Improvement of Left Ventricular Global Longitudinal Strain after 6-Month Therapy with GLP-1RAs Semaglutide and Dulaglutide in Type 2 Diabetes Mellitus: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Screening Protocol and Data Acquisition
2.3. Echocardiographic Evaluation
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Islam, R.M.; Wang, J.; Hird, T.R.; Pavkov, M.E.; Gregg, E.W.; Salim, A.; Tabesh, M.; Koye, D.N.; Harding, J.L.; et al. A systematic review of trends in all-cause mortality among people with diabetes. Diabetologia 2020, 63, 1718–1735. [Google Scholar] [CrossRef] [PubMed]
- Schramm, T.K.; Gislason, G.H.; Køber, L.; Rasmussen, S.; Rasmussen, J.N.; Abildstrøm, S.Z.; Hansen, M.L.; Folke, F.; Buch, P.; Madsen, M.; et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: A population study of 3.3 million people. Circulation 2008, 117, 1945–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budoff, M.J.; Raggi, P.; Beller, G.A.; Berman, D.S.; Druz, R.S.; Malik, S.; Rigolin, V.H.; Weigold, W.G.; Soman, P. Noninvasive Cardiovascular Risk Assessment of the Asymptomatic Diabetic Patient: The Imaging Council of the American College of Cardiology. JACC Cardiovasc. Imaging 2016, 9, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Maffei, E.; Seitun, S.; Nieman, K.; Martini, C.; Guaricci, A.I.; Tedeschi, C.; Weustink, A.C.; Mollet, N.R.; Berti, E.; Grilli, R.; et al. Assessment of coronary artery disease and calcified coronary plaque burden by computed tomography in patients with and without diabetes mellitus. Eur. Radiol. 2011, 21, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Guaricci, A.I.; Lorenzoni, V.; Guglielmo, M.; Mushtaq, S.; Muscogiuri, G.; Cademartiri, F.; Rabbat, M.; Andreini, D.; Serviddio, G.; Gaibazzi, N.; et al. Prognostic relevance of subclinical coronary and carotid atherosclerosis in a diabetic and nondiabetic asymptomatic population. Clin. Cardiol. 2018, 41, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Guaricci, A.I.; Pontone, G.; Fusini, L.; De Luca, M.; Cafarelli, F.P.; Guglielmo, M.; Baggiano, A.; Beltrama, V.; Muscogiuri, G.; Mushtaq, S.; et al. Additional value of inflammatory biomarkers and carotid artery disease in prediction of significant coronary artery disease as assessed by coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1049–1056. [Google Scholar] [CrossRef]
- Esposito, A.; Francone, M.; Andreini, D.; Buffa, V.; Cademartiri, F.; Carbone, I.; Clemente, A.; Guaricci, A.I.; Guglielmo, M.; Indolfi, C.; et al. SIRM-SIC appropriateness criteria for the use of Cardiac Computed Tomography. Part 1: Congenital heart diseases, primary prevention, risk assessment before surgery, suspected CAD in symptomatic patients, plaque and epicardial adipose tissue characterization, and functional assessment of stenosis. Radiol. Med. 2021, 126, 1236–1248. [Google Scholar] [CrossRef]
- Htike, Z.Z.; Zaccardi, F.; Papamargaritis, D.; Webb, D.R.; Khunti, K.; Davies, M.J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes. Metab. 2017, 19, 524–536. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.S.; Park, J.H.; Won, J.C. The Role of Glucagon-Like Peptide 1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors in Reducing Cardiovascular Events in Patients with Type 2 Diabetes. Endocrinol. Metab. 2019, 34, 106–116. [Google Scholar] [CrossRef]
- Brunton, S.A.; Wysham, C.H. GLP-1 receptor agonists in the treatment of type 2 diabetes: Role and clinical experience to date. Postgrad. Med. 2020, 132, 3–14. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Furtado, R.H.M.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2022–2031. [Google Scholar] [CrossRef]
- Sheahan, K.H.; Wahlberg, E.A.; Gilbert, M.P. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad. Med. J. 2020, 96, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Meier, J.J.; Cavender, M.A.; Abd El Aziz, M.; Drucker, D.J. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation 2017, 136, 849–870. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J.; et al. GLP-1 receptor agonists (GLP-1RAs): Cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef]
- Zhang, D.P.; Xu, L.; Wang, L.F.; Wang, H.J.; Jiang, F. Effects of antidiabetic drugs on left ventricular function/dysfunction: A systematic review and network meta-analysis. Cardiovasc. Diabetol. 2020, 19, 10. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.J.; Marwick, T.H.; Haluska, B.A.; Leano, R.; Hordern, M.D.; Hare, J.L.; Fang, Z.Y.; Prins, J.B.; Stanton, T. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart 2015, 101, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Merlo, M.; Porcari, A.; Pagura, L.; Cameli, M.; Vergaro, G.; Musumeci, B.; Biagini, E.; Canepa, M.; Crotti, L.; Imazio, M.; et al. A national survey on prevalence of possible echocardiographic red flags of amyloid cardiomyopathy in consecutive patients undergoing routine echocardiography: Study design and patients characterization-the first insight from the AC-TIVE Study. Eur. J. Prev. Cardiol. 2021, 29, e173–e177. [Google Scholar] [CrossRef]
- Minciună, I.-A.; Hilda Orășan, O.; Minciună, I.; Lazar, A.-L.; Sitar-Tăut, A.V.; Oltean, M.; Tomoaia, R.; Puiu, M.; Sitar-Tăut, D.-A.; Pop, D.; et al. Assessment of subclinical diabetic cardiomyopathy by speckle-tracking imaging. Eur. J. Clin. Investig. 2021, 51, e13475. [Google Scholar] [CrossRef]
- Lorenzo-Almorós, A.; Cepeda-Rodrigo, J.M.; Lorenzo, Ó. Diabetic cardiomyopathy. Rev. Clin. Esp. 2022, 222, 100–111. [Google Scholar] [CrossRef]
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic Cardiomyopathy: Definition, Diagnosis, and Therapeutic Implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef]
- Cosson, S.; Kevorkian, J.P. Left ventricular diastolic dysfunction: An early sign of diabetic cardiomyopathy? Diabetes Metab. 2003, 29, 455–466. [Google Scholar] [CrossRef]
- Ng, A.C.; Delgado, V.; Bertini, M.; van der Meer, R.W.; Rijzewijk, L.J.; Shanks, M.; Nucifora, G.; Smit, J.W.; Diamant, M.; Romijn, J.A.; et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am. J. Cardiol. 2009, 104, 1398–1401. [Google Scholar] [CrossRef]
- Karagöz, A.; Bezgin, T.; Kutlutürk, I.; Külahçıoğlu, S.; Tanboğa, I.H.; Güler, A.; Karabay, C.Y.; Oduncu, V.; Aksoy, H.; Kırma, C. Subclinical left ventricular systolic dysfunction in diabetic patients and its association with retinopathy: A 2D speckle tracking echocardiography study. Herz 2015, 40 (Suppl. 3), 240–246. [Google Scholar] [CrossRef]
- Zoroufian, A.; Razmi, T.; Taghavi-Shavazi, M.; Lotfi-Tokaldany, M.; Jalali, A. Evaluation of subclinical left ventricular dysfunction in diabetic patients: Longitudinal strain velocities and left ventricular dyssynchrony by two-dimensional speckle tracking echocardiography study. Echocardiography 2014, 31, 456–463. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Prins, J.B.; Marwick, T.H. Diabetic cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Endocr. Rev. 2004, 25, 543–567. [Google Scholar] [CrossRef]
- Mandavia, C.H.; Aroor, A.R.; Demarco, V.G.; Sowers, J.R. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci. 2013, 92, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wang, S.; Cai, L. Diabetic cardiomyopathy and its mechanisms: Role of oxidative stress and damage. J. Diabetes Investig. 2014, 5, 623–634. [Google Scholar] [CrossRef]
- Dia, M.; Gomez, L.; Thibault, H.; Tessier, N.; Leon, C.; Chouabe, C.; Ducreux, S.; Gallo-Bona, N.; Tubbs, E.; Bendridi, N.; et al. Reduced reticulum-mitochondria Ca(2+) transfer is an early and reversible trigger of mitochondrial dysfunctions in diabetic cardiomyopathy. Basic Res. Cardiol. 2020, 115, 74. [Google Scholar] [CrossRef]
- Jaquenod De Giusti, C.; Palomeque, J.; Mattiazzi, A. Ca(2+) mishandling and mitochondrial dysfunction: A converging road to prediabetic and diabetic cardiomyopathy. Pflügers Arch. 2022, 474, 33–61. [Google Scholar] [CrossRef]
- DeNicola, M.; Du, J.; Wang, Z.; Yano, N.; Zhang, L.; Wang, Y.; Qin, G.; Zhuang, S.; Zhao, T.C. Stimulation of glucagon-like peptide-1 receptor through exendin-4 preserves myocardial performance and prevents cardiac remodeling in infarcted myocardium. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E630–E643. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.D.; Huang, H.F.; Yang, Q.; Chen, X.Q. Liraglutide improves myocardial fibrosis after myocardial infarction through inhibition of CTGF by activating cAMP in mice. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4648–4656. [Google Scholar] [CrossRef]
- Sokos, G.G.; Nikolaidis, L.A.; Mankad, S.; Elahi, D.; Shannon, R.P. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J. Card. Fail. 2006, 12, 694–699. [Google Scholar] [CrossRef]
- Chen, W.R.; Chen, Y.D.; Tian, F.; Yang, N.; Cheng, L.Q.; Hu, S.Y.; Wang, J.; Yang, J.J.; Wang, S.F.; Gu, X.F. Effects of Liraglutide on Reperfusion Injury in Patients With ST-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2016, 9, e005146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arturi, F.; Succurro, E.; Miceli, S.; Cloro, C.; Ruffo, M.; Maio, R.; Perticone, M.; Sesti, G.; Perticone, F. Liraglutide improves cardiac function in patients with type 2 diabetes and chronic heart failure. Endocrine 2017, 57, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, D.; Frick, M.; Ullman, B.; Nystrom, T. Exenatide infusion decreases atrial natriuretic peptide levels by reducing cardiac filling pressures in type 2 diabetes patients with decompensated congestive heart failure. Diabetol. Metab. Syndr. 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonborg, J.; Kelbaek, H.; Vejlstrup, N.; Botker, H.E.; Kim, W.Y.; Holmvang, L.; Jorgensen, E.; Helqvist, S.; Saunamaki, K.; Terkelsen, C.J.; et al. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ. Cardiovasc. Interv. 2012, 5, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ussher, J.R.; Drucker, D.J. Cardiovascular actions of incretin-based therapies. Circ. Res. 2014, 114, 1788–1803. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhong, J.; Lin, H.; Zhao, Z.; Yan, Z.; He, H.; Ni, Y.; Liu, D.; Zhu, Z. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: A meta-analysis of clinical trials. Diabetes Obes. Metab. 2013, 15, 737–749. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Helmstädter, J.; Keppeler, K.; Küster, L.; Münzel, T.; Daiber, A.; Steven, S. Glucagon-like peptide-1 (GLP-1) receptor agonists and their cardiovascular benefits-The role of the GLP-1 receptor. Br. J. Pharmacol. 2022, 179, 659–676. [Google Scholar] [CrossRef]
- Song, R.; Qian, H.; Wang, Y.; Li, Q.; Li, D.; Chen, J.; Yang, J.; Zhong, J.; Yang, H.; Min, X.; et al. Research Progress on the Cardiovascular Protective Effect of Glucagon-Like Peptide-1 Receptor Agonists. J. Diabetes Res. 2022, 2022, 4554996. [Google Scholar] [CrossRef]
- Ravassa, S.; Zudaire, A.; Carr, R.D.; Diez, J. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1361–H1372. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, M.; Kanemoto, S.; Leshnower, B.G.; Albone, E.F.; Hinmon, R.; Plappert, T.; Gorman, J.H., 3rd; Gorman, R.C. Single dose GLP-1-Tf ameliorates myocardial ischemia/reperfusion injury. J. Surg. Res. 2011, 165, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Guaricci, A.I.; Chiarello, G.; Gherbesi, E.; Fusini, L.; Soldato, N.; Siena, P.; Ursi, R.; Ruggieri, R.; Guglielmo, M.; Muscogiuri, G.; et al. Coronary-specific quantification of myocardial deformation by strain echocardiography may disclose the culprit vessel in patients with non-ST-segment elevation acute coronary syndrome. Eur. Heart J. Open 2022, 2, oeac010. [Google Scholar] [CrossRef]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popović, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 98, 1442–1448. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef]
- Lambadiari, V.; Pavlidis, G.; Kousathana, F.; Varoudi, M.; Vlastos, D.; Maratou, E.; Georgiou, D.; Andreadou, I.; Parissis, J.; Triantafyllidi, H.; et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 8. [Google Scholar] [CrossRef] [Green Version]
- Bizino, M.B.; Jazet, I.M.; Westenberg, J.J.M.; van Eyk, H.J.; Paiman, E.H.M.; Smit, J.W.A.; Lamb, H.J. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: Randomized placebo-controlled trial. Cardiovasc. Diabetol. 2019, 18, 55, Correction in Cardiovasc. Diabetol. 2019, 18, 101. [Google Scholar] [CrossRef] [Green Version]
- Yagi, K.; Imamura, T.; Tada, H.; Chujo, D.; Liu, J.; Shima, Y.; Ohbatake, A.; Miyamoto, Y.; Okazaki, S.; Ito, N.; et al. Diastolic Cardiac Function Improvement by Liraglutide Is Mainly Body Weight Reduction Dependent but Independently Contributes to B-Type Natriuretic Peptide Reduction in Patients with Type 2 Diabetes with Preserved Ejection Fraction. J. Diabetes Res. 2021, 2021, 8838026. [Google Scholar] [CrossRef]
- Raev, D.C. Which left ventricular function is impaired earlier in the evolution of diabetic cardiomyopathy? An echocardiographic study of young type I diabetic patients. Diabetes Care 1994, 17, 633–639. [Google Scholar] [CrossRef]
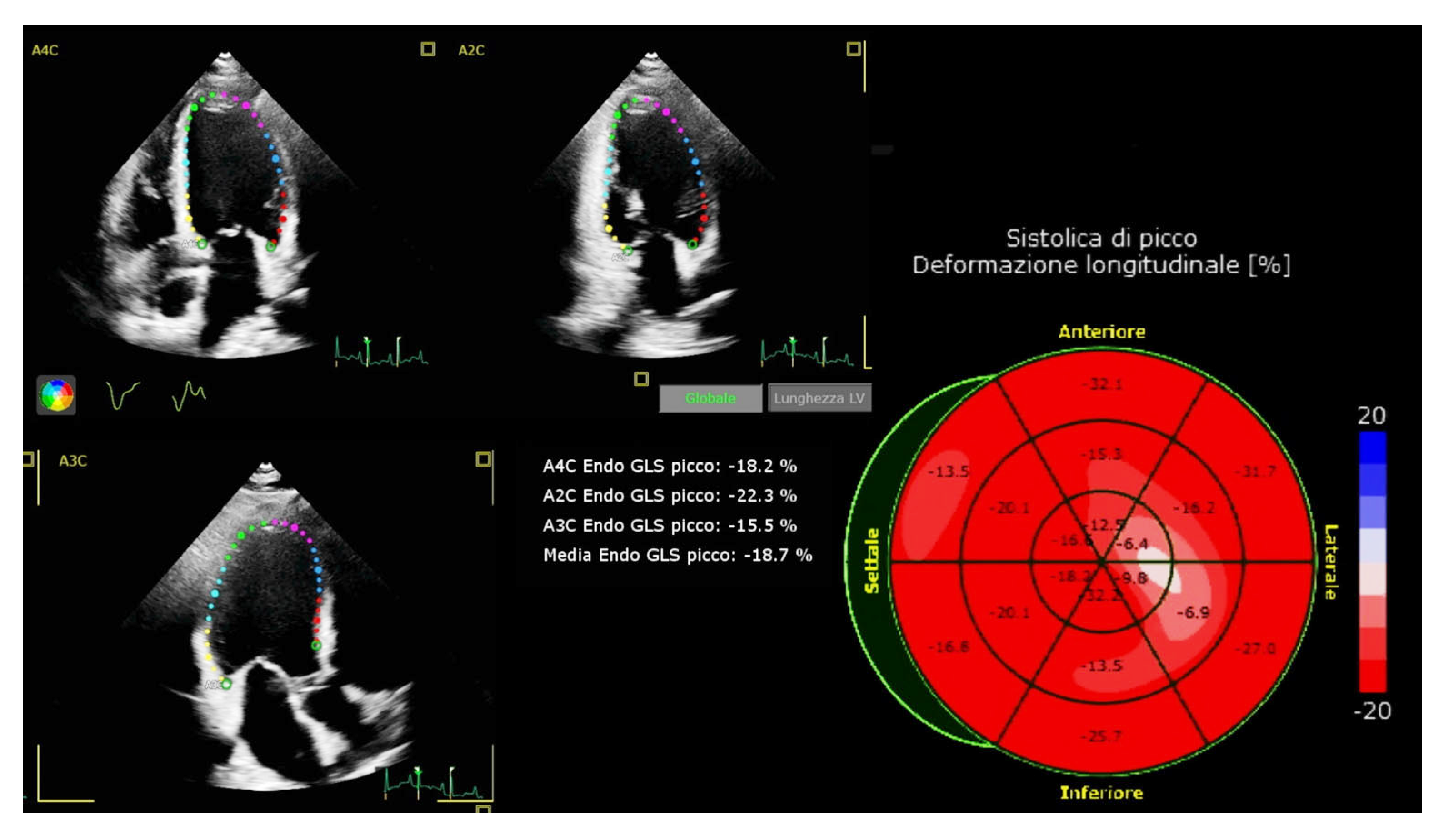
| Variable | All Patients (n = 22) |
|---|---|
| Demographic and anthropometric data | |
| Age, years, mean ± SD | 65 ± 10 |
| Sex, male, n (%) | 14 (64) |
| BSA, m2, mean ± sd | 2.03 ± 0.21 |
| BMI, m2/Kg | 33.9 ± 5.1 |
| Clinical data | |
| SBP, mmHg, mean ± sd | 133 ± 13 |
| DBP, mmHg, mean ± sd | 79 ± 10 |
| Heart rate, bpm | 71 ± 10 |
| Cardiovascular risk factors other than DMT2 | |
| Hypertension, yes, n (%) | 22 (100) |
| Dyslipidaemia, yes, n (%) | 21 (95) |
| Smoking habit, yes, n (%) | 15 (68) |
| Obesity, yes, n (%) | 19 (86) |
| Family history of cardiovascular disease, yes, n (%) | 20 (91) |
| Comorbidities | |
| CAD, yes, n (%) | 2 (9) |
| Previous IM, yes, n (%) | 2 (9) |
| COPD, yes, n (%) | 7 (32) |
| CKD, yes, n (%) | 2 (9) |
| PAD, yes, n (%) | 3 (14) |
| Thyroid disorders, yes, n (%) | 4 (18) |
| Medical therapy | |
| ACE-I/ARB, yes, n (%) | 18 (82) |
| Beta-blockers, yes, n (%) | 10 (45) |
| Diuretics, yes, n (%) | 8 (36) |
| Anticoagulant therapy, yes, n (%) | 2 (9) |
| Anti-thrombotic agents, yes, n (%) | 11 (50) |
| Statins, yes, n (%) | 15 (68) |
| Metformin, yes, n (%) | 21 (95) |
| Insulin, yes, n (%) | 0 (0) |
| Laboratory testing | |
| Creatinine, mg/dL, median [IQR] | 0.87 [0,31] |
| eGFR, mL/min/1.73 m2, median [IQR] | 83 [26] |
| Uric acid, mg/dL, median [IQR] | 5.6 [2,9] |
| Plasma sodium, mEq/L, mean ± sd | 139.5 ± 1.8 |
| Plasma potassium, mEq/L, median [IQR] | 4.2 [0,4] |
| Haemoglobin, g/dL, mean ± sd | 13.6 ± 1.4 |
| Glycated haemoglobin, mMol/Mol, median [IQR] | 49 [16] |
| Fasting plasma glucose, mg/dL, median [IQR] | 109 [25] |
| Total bilirubin, mg/dL, median [IQR] | 0.6 [0,2] |
| ALT, mg/dL, median [IQR] | 25 [14] |
| Variable | Baseline (n = 22) | After 6 Months (n = 22) | Difference (n = 22) | p Value |
|---|---|---|---|---|
| LV septum, mm, median [IQR] | 12 [1] | 12 [1] | 0 [0] | 0.257 |
| LV PW, mm, median [IQR] | 11 [1] | 11 [1] | 0 [0] | 0.206 |
| LV EDD, mm, mean ± sd | 47 ± 5 | 46 ± 5 | 1 ± 3 | 0.158 |
| LV Mass, g, mean ± sd | 154 ± 22 | 153 ± 21 | 1 ± 2 | 0.560 |
| LV EDV, mL, median [IQR] | 98 [24] | 98 [23] | 0 [11] | 0.835 |
| LV ESV, mL, median [IQR] | 40 [19] | 41 [16] | 0 [6] | 0.615 |
| LV EF, %, median [IQR] | 59 [7] | 58 [8] | 1 [6] | 0.776 |
| LA APD, mm, median [IQR] | 41 [3] | 40 [3] | 0 [2] | 0.273 |
| LA Area, cm2, median [IQR] | 17 [5] | 17 [4] | 1 [2] | 0.024 |
| LA Volume, mL, mean ± sd | 50 ± 13 | 48 ± 13 | 2 ± 7 | 0.278 |
| RVOT, mm, mean ± sd | 31 ± 4 | 32 ± 3 | −1 ± 10 | 0.294 |
| RV basal, median [IQR] | 35 [4] | 36 [4] | −1 [2] | 0.059 |
| RV EDA, cm2, mean ± sd | 15 ± 2 | 15 ± 2 | 0 ±2 | 0.102 |
| RV ESA, cm2, mean ± sd | 8 ± 1 | 7 ± 1 | 1 ± 1 | 0.005 |
| RV FAC, %, mean ± sd | 46 ± 6 | 48 ± 8 | −2 ± 8 | 0.172 |
| E wave, cm/s, mean ± sd | 64 ± 13 | 64 ± 13 | 0 ± 16 | 0.863 |
| A wave, cm/s, mean ± sd | 85 ± 18 | 83 ± 14 | 2 ± 13 | 0.545 |
| E/A, median [IQR] | 0.75 [0,28] | 0.78 [0,19] | −0.02 [0,15] | 0.433 |
| DT, msec, mean ± sd | 175 ± 45 | 166 ± 39 | 9 ± 34 | 0.236 |
| S’ septal, cm/s, mean ± sd | 8 ± 2 | 8 ± 1 | 0 ± 2 | 0.390 |
| E’ septal, cm/s, mean ± sd | 9 ± 2 | 8 ± 2 | 1 ± 2 | 0.280 |
| A’ septal, cm/s, mean ± sd | 10 ± 2 | 11 ± 2 | −1 ± 2 | 0.196 |
| S’ mitral, cm/s, mean ± sd | 9 ± 2 | 9 ± 2 | 0 ± 2 | 0.853 |
| E’ mitral, cm/s, mean ± sd | 10 ± 3 | 10 ± 3 | 0 ± 2 | 0.741 |
| A’ mitral, cm/s, mean ± sd | 11 ± 3 | 11 ± 3 | 0 ± 2 | 0.343 |
| E/E’, mean ± sd | 7 ± 2 | 7 ± 2 | 0 ± 2 | 0.589 |
| S’ tricuspid, cm/s, median [IQR] | 12 [2] | 12 [3] | 0 [2] | 0.751 |
| E’ tricuspid, cm/s, median [IQR] | 11 [2] | 11 [2] | 0 [1] | 0.842 |
| A’ tricuspid, cm/s, median [IQR] | 9 [5] | 13 [6] | −1 [5] | 0.003 |
| TAPSE, mm, mean ± sd | 21 ± 2 | 22 ± 2 | −1 ± 2 | 0.392 |
| SPAP, mmHg, median [IQR] | 26 [10] | 26 [9] | 0 [4] | 0.362 |
| IVC diameter, mm, median [IQR] | 18 [2] | 18 [3] | 0 [1] | 0.361 |
| IVC collapse, no, n (%) | 22 (100) | 21 (95) | - | nv |
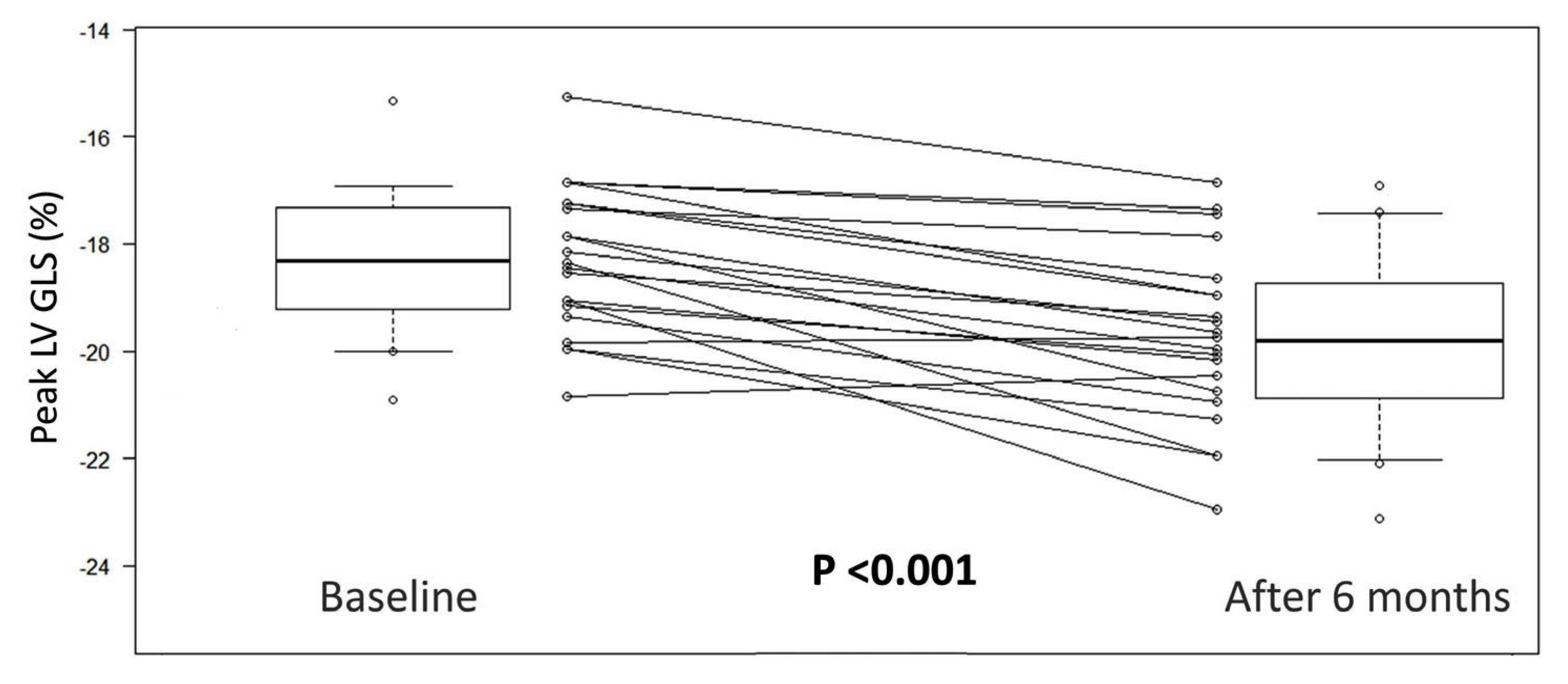
| LV GLS, %, mean ± sd | −18.0 ± 1.8 | −19.5 ± 2.1 | −1.4 ± 1.1 | <0.001 |
| Variabile | Coefficient | p Value |
|---|---|---|
| Age | 0.239 | 0.285 |
| Sex | −0.045 | 0.843 |
| BMI | 0.364 | 0.096 |
| Smoking habit | −0.431 | 0.045 |
| SBP | 0.059 | 0.796 |
| DBP | −0.237 | 0.287 |
| Heart Rate | 0.054 | 0.817 |
| Therapy with metformin | −0.209 | 0.351 |
| Gylicated haemoglobin | 0.019 | 0.933 |
| Fasting plasma glucose | −0.007 | 0.975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile, P.; Guaricci, A.I.; Piazzolla, G.; Volpe, S.; Vozza, A.; Benedetto, M.; Carella, M.C.; Santoro, D.; Monitillo, F.; Baggiano, A.; et al. Improvement of Left Ventricular Global Longitudinal Strain after 6-Month Therapy with GLP-1RAs Semaglutide and Dulaglutide in Type 2 Diabetes Mellitus: A Pilot Study. J. Clin. Med. 2023, 12, 1586. https://doi.org/10.3390/jcm12041586
Basile P, Guaricci AI, Piazzolla G, Volpe S, Vozza A, Benedetto M, Carella MC, Santoro D, Monitillo F, Baggiano A, et al. Improvement of Left Ventricular Global Longitudinal Strain after 6-Month Therapy with GLP-1RAs Semaglutide and Dulaglutide in Type 2 Diabetes Mellitus: A Pilot Study. Journal of Clinical Medicine. 2023; 12(4):1586. https://doi.org/10.3390/jcm12041586
Chicago/Turabian StyleBasile, Paolo, Andrea Igoren Guaricci, Giuseppina Piazzolla, Sara Volpe, Alfredo Vozza, Marina Benedetto, Maria Cristina Carella, Daniela Santoro, Francesco Monitillo, Andrea Baggiano, and et al. 2023. "Improvement of Left Ventricular Global Longitudinal Strain after 6-Month Therapy with GLP-1RAs Semaglutide and Dulaglutide in Type 2 Diabetes Mellitus: A Pilot Study" Journal of Clinical Medicine 12, no. 4: 1586. https://doi.org/10.3390/jcm12041586
APA StyleBasile, P., Guaricci, A. I., Piazzolla, G., Volpe, S., Vozza, A., Benedetto, M., Carella, M. C., Santoro, D., Monitillo, F., Baggiano, A., Mushtaq, S., Fusini, L., Fazzari, F., Forleo, C., Ribecco, N., Pontone, G., Sabbà, C., & Ciccone, M. M. (2023). Improvement of Left Ventricular Global Longitudinal Strain after 6-Month Therapy with GLP-1RAs Semaglutide and Dulaglutide in Type 2 Diabetes Mellitus: A Pilot Study. Journal of Clinical Medicine, 12(4), 1586. https://doi.org/10.3390/jcm12041586










