Are Predictors for Overall Mortality in COPD Patients Robust over Time?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurements
2.2.1. Physical Capacity
2.2.2. Respiratory Variables
2.2.3. Blood Gas Analysis
2.2.4. COPD-Specific Health Status
2.2.5. Comorbidities and Survival
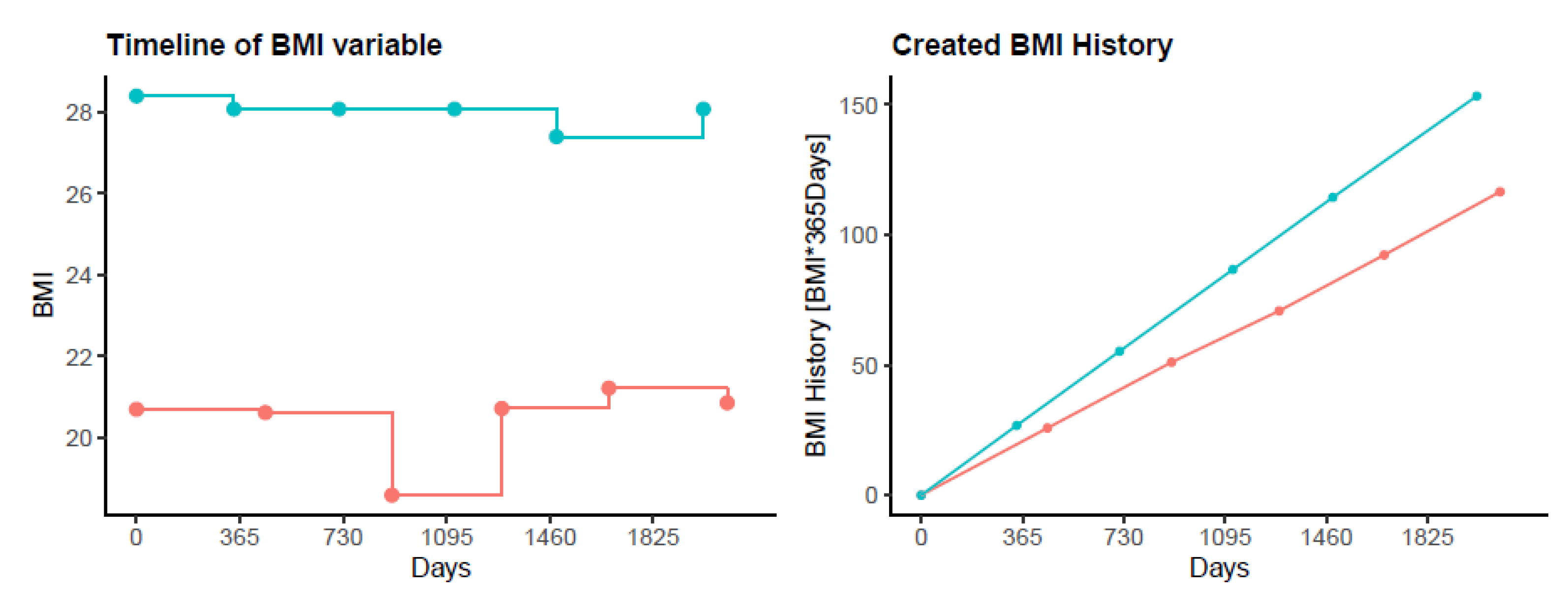
2.2.6. Exacerbation History
2.3. Data Analysis and Statistics
3. Results
3.1. Study Participants
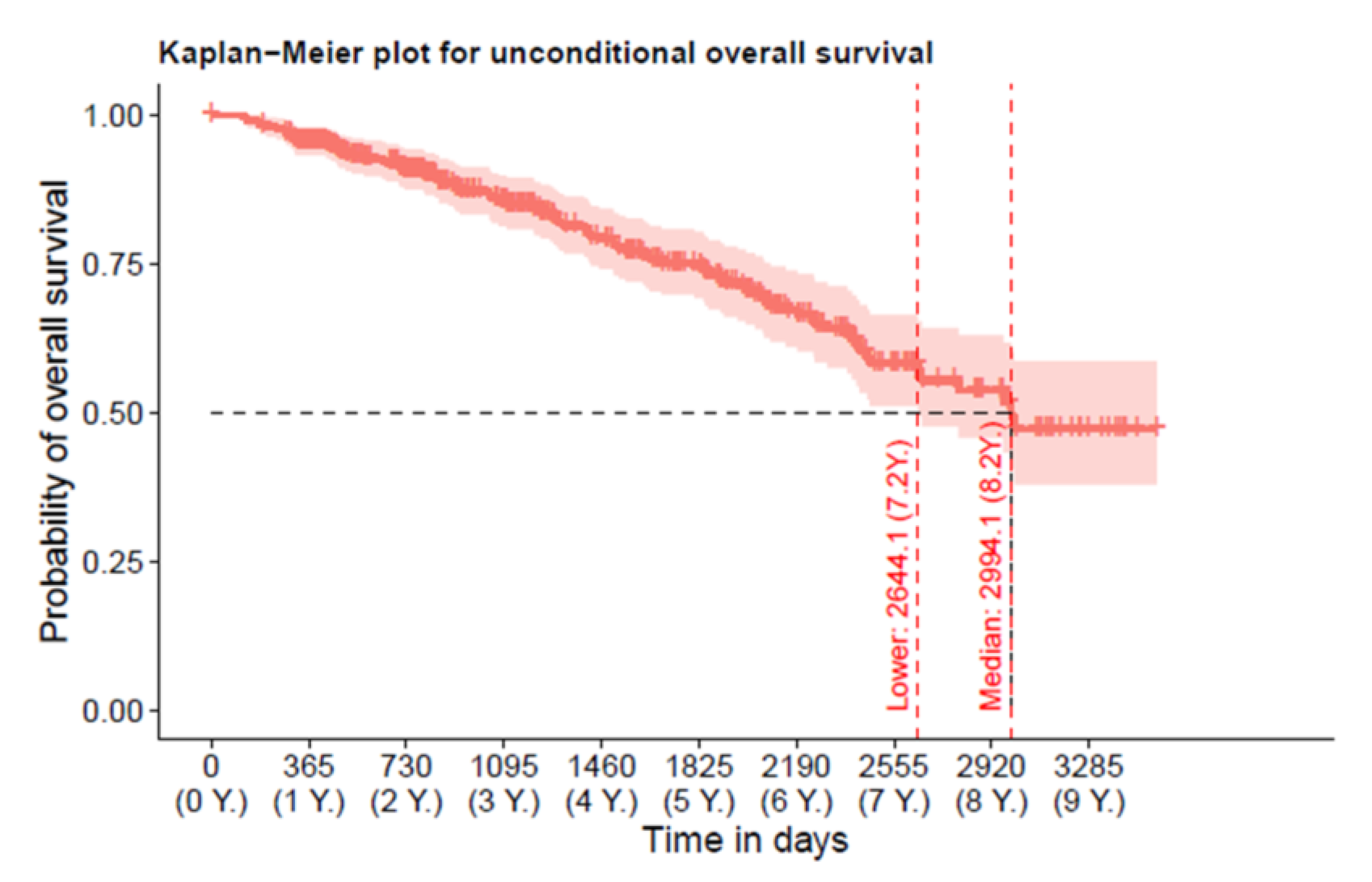
3.2. Overall-Mortality
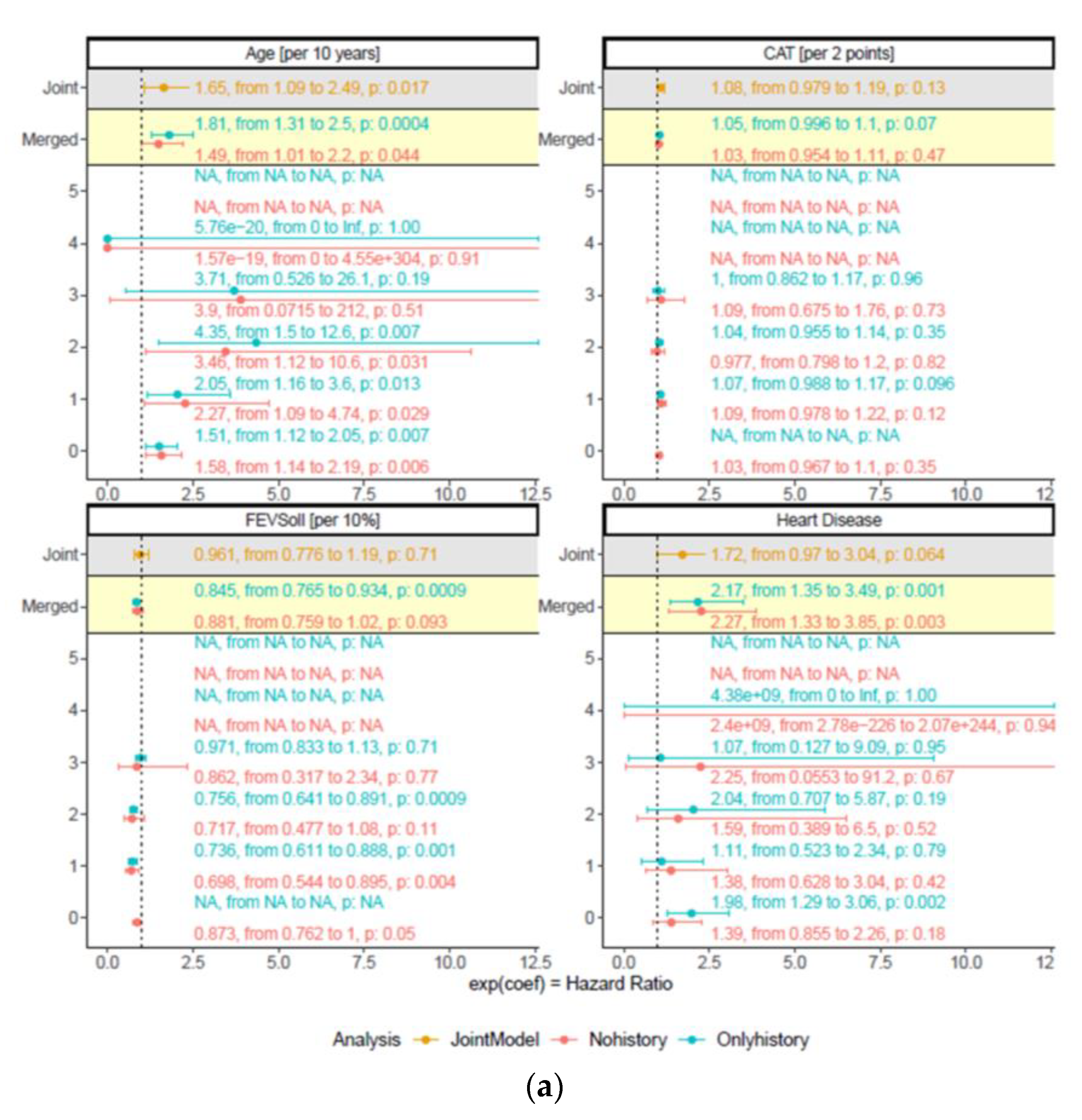
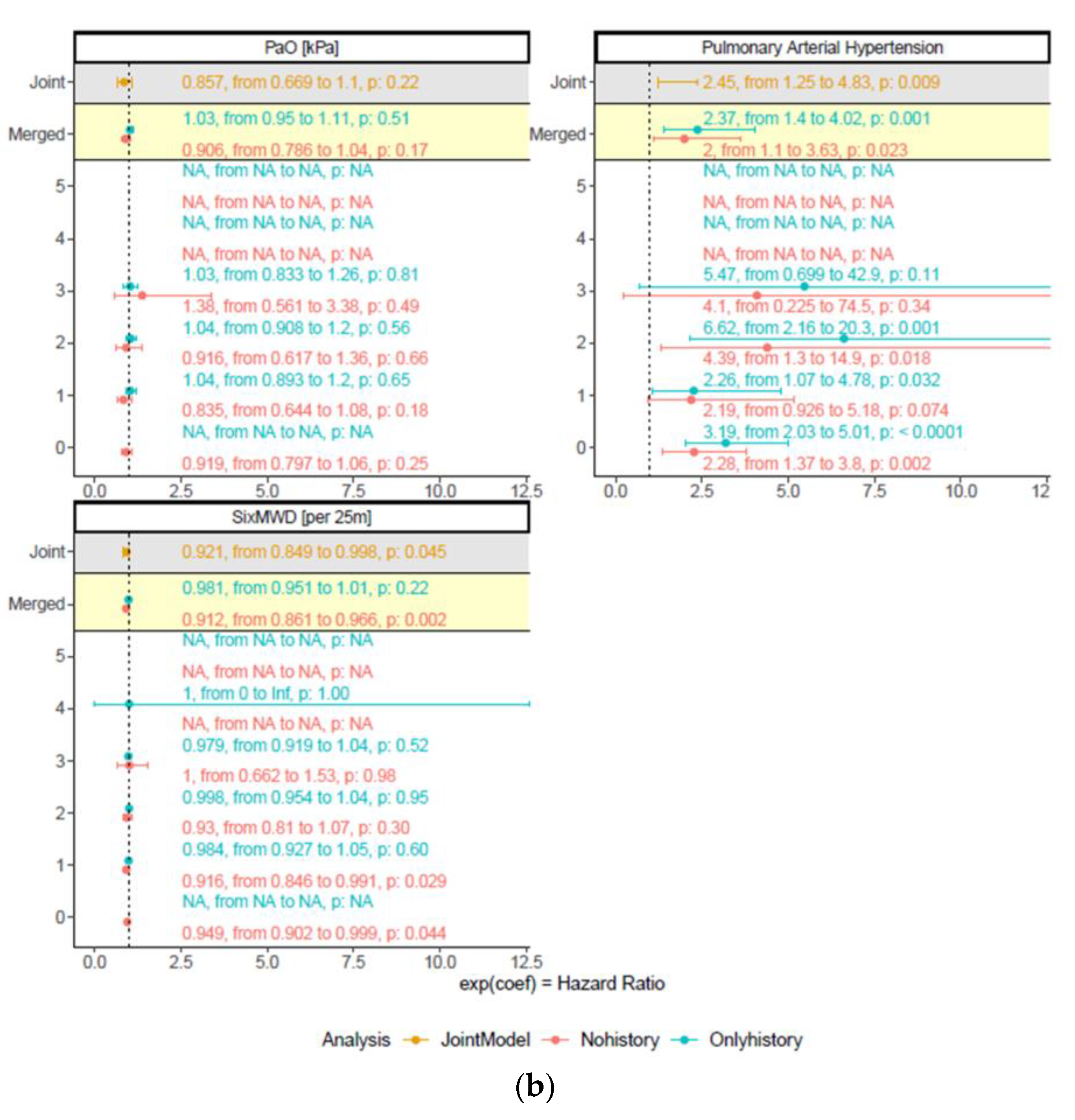
3.3. Robustness of Predictors for Mortality over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Sin, D.D.; Anthonisen, N.R.; Soriano, J.B.; Agusti, A.G. Mortality in COPD: Role of comorbidities. Eur. Respir. J. 2006, 28, 1245–1257. [Google Scholar] [CrossRef]
- Stallberg, B.; Janson, C.; Johansson, G.; Larsson, K.; Stratelis, G.; Telg, G.; Lisspers, K.H. Management, morbidity and mortality of COPD during an 11-year period: An observational retrospective epidemiological register study in Sweden (PATHOS). Prim. Care Resp. J. 2014, 23, 38–45. [Google Scholar] [CrossRef]
- Scott, D.A.; Woods, B.; Thompson, J.C.; Clark, J.F.; Hawkins, N.; Chambers, M.; Celli, B.R.; Calverley, P. Mortality and drug therapy in patients with chronic obstructive pulmonary disease: A network meta-analysis. BMC Pulm Med. 2015, 15, 145. [Google Scholar] [CrossRef]
- Celli, B.R.; Cote, C.G.; Lareau, S.C.; Meek, P.M. Predictors of Survival in COPD: More than just the FEV1. Respir. Med. 2008, 102 (Suppl. S1), S27–S35. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes, d.O.; Mendez, R.A.; Pinto, P.V.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Fukuchi, Y.; Jenkins, C.; Rodriguez-Roisin, R.; van Weel, C.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease—GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.; Solway, S.; Gibbons, W.J. ATS statement on six-minute walk test. Am. J. Respir. Crit. Care Med. 2003, 167, 1287. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease: Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Disease. 2016. Available online: http://www.goldcopd.com (accessed on 22 September 2022).
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Kon, S.S.; Canavan, J.L.; Jones, S.E.; Nolan, C.M.; Clark, A.L.; Dickson, M.J.; Haselden, B.M.; Polkey, M.I.; Man, W.D. Minimum clinically important difference for the COPD Assessment Test: A prospective analysis. Lancet Respir. Med. 2014, 2, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef] [PubMed]
- WHO. International Classification of Diseases (ICD). Available online: http://www.who.int/classifications/icd/en/ (accessed on 21 September 2013).
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Mullerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; Macnee, W.; et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R. Predictors of mortality in COPD. Respir. Med. 2010, 104, 773–779. [Google Scholar] [CrossRef]
- Papaioannou, A.I.; Loukides, S.; Gourgoulianis, K.I.; Kostikas, K. Global assessment of the COPD patient: Time to look beyond FEV1? Respir. Med. 2009, 103, 650–660. [Google Scholar] [CrossRef]
- Ong, K.C.; Earnest, A.; Lu, S.J. A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest 2005, 128, 3810–3816. [Google Scholar] [CrossRef]
- Soler-Cataluna, J.J.; Martinez-Garcia, M.A.; Sanchez, L.S.; Tordera, M.P.; Sanchez, P.R. Severe exacerbations and BODE index: Two independent risk factors for death in male COPD patients. Respir. Med. 2009, 103, 692–699. [Google Scholar] [CrossRef]
- de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Divo, M.; Zulueta, J.J.; Berto, J.; Zagaceta, J.; Sanchez-Salcedo, P.; Cabrera, C.; et al. Prognostic evaluation of COPD patients: GOLD 2011 versus BODE and the COPD comorbidity index COTE. Thorax 2014, 69, 799–804. [Google Scholar] [CrossRef]
- Abu Hussein, N.; Ter Riet, G.; Schoenenberger, L.; Bridevaux, P.O.; Chhajed, P.N.; Fitting, J.W.; Geiser, T.; Jochmann, A.; Joos Zellweger, L.; Kohler, M.; et al. The ADO index as a predictor of two-year mortality in general practice-based chronic obstructive pulmonary disease cohorts. Respiration 2014, 88, 208–214. [Google Scholar] [CrossRef]
- Henoch, I.; Ekberg-Jansson, A.; Lofdahl, C.G.; Strang, P. Early Predictors of Mortality in Patients with COPD, in Relation to Respiratory and Non-Respiratory Causes of Death—A National Register Study. Int. J. Chron Obs. Pulmon. Dis. 2020, 15, 1495–1505. [Google Scholar] [CrossRef]
| N = 297 | |
|---|---|
| Age, y | 62.5 (7.6) |
| Female/Male, N | 101/196 |
| BMI, kg/m2 | 26.6 (6.1) |
| Comorbidities | |
| Arterial hypertension, N (%) | 140 (47) |
| Coronary artery disease, N (%) | 55 (19) |
| Heart failure, N (%) | 23 (8) |
| Arteriosclerosis, N (%) | 83 (28) |
| Lung cancer, N (%) | 4 (1) |
| Cor pulmonale, N (%) | 12 (4) |
| Pulmonary hypertension, N (%) | 36 (12) |
| Chronic renal failure, N (%) | 17 (6) |
| Diabetes, N (%) | 39 (13) |
| Smoking history | |
| Never smokers, N (%) | 11 (4) |
| Current smokers, N (%) | 74 (25) |
| Ex-smokers, N (%) | 212 (71) |
| Pack years of smoking, N | 45.6 (27.7) |
| Respiratory variables | |
| FVC, % pred. | 81.2 (20.7) |
| FEV1, % pred. | 48.8 (21.4) |
| RV/TLC, % | 54.9 (11.8) |
| PaO2, kPa | 9.1 (1.6) |
| CAT score | 15 (11/21) |
| mMRC score | 2.0 (1.0/2.0) |
| Number of exacerbations in the previous year, N | 1.0 (1.4) |
| 6MWD, m | 415.7 (130.1) |
| Coef. (95% CI) | p-Value | |
|---|---|---|
| Age at baseline, per 10 year | 1.65 (1.09/2.49) | 0.017 |
| CAT | 1.08 (0.98/1.19) | 0.13 |
| FEV1, % pred. | 0.96 (0.78/1.19) | 0.71 |
| Coronary artery disease | 1.72 (0.97/3.04) | 0.064 |
| PaO2, kPa | 0.86 (0.67/1.10) | 0.22 |
| Pulmonary hypertension | 2.45 (1.25/4.83) | 0.009 |
| 6MWD, m | 0.92 (0.85/0.998) | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sievi, N.A.; Sepin, J.; Roeder, M.; Brack, T.; Brutsche, M.H.; Frey, M.; Irani, S.; Leuppi, J.D.; Thurnheer, R.; Clarenbach, C.F.; et al. Are Predictors for Overall Mortality in COPD Patients Robust over Time? J. Clin. Med. 2023, 12, 1587. https://doi.org/10.3390/jcm12041587
Sievi NA, Sepin J, Roeder M, Brack T, Brutsche MH, Frey M, Irani S, Leuppi JD, Thurnheer R, Clarenbach CF, et al. Are Predictors for Overall Mortality in COPD Patients Robust over Time? Journal of Clinical Medicine. 2023; 12(4):1587. https://doi.org/10.3390/jcm12041587
Chicago/Turabian StyleSievi, Noriane A, Jerome Sepin, Maurice Roeder, Thomas Brack, Martin H Brutsche, Martin Frey, Sarosh Irani, Jörg D. Leuppi, Robert Thurnheer, Christian F. Clarenbach, and et al. 2023. "Are Predictors for Overall Mortality in COPD Patients Robust over Time?" Journal of Clinical Medicine 12, no. 4: 1587. https://doi.org/10.3390/jcm12041587
APA StyleSievi, N. A., Sepin, J., Roeder, M., Brack, T., Brutsche, M. H., Frey, M., Irani, S., Leuppi, J. D., Thurnheer, R., Clarenbach, C. F., & Kohler, M. (2023). Are Predictors for Overall Mortality in COPD Patients Robust over Time? Journal of Clinical Medicine, 12(4), 1587. https://doi.org/10.3390/jcm12041587







