The β1 Adrenergic Blocker Nebivolol Ameliorates Development of Endotoxic Acute Lung Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Histopathological Analyses
2.2. Immunohistochemical Analyses
2.3. Biochemical Analyses
2.4. Quantitative Polymerase Chain Reaction Analysis
2.5. Statistical Analyses
3. Results
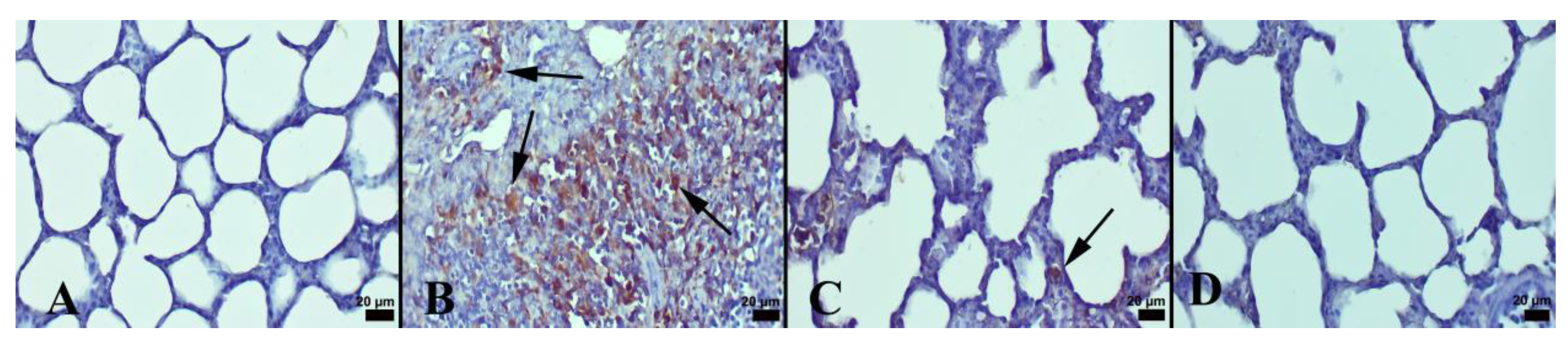
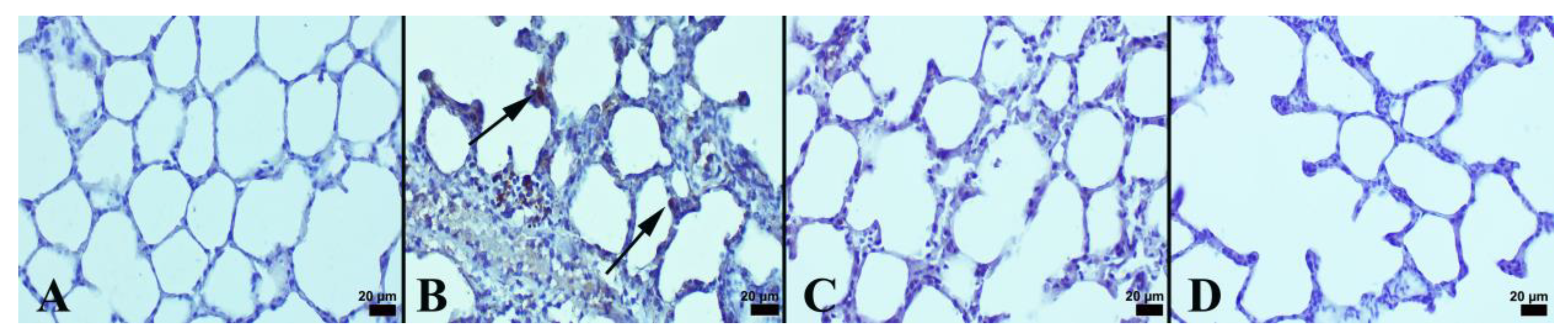
3.1. Immunohistochemical and Histopathological Results
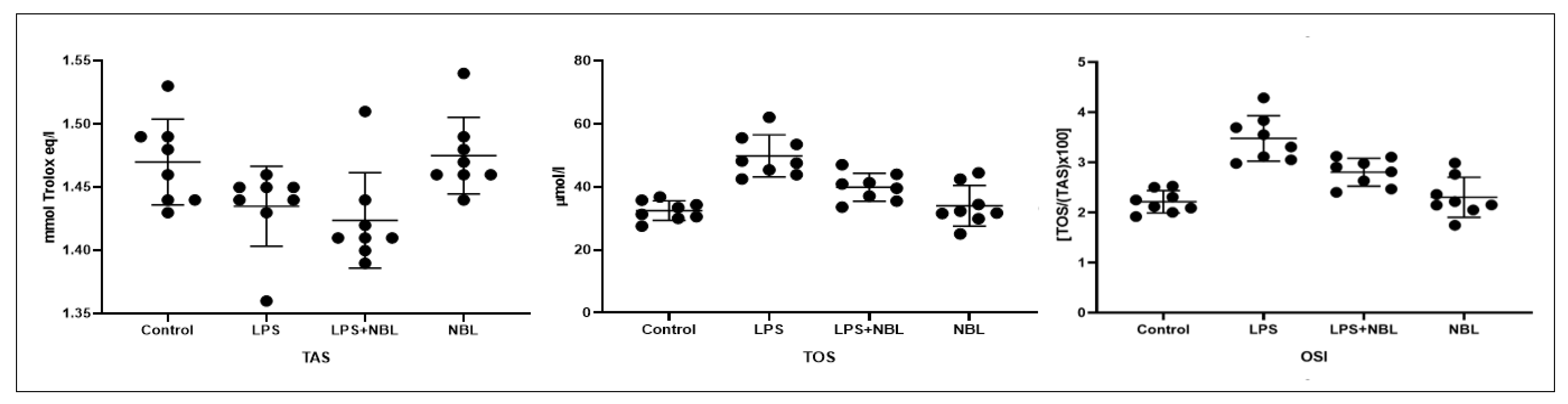
3.2. Biochemical Results
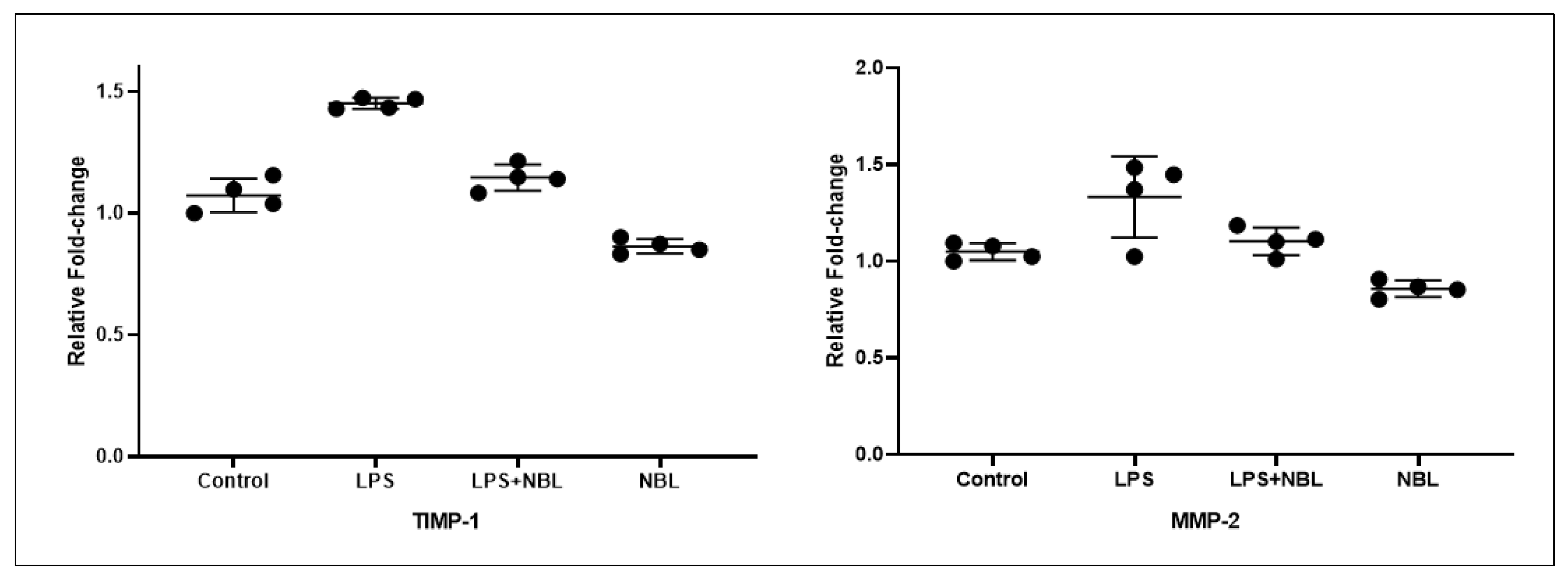
3.3. Gene Expression Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aziz, M.; Ode, Y.; Zhou, M.; Ochani, M.; Holodick, N.E.; Rothstein, T.L.; Wang, P. B-1a cells protect mice from sepsis-induced acute lung injury. Mol. Med. 2018, 24, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokra, D.; Mikolka, P.; Kosutova, P.; Mokry, J. Corticosteroids in Acute Lung Injury: The Dilemma Continues. Int. J. Mol. Sci. 2019, 20, 4765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, K.; He, W.; Guan, W.; Hou, F.; Yan, P.; Xu, J.; Zhou, T.; Liu, Y.; Xie, L. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Nova, Z.; Skovierova, H.; Calkovska, A. Alveolar-Capillary Membrane-Related Pulmonary Cells as a Target in Endotoxin-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, 831. [Google Scholar] [CrossRef] [Green Version]
- Chae, B.S. Pretreatment of Low-Dose and Super-Low-Dose LPS on the Production of In Vitro LPS-Induced Inflammatory Mediators. Toxicol. Res. 2018, 34, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.H.; Duan, J.X.; Liu, S.K.; Xiong, J.B.; Guan, X.X.; Zhong, W.J.; Sun, C.C.; Zhang, C.Y.; Luo, X.Q.; Zhang, Y.F.; et al. A COX-2/sEH dual inhibitor PTUPB alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation. Theranostics 2020, 10, 4749–4761. [Google Scholar] [CrossRef]
- Li, X.; Jamal, M.; Guo, P.; Jin, Z.; Zheng, F.; Song, X.; Zhan, J.; Wu, H. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed. Pharmacother. 2019, 118, 109363. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Deng, J.S.; Chang, Y.S.; Huang, G.J. Ginsenoside Rh2 Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 Signaling Pathways in Mice. Nutrients 2018, 10, 1208. [Google Scholar] [CrossRef]
- Lagente, V.; Manoury, B.; Nénan, S.; Le Quément, C.; Martin-Chouly, C.; Boichot, E. Role of matrix metalloproteinases in the development of airway inflammation and remodeling. Braz. J. Med. Biol. Res. 2005, 38, 1521–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrix, A.Y.; Kheradmand, F. The Role of Matrix Metalloproteinases in Development, Repair, and Destruction of the Lungs. Prog. Mol. Biol. Transl. Sci. 2017, 148, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Jublanc, C.; Beaudeux, J.L.; Aubart, F.; Raphael, M.; Chadarevian, R.; Chapman, M.J.; Bonnefont-Rousselot, D.; Bruckert, E. Serum levels of adhesion molecules ICAM-1 and VCAM-1 and tissue inhibitor of metalloproteinases, TIMP-1, are elevated in patients with autoimmune thyroid disorders: Relevance to vascular inflammation. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Lerchenberger, M.; Uhl, B.; Stark, K.; Zuchtriegel, G.; Eckart, A.; Miller, M.; Puhr-Westerheide, D.; Praetner, M.; Rehberg, M.; Khandoga, A.G.; et al. Matrix metalloproteinases modulate ameboid-like migration of neutrophils through inflamed interstitial tissue. Blood 2013, 122, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Fongemie, J.; Felix-Getzik, E. A Review of Nebivolol Pharmacology and Clinical Evidence. Drugs 2015, 75, 1349–1371. [Google Scholar] [CrossRef] [Green Version]
- Serg, M.; Kampus, P.; Kals, J.; Zagura, M.; Zilmer, M.; Zilmer, K.; Kullisaar, T.; Eha, J. Nebivolol and metoprolol: Long-term effects on inflammation and oxidative stress in essential hypertension. Scand. J. Clin. Lab. Invest. 2012, 72, 427–432. [Google Scholar] [CrossRef]
- Hussain, M.; Saeed, M.; Babar, M.Z.M.; Atif, M.A.; Akhtar, L. Nebivolol Attenuates Neutrophil Lymphocyte Ratio: A Marker of Subclinical Inflammation in Hypertensive Patients. Int. J. Hypertens. 2017, 2017, 7643628. [Google Scholar] [CrossRef] [Green Version]
- Merchant, N.; Rahman, S.T.; Ferdinand, K.C.; Haque, T.; Umpierrez, G.E.; Khan, B.V. Effects of nebivolol in obese African Americans with hypertension (NOAAH): Markers of inflammation and obesity in response to exercise-induced stress. J. Hum. Hypertens. 2011, 25, 196–202. [Google Scholar] [CrossRef]
- Abuelezz, S.A. Nebivolol attenuates oxidative stress and inflammation in a guinea pig model of ovalbumin-induced asthma: A possible mechanism for its favorable respiratory effects. Can. J. Physiol. Pharmacol. 2018, 96, 258–265. [Google Scholar] [CrossRef]
- Pekgöz, S.; Asci, H.; Erzurumlu, Y.; Savran, M.; Ilhan, I.; Hasseyid, N.; Ciris, M. Nebivolol alleviates liver damage caused by methotrexate via AKT1/Hif1α/eNOS signaling. Drug Chem. Toxicol. 2022, 45, 2153–2159. [Google Scholar] [CrossRef]
- Gandhi, C.; Zalawadia, R.; Balaraman, R. Nebivolol reduces experimentally induced warm renal ischemia reperfusion injury in rats. Ren. Fail. 2008, 30, 921–930. [Google Scholar] [CrossRef]
- Li, Z.; Liu, B.; Zhao, D.; Wang, B.; Liu, Y.; Zhang, Y.; Tian, F.; Li, B. Protective effects of Nebivolol against interleukin-1β (IL-1β)-induced type II collagen destruction mediated by matrix metalloproteinase-13 (MMP-13). Cell Stress Chaperones 2017, 22, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Samuvel, D.J.; Shunmugavel, A.; Singh, A.K.; Singh, I.; Khan, M. S-Nitrosoglutathione ameliorates acute renal dysfunction in a rat model of lipopolysaccharide-induced sepsis. J. Pharm. Pharmacol. 2016, 68, 1310–1319. [Google Scholar] [CrossRef] [Green Version]
- Dursun, M.; Sahin, S.; Besiroglu, H.; Otunctemur, A.; Ozbek, E.; Cakir, S.S.; Cekmen, M.; Somay, A. Protective effect of nebivolol on gentamicin-induced nephrotoxicity in rats. Bratisl. Lek. Listy 2018, 119, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Q.; Han, Y.; Liang, Y.; Xu, Z.; Ren, T. Prevention of LPS-induced acute lung injury in mice by progranulin. Mediat. Inflamm. 2012, 2012, 540794. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Savas, H.B.; Gultekin, F.; Ciris, I.M. Positive effects of meal frequency and calorie restriction on antioxidant systems in rats. North Clin. Istanb. 2017, 4, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.Q.; Wu, H.B.; Zhang, M.; Wang, J.A. Effects of Zinc Finger Protein A20 on Lipopolysaccharide (LPS)-Induced Pulmonary Inflammation/Anti-Inflammatory Mediators in an Acute Lung Injury/Acute Respiratory Distress Syndrome Rat Model. Med. Sci. Monit. 2017, 23, 3536–3545. [Google Scholar] [CrossRef] [Green Version]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Reutershan, J.; Morris, M.A.; Solga, M.; Rose, C.E., Jr.; Ley, K. ICAM-1 and LFA-1 play critical roles in LPS-induced neutrophil recruitment into the alveolar space. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L200–L207. [Google Scholar] [CrossRef] [Green Version]
- Petrovich, E.; Feigelson, S.W.; Stoler-Barak, L.; Hatzav, M.; Solomon, A.; Bar-Shai, A.; Ilan, N.; Li, J.P.; Engelhardt, B.; Vlodavsky, I.; et al. Lung ICAM-1 and ICAM-2 support spontaneous intravascular effector lymphocyte entrapment but are not required for neutrophil entrapment or emigration inside endotoxin-inflamed lungs. FASEB J. 2016, 30, 1767–1778. [Google Scholar] [CrossRef] [Green Version]
- Kamochi, M.; Kamochi, F.; Kim, Y.B.; Sawh, S.; Sanders, J.M.; Sarembock, I.; Green, S.; Young, J.S.; Ley, K.; Fu, S.M.; et al. P-selectin and ICAM-1 mediate endotoxin-induced neutrophil recruitment and injury to the lung and liver. Am. J. Physiol. 1999, 277, L310–L319. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.C.; Sauter, G.; Preyer, M.; Poerner, T.; Kempf, V.A.; Risler, T.; Brehm, B.R. Influence of nebivolol and metoprolol on inflammatory mediators in human coronary endothelial or smooth muscle cells. Effects on neointima formation after balloon denudation in carotid arteries of rats treated with nebivolol. Cell Physiol. Biochem. 2007, 19, 129–136. [Google Scholar] [CrossRef]
- Garbin, U.; Fratta Pasini, A.; Stranieri, C.; Manfro, S.; Mozzini, C.; Boccioletti, V.; Pasini, A.; Cominacini, M.; Evangelista, S.; Cominacini, L. Effects of nebivolol on endothelial gene expression during oxidative stress in human umbilical vein endothelial cells. Mediat. Inflamm. 2008, 2008, 367590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef] [Green Version]
- Lagente, V.; Martin-Chouly, C.; Boichot, E.; Martins, M.A.; Silva, P.M. Selective PDE4 inhibitors as potent anti-inflammatory drugs for the treatment of airway diseases. Mem. Inst. Oswaldo. Cruz. 2005, 100 (Suppl. S1), 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manicone, A.M.; McGuire, J.K. Matrix metalloproteinases as modulators of inflammation. Semin. Cell Dev. Biol. 2008, 19, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ortho, M.P.; Jarreau, P.H.; Delacourt, C.; Macquin-Mavier, I.; Levame, M.; Pezet, S.; Harf, A.; Lafuma, C. Matrix metalloproteinase and elastase activities in LPS-induced acute lung injury in guinea pigs. Am. J. Physiol. 1994, 266, L209–L216. [Google Scholar] [CrossRef]
- Corbel, M.; Lagente, V.; Théret, N.; Germain, N.; Clément, B.; Boichot, E. Comparative effects of betamethasone, cyclosporin and nedocromil sodium in acute pulmonary inflammation and metalloproteinase activities in bronchoalveolar lavage fluid from mice exposed to lipopolysaccharide. Pulm. Pharmacol. Ther. 1999, 12, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yang, N.; Pan, G.; Jin, B.; Wang, S.; Ji, W. Elevated IL-33 promotes expression of MMP2 and MMP9 via activating STAT3 in alveolar macrophages during LPS-induced acute lung injury. Cell. Mol. Biol. Lett. 2018, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef] [PubMed]
- Hannocks, M.J.; Zhang, X.; Gerwien, H.; Chashchina, A.; Burmeister, M.; Korpos, E.; Song, J.; Sorokin, L. The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 2019, 75–76, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Ceron, C.S.; Rizzi, E.; Guimarães, D.A.; Martins-Oliveira, A.; Gerlach, R.F.; Tanus-Santos, J.E. Nebivolol attenuates prooxidant and profibrotic mechanisms involving TGF-β and MMPs, and decreases vascular remodeling in renovascular hypertension. Free Radic. Biol. Med. 2013, 65, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzi, E.; Guimaraes, D.A.; Ceron, C.S.; Prado, C.M.; Pinheiro, L.C.; Martins-Oliveira, A.; Gerlach, R.F.; Tanus-Santos, J.E. β1-Adrenergic blockers exert antioxidant effects, reduce matrix metalloproteinase activity, and improve renovascular hypertension-induced cardiac hypertrophy. Free Radic. Biol. Med. 2014, 73, 308–317. [Google Scholar] [CrossRef] [Green Version]
- do Vale, G.T.; da Silva, C.B.P.; Sousa, A.H.; Gonzaga, N.A.; Parente, J.M.; Araújo, K.M.; Castro, M.M.; Tirapelli, C.R. Nebivolol Prevents Up-Regulation of Nox2/NADPH Oxidase and Lipoperoxidation in the Early Stages of Ethanol-Induced Cardiac Toxicity. Cardiovasc. Toxicol. 2021, 21, 224–235. [Google Scholar] [CrossRef]
- Skrzypiec-Spring, M.; Urbaniak, J.; Sapa-Wojciechowska, A.; Pietkiewicz, J.; Orda, A.; Karolko, B.; Danielewicz, R.; Bil-Lula, I.; Woźniak, M.; Schulz, R.; et al. Matrix Metalloproteinase-2 Inhibition in Acute Ischemia-Reperfusion Heart Injury-Cardioprotective Properties of Carvedilol. Pharmaceuticals 2021, 14, 1276. [Google Scholar] [CrossRef]
- de Brouwer, P.; Bikker, F.J.; Brand, H.S.; Kaman, W.E. Is TIMP-1 a biomarker for periodontal disease? A systematic review and meta-analysis. J. Periodontal. Res. 2022, 57, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Schoeps, B.; Frädrich, J.; Krüger, A. Cut loose TIMP-1: An emerging cytokine in inflammation. Trends Cell Biol. 2022. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Marini, F.; Landi, F.; Marzetti, E. Circulating Inflammatory, Mitochondrial Dysfunction, and Senescence-Related Markers in Older Adults with Physical Frailty and Sarcopenia: A BIOSPHERE Exploratory Study. Int. J. Mol. Sci. 2022, 23, 14006. [Google Scholar] [CrossRef] [PubMed]
- Osipova, O.A.; Gosteva, E.V.; Ilnitski, A.N.; Prashchayeu, K.I.; Rozhdestvenskaya, O.A.; Fesenko, E.V. Effect of pharmacotherapy on collagen metabolism in patients with heart failure with middle range ejection fraction of senile age. Adv. Gerontol. 2020, 33, 956–963. (In Russian) [Google Scholar] [PubMed]
- Guo, Y.; Liu, Y.; Zhao, S.; Xu, W.; Li, Y.; Zhao, P.; Wang, D.; Cheng, H.; Ke, Y.; Zhang, X. Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat. Commun. 2021, 12, 7094. [Google Scholar] [CrossRef] [PubMed]
- Boskabadi, J.; Askari, V.R.; Hosseini, M.; Boskabady, M.H. Immunomodulatory properties of captopril, an ACE inhibitor, on LPS-induced lung inflammation and fibrosis as well as oxidative stress. Inflammopharmacology 2019, 27, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Olawi, N.; Krüger, M.; Grimm, D.; Infanger, M.; Wehland, M. Nebivolol in the treatment of arterial hypertension. Basic Clin. Pharm. Toxicol. 2019, 125, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.U.; Zhao, W.; Zhao, T.; Al Darazi, F.; Ahokas, R.A.; Sun, Y.; Bhattacharya, S.K.; Gerling, I.C.; Weber, K.T. Nebivolol: A multifaceted antioxidant and cardioprotectant in hypertensive heart disease. J. Cardiovasc. Pharmacol. 2013, 62, 445–451. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, X.; Yan, J.; Jiang, J.; Jiang, H. Propofol inhibits LPS-induced apoptosis in lung epithelial cell line, BEAS-2B. Biomed. Pharmacother. 2017, 87, 180–187. [Google Scholar] [CrossRef]
- Morsy, M.A.; Heeba, G.H. Nebivolol Ameliorates Cisplatin-Induced Nephrotoxicity in Rats. Basic Clin. Pharmacol. Toxicol. 2016, 118, 449–455. [Google Scholar] [CrossRef] [Green Version]


| Score | Alveolar Septae Hyperemia | Alveolar Edema | Alveolar Hemorrhage | Intra-Alveolar Neutrophil Filtrations per Field |
|---|---|---|---|---|
| 0 | Normal thin septae | No edema | No hemorrhage | Less than 5 in the fields |
| 1 | Slight hyperemic alveolar septae (in less than 1/3 of the fields) | Slight edema (in less than 1/3 of the fields) | Less than 10 erythrocytes in the fields | 5 to 10 in the fields |
| 2 | Moderate hyperemic alveolar septae (in 1/3 to 2/3 of the fields) | Moderate edema (in 1/3 to 2/3 of the fields) | 11 -20 erythrocytes in the fields | 10 to 20 in the fields |
| 3 | Severe hyperemic alveolar septae (in greater than 2/3 of the fields) | Severe edema (in greater than 2/3 of the fields) | More than 21 erythrocytes in the fields | More than 20 in the fields |
| Gene | Specific Primer Sequence | Tm |
|---|---|---|
| GAPDH (HouseKeeping) | F: 5′-CAAGGTCATCCCAGAGCTGAA-3′ | 62.8 °C |
| R: 5′-CATGTAGGCCATGAGGTCCAC-3′ | ||
| TIMP-1 | F: 5′-ATGTCCACAAGTCCCAGAAC-3′ | 57.6 °C |
| R: 5′-AGCAGGGCTCAGATTATGC-3′ | ||
| MMP-2 | F: 5′-CTGTCCCGACCAAGGATATAG-3′ | 57.0 °C |
| R: 5′-CTTGGTGTAGGTGTAGATAGGG-3 |
| Groups | Histopathological Scores | Cas-3 Scores | ICAM-1 Scores |
|---|---|---|---|
| Control | 0.25 ± 0.16 ***, ### | 0.25 ± 0.16 *** | 0.87 ± 0.35 ***, ### |
| LPS | 2.62 ± 0.51 | 1.62 ± 0.51 | 2.75 ± 0.46 |
| LPS + NBL | 1.25 ± 0.46 *** | 0.62 ± 0.51 *** | 1.37 ± 0.51 *** |
| NBL | 0.12 ± 0.12 ***, ### | 0.12 ± 0.12 *** | 0.62 ± 0.51 ***, ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurlu Temel, E.; Savran, M.; Erzurumlu, Y.; Hasseyid, N.; Buyukbayram, H.I.; Okuyucu, G.; Sevuk, M.A.; Ozmen, O.; Beyan, A.C. The β1 Adrenergic Blocker Nebivolol Ameliorates Development of Endotoxic Acute Lung Injury. J. Clin. Med. 2023, 12, 1721. https://doi.org/10.3390/jcm12051721
Nurlu Temel E, Savran M, Erzurumlu Y, Hasseyid N, Buyukbayram HI, Okuyucu G, Sevuk MA, Ozmen O, Beyan AC. The β1 Adrenergic Blocker Nebivolol Ameliorates Development of Endotoxic Acute Lung Injury. Journal of Clinical Medicine. 2023; 12(5):1721. https://doi.org/10.3390/jcm12051721
Chicago/Turabian StyleNurlu Temel, Esra, Mehtap Savran, Yalcın Erzurumlu, Nursel Hasseyid, Halil Ibrahim Buyukbayram, Gozde Okuyucu, Mehmet Abdulkadir Sevuk, Ozlem Ozmen, and Ayse Coskun Beyan. 2023. "The β1 Adrenergic Blocker Nebivolol Ameliorates Development of Endotoxic Acute Lung Injury" Journal of Clinical Medicine 12, no. 5: 1721. https://doi.org/10.3390/jcm12051721





