Adaptation of the Brain to Hyponatremia and Its Clinical Implications
Abstract
1. Introduction
2. Brain Adaptation to Acute Hyponatremia
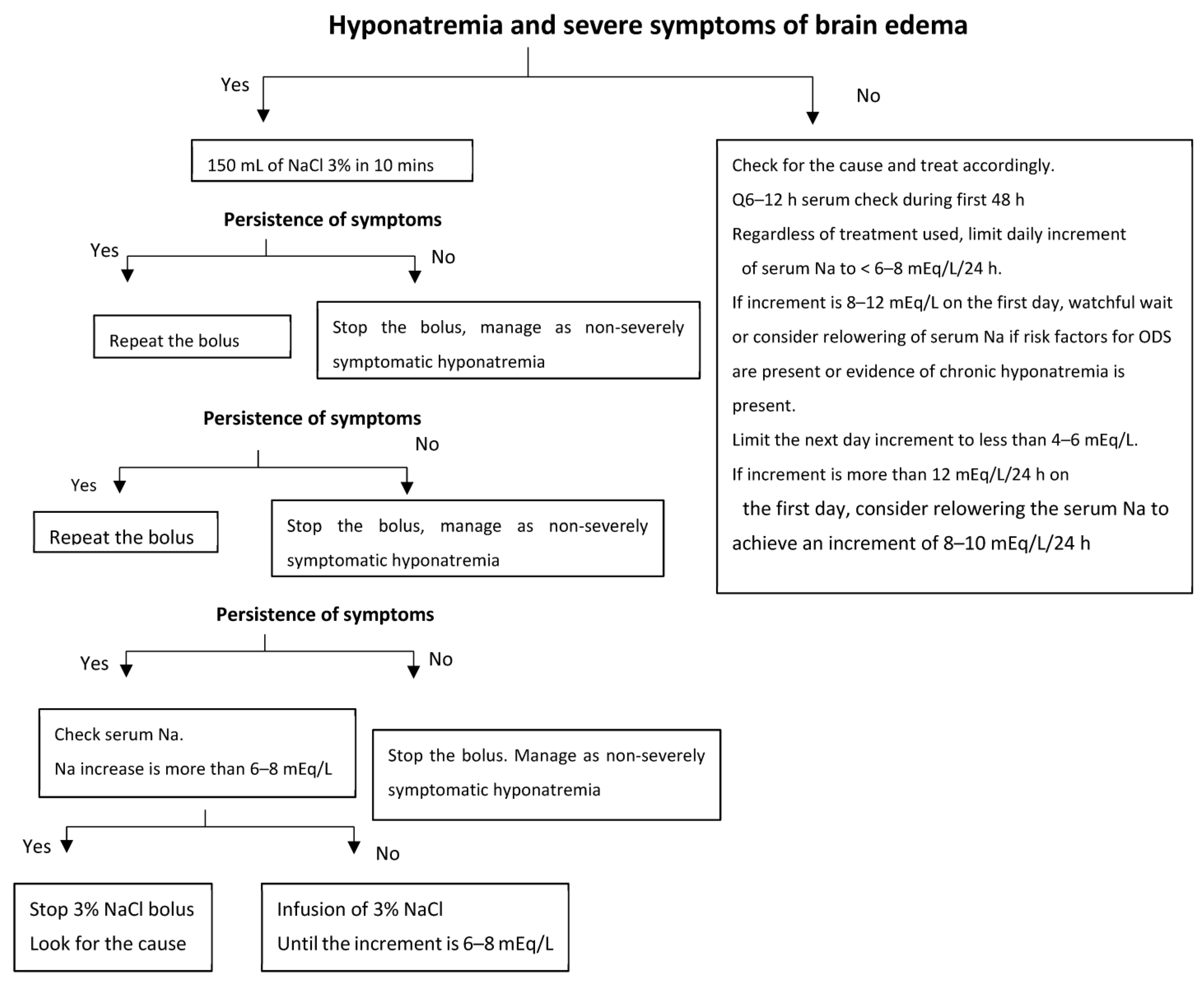
3. Symptoms of Acute Hyponatremia
4. Treatment of Acute Hyponatremia
5. Brain Adaptation to Chronic Hyponatremia
6. Symptoms and Treatment of Chronic Hyponatremia
7. Osmotic Demyelination Syndrome (ODS)
8. Physiopathology of ODS
9. Clinical Course and Prognosis of Osmotic Demyelination
10. Treatment of Osmotic Demyelination
11. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stillwell, W. (Ed.) Chapter 1—Introduction to Biological Membranes. In An Introduction to Biological Membranes, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–15. [Google Scholar]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Busch, G.L.; Ritter, M.; Volkl, H.; Waldegger, S.; Gulbins, E.; Haussinger, D. Functional Significance of Cell Volume Regulatory Mechanisms. Physiol. Rev. 1998, 78, 247–306. [Google Scholar] [CrossRef] [PubMed]
- King, L.S.; Agre, P. Pathophysiology of the Aquaporin Water Channels. Annu. Rev. Physiol. 1996, 58, 619–648. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, W. (Ed.) Chapter 2—Membrane History. In An Introduction to Biological Membranes, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 17–33. [Google Scholar]
- Edelman, I.S.; Leibman, J.; O’Meara, M.P.; Birkenfeld, L.W. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J. Clin. Investig. 1958, 37, 1236–1256. [Google Scholar] [CrossRef]
- Gankam-Kengne, F.; Ayers, C.; Khera, A.; de Lemos, J.; Maalouf, N.M. Mild hyponatremia is associated with an increased risk of death in an ambulatory setting. Kidney Int. 2013, 83, 700–706. [Google Scholar] [CrossRef]
- Wald, R.; Jaber, B.L.; Price, L.L.; Upadhyay, A.; Madias, N.E. Impact of hospital-associated hyponatremia on selected outcomes. Arch. Intern. Med. 2010, 170, 294–302. [Google Scholar] [CrossRef]
- Hawkins, R.C. Age and gender as risk factors for hyponatremia and hypernatremia. Clin. Chim. Acta 2003, 337, 169–172. [Google Scholar] [CrossRef]
- Waikar, S.S.; Mount, D.B.; Curhan, G.C. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am. J. Med. 2009, 122, 857–865. [Google Scholar] [CrossRef]
- Pasantes-Morales, H.; Lezama, R.A.; Ramos-Mandujano, G.; Tuz, K.L. Mechanisms of cell volume regulation in hypo-osmolality. Am. J. Med. 2006, 119 (Suppl. S1), S4–S11. [Google Scholar] [CrossRef]
- Pasantes-Morales, H.; Franco, R.; Ordaz, B.; Ochoa, L.D. Mechanisms counteracting swelling in brain cells during hyponatremia. Arch. Med. Res. 2002, 33, 237–244. [Google Scholar] [CrossRef]
- Kengne, F.G.; Decaux, G. Hyponatremia and the Brain. Kidney Int. Rep. 2018, 3, 24–35. [Google Scholar] [CrossRef]
- Melton, J.E.; Patlak, C.S.; Pettigrew, K.D.; Cserr, H.F. Volume regulatory loss of Na, Cl, and K from rat brain during acute hyponatremia. Am. J. Physiol. 1987, 252, F661–F669. [Google Scholar] [CrossRef]
- Holliday, M.A.; Kalayci, M.N.; Harrah, J. Factors that limit brain volume changes in response to acute and sustained hyper- and hyponatremia. J. Clin. Investig. 1968, 47, 1916–1928. [Google Scholar] [CrossRef]
- Melton, J.E.; Nattie, E.E. Brain and CSF water and ions during dilutional and isosmotic hyponatremia in the rat. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 1983, 244, R724–R732. [Google Scholar] [CrossRef]
- Verbalis, J.G.; Gullans, S.R. Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain Res. 1991, 567, 274–282. [Google Scholar] [CrossRef]
- Videen, J.S.; Michaelis, T.; Pinto, P.; Ross, B.D. Human cerebral osmolytes during chronic hyponatremia. A proton magnetic resonance spectroscopy study. J. Clin. Investig. 1995, 95, 788–793. [Google Scholar] [CrossRef]
- Olson, J.E. Osmolyte contents of cultured astrocytes grown in hypoosmotic medium. Biochim. Biophys. Acta 1999, 1453, 175–179. [Google Scholar] [CrossRef]
- Isaacks, R.E.; Bender, A.S.; Kim, C.Y.; Norenberg, M.D. Effect of Osmolality and myo-Inositol Deprivation on the Transport Properties of myo-Inositol in Primary Astrocyte Cultures. Neurochem. Res. 1997, 22, 1461–1469. [Google Scholar] [CrossRef]
- Barakat, L.; Wang, D.; Bordey, A. Carrier-mediated uptake and release of taurine from Bergmann glia in rat cerebellar slices. J. Physiol. 2002, 541, 753–767. [Google Scholar] [CrossRef]
- Arieff, A.I.; Llach, F.; Massry, S.G. Neurological manifestations and morbidity of hyponatremia. Correlation with brain water and electrolytes. Medicine 1976, 55, 121–129. [Google Scholar] [CrossRef]
- Soupart, A.; Penninckx, R.; Stenuit, A.; Decaux, G. Lack of major hypoxia and significant brain damage in rats despite dramatic hyponatremic encephalopathy. J. Lab. Clin. Med. 1997, 130, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G. An experimental model of syndrome of inappropriate antidiuretic hormone secretion in the rat. Am. J. Physiol. 1984, 247 Pt 1, E540–E553. [Google Scholar] [CrossRef] [PubMed]
- Arieff, A.I. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N. Engl. J. Med. 1986, 314, 1529–1535. [Google Scholar] [CrossRef]
- Ayus, J.C.; Varon, J.; Arieff, A.I. Hyponatremia, Cerebral Edema, and Noncardiogenic Pulmonary Edema in Marathon Runners. Ann. Intern. Med. 2000, 132, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Hew, T.D.; Chorley, J.N.; Cianca, J.C.; Divine, J.G. The incidence, risk factors, and clinical manifestations of hyponatremia in marathon runners. Clin. J. Sport Med. 2003, 13, 41–47. [Google Scholar] [CrossRef]
- Bentsen, G.; Breivik, H.; Lundar, T.; Stubhaug, A. Hypertonic saline (7.2%) in 6% hydroxyethyl starch reduces intracranial pressure and improves hemodynamics in a placebo-controlled study involving stable patients with subarachnoid hemorrhage. Crit. Care Med. 2006, 34, 2912–2917. [Google Scholar] [CrossRef]
- Koenig, M.A.; Bryan, M.; Lewin, J.L., 3rd; Mirski, M.A.; Geocadin, R.G.; Stevens, R.D. Reversal of transtentorial herniation with hypertonic saline. Neurology 2008, 70, 1023–1029. [Google Scholar] [CrossRef]
- Annoni, F.; Fontana, V.; Brimioulle, S.; Creteur, J.; Vincent, J.L.; Taccone, F.S. Early Effects of Enteral Urea on Intracranial Pressure in Patients with Acute Brain Injury and Hyponatremia. J. Neurosurg. Anesthesiol. 2017, 29, 400–405. [Google Scholar] [CrossRef]
- Spasovski, G.; Vanholder, R.; Allolio, B.; Annane, D.; Ball, S.; Bichet, D.; Decaux, G.; Fenske, W.; Hoorn, E.J.; Ichai, C.; et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur. J. Endocrinol. 2014, 170, G1–G47. [Google Scholar] [CrossRef]
- Adler, S.; Verbalis, J.G.; Williams, D. Effect of acute and chronic hyponatremia on brain buffering in rats. Am. J. Physiol. 1993, 264 Pt 2, F968–F974. [Google Scholar] [CrossRef]
- Verbalis, J. Hyponatremia induced by vasopressin or desmopressin in female and male rats. J. Am. Soc. Nephrol. 1993, 3, 1600–1606. [Google Scholar] [CrossRef]
- Clark, E.C.; Thomas, D.; Baer, J.; Sterns, R.H. Depletion of glutathione from brain cells in hyponatremia. Kidney Int. 1996, 49, 470–476. [Google Scholar] [CrossRef]
- Lien, Y.H.; Shapiro, J.I.; Chan, L. Study of brain electrolytes and organic osmolytes during correction of chronic hyponatremia. Implications for the pathogenesis of central pontine myelinolysis. J. Clin. Investig. 1991, 88, 303–309. [Google Scholar] [CrossRef]
- Lien, Y.H. Role of organic osmolytes in myelinolysis. A topographic study in rats after rapid correction of hyponatremia. J. Clin. Investig. 1995, 95, 1579–1586. [Google Scholar] [CrossRef]
- Fujisawa, H.; Sugimura, Y.; Takagi, H.; Mizoguchi, H.; Takeuchi, H.; Izumida, H.; Nakashima, K.; Ochiai, H.; Takeuchi, S.; Kiyota, A.; et al. Chronic Hyponatremia Causes Neurologic and Psychologic Impairments. J. Am. Soc. Nephrol. 2016, 27, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Renneboog, B.; Musch, W.; Vandemergel, X.; Manto, M.U.; Decaux, G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am. J. Med. 2006, 119, 71.e1–71.e8. [Google Scholar] [CrossRef]
- Decaux, G. Is asymptomatic hyponatremia really asymptomatic? Am. J. Med. 2006, 119 (Suppl. S1), S79–S82. [Google Scholar] [CrossRef]
- Barsony, J.; Manigrasso, M.B.; Xu, Q.; Tam, H.; Verbalis, J.G. Chronic hyponatremia exacerbates multiple manifestations of senescence in male rats. Age 2012, 35, 271–288. [Google Scholar] [CrossRef]
- Sterns, R.H.; Riggs, J.E.; Schochet, S.S., Jr. Osmotic demyelination syndrome following correction of hyponatremia. N. Engl. J. Med. 1986, 314, 1535–1542. [Google Scholar] [CrossRef]
- Sterns, R.H. Neurological deterioration following treatment for hyponatremia. Am. J. Kidney Dis. 1989, 13, 434–437. [Google Scholar] [CrossRef]
- Sterns, R.H.; Cappuccio, J.D.; Silver, S.M.; Cohen, E.P. Neurologic sequelae after treatment of severe hyponatremia: A multicenter perspective. J. Am. Soc. Nephrol. 1993, 4, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G.; Gullans, S.R. Rapid correction of hyponatremia produces differential effects on brain osmolyte and electrolyte reaccumulation in rats. Brain Res. 1993, 606, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G.; Martinez, A.J. Determinants of brain myelinolysis following correction of chronic hyponatremia in rats. Vasopressin 1991, 208, 539–547. [Google Scholar]
- Verbalis, J.G.; Martinez, A.J. Neurological and neuropathological sequelae of correction of chronic hyponatremia. Kidney Int. 1991, 39, 1274–1282. [Google Scholar] [CrossRef]
- Soupart, A.; Penninckx, R.; Stenuit, A.; Perier, O.; Decaux, G. Treatment of chronic hyponatremia in rats by intravenous saline: Comparison of rate versus magnitude of correction. Kidney Int. 1992, 41, 1662–1667. [Google Scholar] [CrossRef]
- Kleinschmidt-DeMasters, B.K.; Norenberg, M.D. Rapid Correction of hyponatremia causes demyelination: Relation to central pontine myelinolysis. Science 1981, 211, 1068–1070. [Google Scholar] [CrossRef]
- Chua, G.C.; Sitoh, Y.Y.; Lim, C.C.; Chua, H.C.; Ng, P.Y. MRI findings in osmotic myelinolysis. Clin. Radiol. 2002, 57, 800–806. [Google Scholar] [CrossRef]
- Ho, V.B.; Fitz, C.R.; Yoder, C.C.; Geyer, C.A. Resolving MR features in osmotic myelinolysis (central pontine and extrapontine myelinolysis). AJNR Am. J. Neuroradiol. 1993, 14, 163–167. [Google Scholar]
- Graff-Radford, J.; Fugate, J.E.; Kaufmann, T.J.; Mandrekar, J.N.; Rabinstein, A.A. Clinical and Radiologic Correlations of Central Pontine Myelinolysis Syndrome. Mayo Clin. Proc. 2011, 86, 1063–1067. [Google Scholar] [CrossRef]
- De Souza, A.; Desai, P.K. More often striatal myelinolysis than pontine? A consecutive series of patients with osmotic demyelination syndrome. Neurol. Res. 2012, 34, 262–271. [Google Scholar] [CrossRef]
- Aegisdottir, H.; Cooray, C.; Wirdefeldt, K.; Piehl, F.; Sveinsson, O. Incidence of osmotic demyelination syndrome in Sweden: A nationwide study. Acta Neurol. Scand. 2019, 140, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.; Verbalis, J.G.; Williams, D. Effect of rapid correction of hyponatremia on the blood-brain barrier of rats. Brain Res. 1995, 679, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.A.; Tian, Y.; Adler, S.; Verbalis, J.G. Blood-brain barrier disruption and complement activation in the brain following rapid correction of chronic hyponatremia. Exp. Neurol. 2000, 165, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Gankam-Kengne, F.; Soupart, A.; Pochet, R.; Brion, J.P.; Decaux, G. Minocycline protects against neurologic complications of rapid correction of hyponatremia. J. Am. Soc. Nephrol. 2010, 21, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sugimura, Y.; Iwama, S.; Nobuaki, O.; Nagasaki, H.; Arima, H.; Sawada, M.; Oiso, Y. Minocycline prevents osmotic demyelination syndrome by inhibiting the activation of microglia. J. Am. Soc. Nephrol. 2010, 21, 2090–2098. [Google Scholar] [CrossRef]
- Gankam Kengne, F.; Nicaise, C.; Soupart, A.; Boom, A.; Schiettecatte, J.; Pochet, R.; Brion, J.P.; Decaux, G. Astrocytes are an early target in osmotic demyelination syndrome. J. Am. Soc. Nephrol. 2011, 22, 1834–1845. [Google Scholar] [CrossRef]
- Gankam-Kengne, F.; Couturier, B.S.; Soupart, A.; Brion, J.P.; Decaux, G. Osmotic Stress-Induced Defective Glial Proteostasis Contributes to Brain Demyelination after Hyponatremia Treatment. J. Am. Soc. Nephrol. 2017, 28, 1802–1813. [Google Scholar] [CrossRef]
- Iwama, S.; Sugimura, Y.; Suzuki, H.; Murase, T.; Ozaki, N.; Nagasaki, H.; Arima, H.; Murata, Y.; Sawada, M.; Oiso, Y. Time-dependent changes in proinflammatory and neurotrophic responses of microglia and astrocytes in a rat model of osmotic demyelination syndrome. GLIA 2011, 59, 452–462. [Google Scholar] [CrossRef]
- Lohrberg, M.; Winkler, A.; Franz, J.; van der Meer, F.; Ruhwedel, T.; Sirmpilatze, N.; Dadarwal, R.; Handwerker, R.; Esser, D.; Wiegand, K.; et al. Lack of astrocytes hinders parenchymal oligodendrocyte precursor cells from reaching a myelinating state in osmolyte-induced demyelination. Acta Neuropathol. Commun. 2020, 8, 224. [Google Scholar] [CrossRef]
- Soupart, A.; Penninckx, R.; Stenuit, A.; Decaux, G. Azotemia (48 h) decreases the risk of brain damage in rats after correction of chronic hyponatremia. Brain Res. 2000, 852, 167–172. [Google Scholar] [CrossRef]
- Soupart, A.; Stenuit, A.; Perier, O.; Decaux, G. Limits of brain tolerance to daily increments in serum sodium in chronically hyponatraemic rats treated with hypertonic saline or urea: Advantages of urea. Clin. Sci. 1991, 80, 77–84. [Google Scholar] [CrossRef]
- Menger, H.; Jörg, J. Outcome of central pontine and extrapontine myelinolysis (n = 44). J. Neurol. 1999, 246, 700–705. [Google Scholar] [CrossRef]
- Louis, G.; Megarbane, B.; Lavoue, S.; Lassalle, V.; Argaud, L.; Poussel, J.F.; Georges, H.; Bollaert, P.E. Long-term outcome of patients hospitalized in intensive care units with central or extrapontine myelinolysis. Crit. Care Med. 2011, 40, 970–972. [Google Scholar] [CrossRef]
- Kallakatta, R.N.; Radhakrishnan, A.; Fayaz, R.K.; Unnikrishnan, J.P.; Kesavadas, C.; Sarma, S.P. Clinical and functional outcome and factors predicting prognosis in osmotic demyelination syndrome (central pontine and/or extrapontine myelinolysis) in 25 patients. J. Neurol. Neurosurg. Psychiatry 2011, 82, 326–331. [Google Scholar] [CrossRef]
- Soupart, A.; Ngassa, M.; Decaux, G. Therapeutic relowering of the serum sodium in a patient after excessive correction of hyponatremia. Clin. Nephrol. 1999, 51, 383–386. [Google Scholar]
- Gankam Kengne, F.; Soupart, A.; Pochet, R.; Brion, J.P.; Decaux, G. Re-induction of hyponatremia after rapid overcorrection of hyponatremia reduces mortality in rats. Kidney Int. 2009, 76, 614–621. [Google Scholar] [CrossRef]
- Rondon-Berrios, H. Therapeutic Relowering of Plasma Sodium after Overly Rapid Correction of Hyponatremia: What Is the Evidence? Clin. J. Am. Soc. Nephrol. 2020, 15, 282–284. [Google Scholar] [CrossRef]

| Acute Hyponatremia | Chronic Hyponatremia |
|---|---|
| Nausea and vomiting | Nausea |
| Headaches | Fatigue |
| Seizures | Gait and attention deficit |
| Coma and death | Falls and bone fractures |
| Respiratory arrest Non-cardiogenic pulmonary edema |
| Treatment of Severe Hyponatremia | |
|---|---|
| Signs or symptoms of Brain Edema | No signs or symptoms of brain edema |
| ICU admission Hypertonic saline boluses (100 mL NaCl 3%) Repeat if symptoms persist Measure serum Na q1–2 h Stop when symptoms abate Stop when sodium increment is 6 mEq/L Keep checking serum Na q3–4 h | Etiological evaluation Determine chronicity (>48 h) Assess for risk factors for ODS (chronic hyponatremia, hypokalemia, liver disease, alcoholism) Treatment of the cause and avoid hypertonic saline If risk factors for ODS are present q6–12 h serum Na check Limit increment of Na to < 6 mEq/L/24 h * If no risk factors for ODS Limit increment of Na to < 8 mEq/L/24 h * Additional measures: If SIADH, use of urea is better Relower the serum Na if increment of > 12 mEq/L/day Desmopressin + D5W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gankam Kengne, F. Adaptation of the Brain to Hyponatremia and Its Clinical Implications. J. Clin. Med. 2023, 12, 1714. https://doi.org/10.3390/jcm12051714
Gankam Kengne F. Adaptation of the Brain to Hyponatremia and Its Clinical Implications. Journal of Clinical Medicine. 2023; 12(5):1714. https://doi.org/10.3390/jcm12051714
Chicago/Turabian StyleGankam Kengne, Fabrice. 2023. "Adaptation of the Brain to Hyponatremia and Its Clinical Implications" Journal of Clinical Medicine 12, no. 5: 1714. https://doi.org/10.3390/jcm12051714
APA StyleGankam Kengne, F. (2023). Adaptation of the Brain to Hyponatremia and Its Clinical Implications. Journal of Clinical Medicine, 12(5), 1714. https://doi.org/10.3390/jcm12051714




