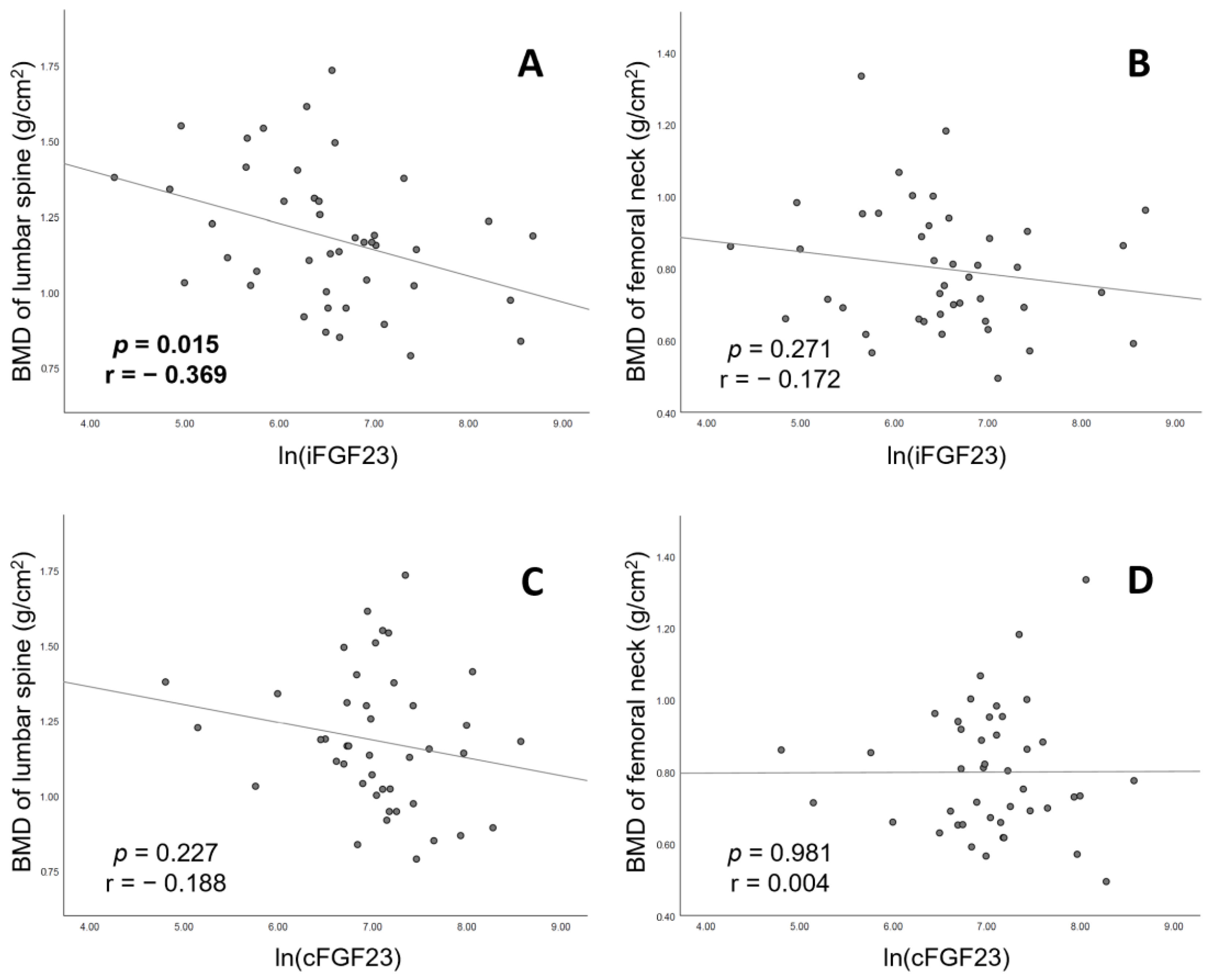
| ln(iFGF23) (per 1 unit) | −0.09 (−0.16, −0.02) | 0.015 | −0.03 (−0.09, 0.03) | 0.271 |
| ln(cFGF23) (per 1 unit) | −0.06 (−0.16, 0.04) | 0.227 | 0.00 (−0.08, 0.08) | 0.981 |
| ln(DDK1) (per 1 unit) | −0.01 (−0.08, 0.06) | 0.772 | −0.01 (−0.06, 0.04) | 0.720 |
| ln(Sclerostin) (per 1 unit) | 0.14 (0.03, 0.24) | 0.012 | 0.05 (−0.03, 0.14) | 0.224 |
| ln(α-Klotho) (per 1 unit) | 0.01 (−0.08, 0.10) | 0.864 | −0.00 (−0.07, 0.07) | 0.912 |
| ln(vitD) (per 1 unit) | −0.01 (−0.05, 0.04) | 0.808 | 0.02 (−0.01, 0.05) | 0.212 |
| Creatinine (per 1 mg/dL) | 0.01 (−0.02, 0.05) | 0.414 | 0.04 (0.01, 0.06) | 0.004 |
| Albumin level (per 1 g/dL) | 0.14 (−0.12, 0.40) | 0.268 | 0.28 (0.13, 0.46) | 0.004 |
| ln(Triglyceride) (per 1 unit) | 0.08 (−0.03, 0.19) | 0.140 | 0.08 (−0.00, 0.16) | 0.059 |
| Cholesterol (per 10 mg/dL) | −0.00 (−0.00, 0.00) | 0.336 | −0.00 (−0.02, 0.01) | 0.264 |
| Kt/V (per 1 unit) | −0.49 (−0.84, −0.14) | 0.007 | −0.38 (−0.64, −0.11) | 0.007 |
| Hemoglobin (per 1 g/dL) | 0.01 (−0.06, 0.09) | 0.747 | −0.01 (−0.06, 0.05) | 0.795 |
| ln(iPTH) (per 1 unit) | −0.03 (−0.08, 0.02) | 0.275 | −0.01 (−0.05, 0.03) | 0.721 |
| ln(Alk-P) (per 1 unit) | −0.05 (−0.19, 0.09) | 0.463 | 0.02 (−0.09, 0.13) | 0.698 |
| Calcium (per 1 mg/dL) | −0.02 (−0.07, 0.04) | 0.497 | −0.02 (−0.06, 0.02) | 0.413 |
| Phosphate (per 1 mg/dL) | 0.01 (−0.05, 0.08) | 0.689 | 0.03 (−0.02, 0.08) | 0.242 |
| Cardiothoracic ratio (per 1%) | −0.25 (−1.55, 1.04) | 0.692 | −0.17 (−1.15, 0.82) | 0.735 |
| BMD of Lumbar Spine | BMD of Femoral Neck | |||
|---|---|---|---|---|
| Estimate (95% CI) | p-Value | Estimate (95% CI) | p-Value | |
| ln(iFGF23) | ||||
| Crude | −0.09 (−0.16, −0.02) | 0.015 | −0.03 (−0.09, 0.03) | 0.271 |
| Model 1 | −0.12 (−0.19, −0.04) | 0.003 | −0.06 (−0.12, −0.01) | 0.028 |
| Model 2 | −0.11 (−0.18, −0.03) | 0.006 | −0.06 (−0.12, −0.01) | 0.032 |
| Model 3 | −0.10 (−0.18, −0.02) | 0.012 | −0.06 (−0.12, −0.003) | 0.037 |
| ln(cFGF23) | ||||
| Crude | −0.06 (−0.16, 0.04) | 0.226 | 0.00 (−0.08, 0.08) | 0.980 |
| Model 1 | −0.08 (−0.18, 0.03) | 0.145 | −0.03 (−0.11, 0.04) | 0.369 |
| Model 2 | −0.07 (−0.18, 0.04) | 0.184 | −0.04 (−0.12, 0.04) | 0.343 |
| Model 3 | −0.05 (−0.17, 0.07) | 0.387 | −0.04 (−0.13, 0.06) | 0.430 |
| Study | Year | Number | Study Design | FGF23 Type * | BMD Method | Parameter Adjusting | Results |
|---|---|---|---|---|---|---|---|
| Urena Torres, P., et al. [32] | 2008 | 99 | Cross-sectional | cFGF23 | DXA | No | cFGF23 was not correlated with BMD. |
| Park, S.-Y., et al. [33] | 2010 | 54 | Cross-sectional | iFGF23 | DXA | Yes | iFGF23 was not correlated with BMD. |
| Malluche, H., et al. [28] | 2014 | 81 | Prospective | cFGF23 | QTC | Yes | cFGF23 was a predictive factor of lower BMD of the spine. |
| DXA | |||||||
| Wu, Q., et al. [29] | 2014 | 64 | Cross-sectional | iFGF23 | DXA | No | iFGF23 was positively associated with osteopenia and osteoporosis. |
| Zheng, S., et al. [34] | 2018 | 125 | Cross-sectional | iFGF23 | DXA | Yes | iFGF23 was not associated with osteopenia and osteoporosis. |
| Bouksila, M., et al. [30] | 2019 | 100 | Cross-sectional | iFGF23 | DXA | No | iFGF23 was positively associated with osteopenia and osteoporosis. |
| Slouma, M., et al. [31] | 2020 | 90 | Cross-sectional | iFGF23 | DXA | No | iFGF23 was associated with osteopenia and osteoporosis of lumbar spine. |
| Our study | 2021 | 43 | Cross-sectional | iFGF23 | DXA | Yes | iFGF23 had a negative correlation with BMD levels of both lumbar spine and femoral neck. |
| cFGF23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-T.; Fang, Y.-W.; Chen, M.; Liou, H.-H.; Lee, C.-J.; Tsai, M.-H. Serum Intact Fibroblast Growth Factor 23 Levels Are Negatively Associated with Bone Mineral Density in Chronic Hemodialysis Patients. J. Clin. Med. 2023, 12, 1550. https://doi.org/10.3390/jcm12041550
Lee W-T, Fang Y-W, Chen M, Liou H-H, Lee C-J, Tsai M-H. Serum Intact Fibroblast Growth Factor 23 Levels Are Negatively Associated with Bone Mineral Density in Chronic Hemodialysis Patients. Journal of Clinical Medicine. 2023; 12(4):1550. https://doi.org/10.3390/jcm12041550
Chicago/Turabian StyleLee, Wen-Teng, Yu-Wei Fang, Mingchih Chen, Hung-Hsiang Liou, Chung-Jen Lee, and Ming-Hsien Tsai. 2023. "Serum Intact Fibroblast Growth Factor 23 Levels Are Negatively Associated with Bone Mineral Density in Chronic Hemodialysis Patients" Journal of Clinical Medicine 12, no. 4: 1550. https://doi.org/10.3390/jcm12041550
APA StyleLee, W.-T., Fang, Y.-W., Chen, M., Liou, H.-H., Lee, C.-J., & Tsai, M.-H. (2023). Serum Intact Fibroblast Growth Factor 23 Levels Are Negatively Associated with Bone Mineral Density in Chronic Hemodialysis Patients. Journal of Clinical Medicine, 12(4), 1550. https://doi.org/10.3390/jcm12041550







