Influence of Left Ventricular Diastolic Dysfunction on the Diagnostic Performance of Coronary Computed Tomography Angiography-Derived Fractional Flow Reserve
Abstract
:1. Introduction
2. Materials and Methods
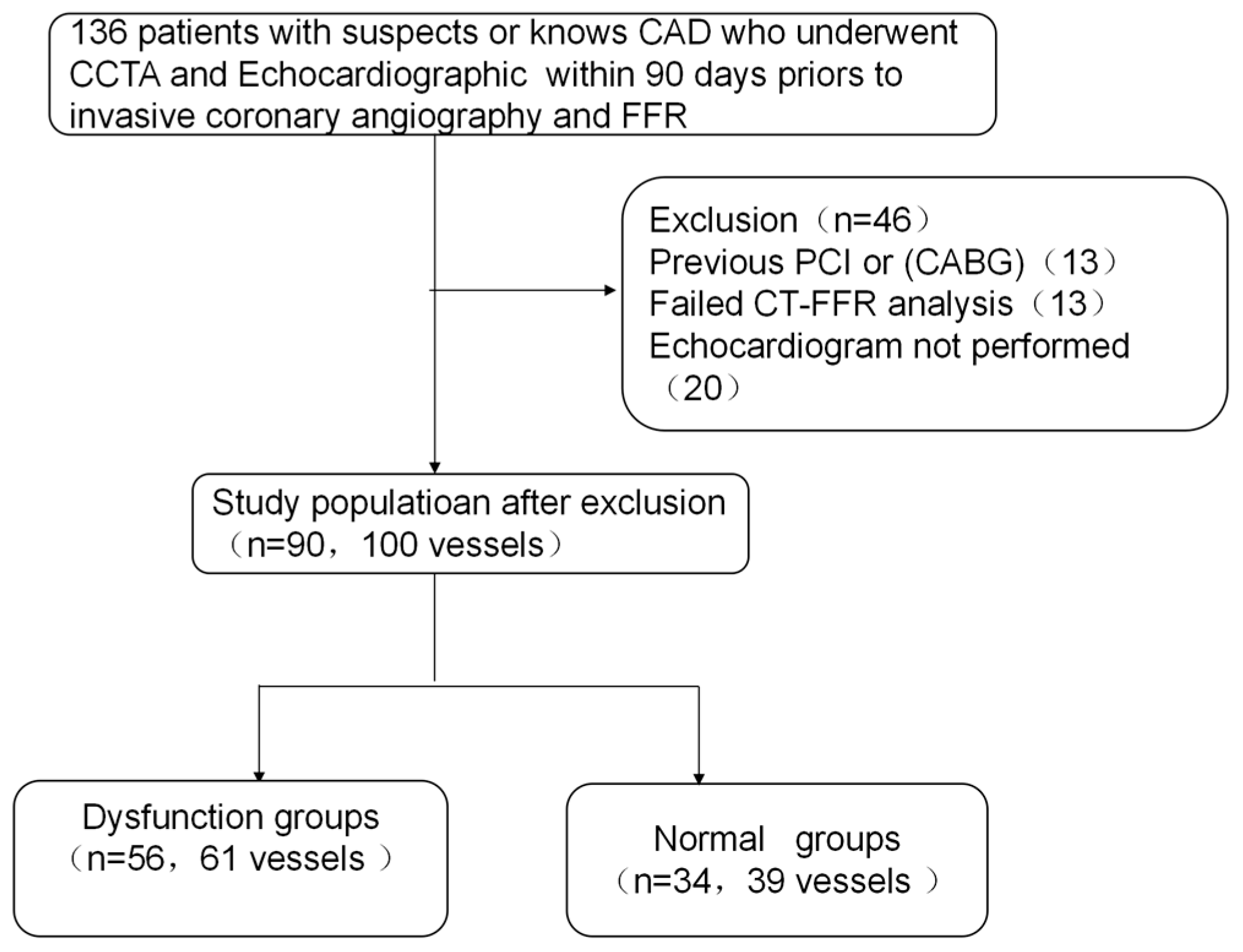
2.1. Study Population
2.2. Coronary Computed Tomography Angiography Analysis
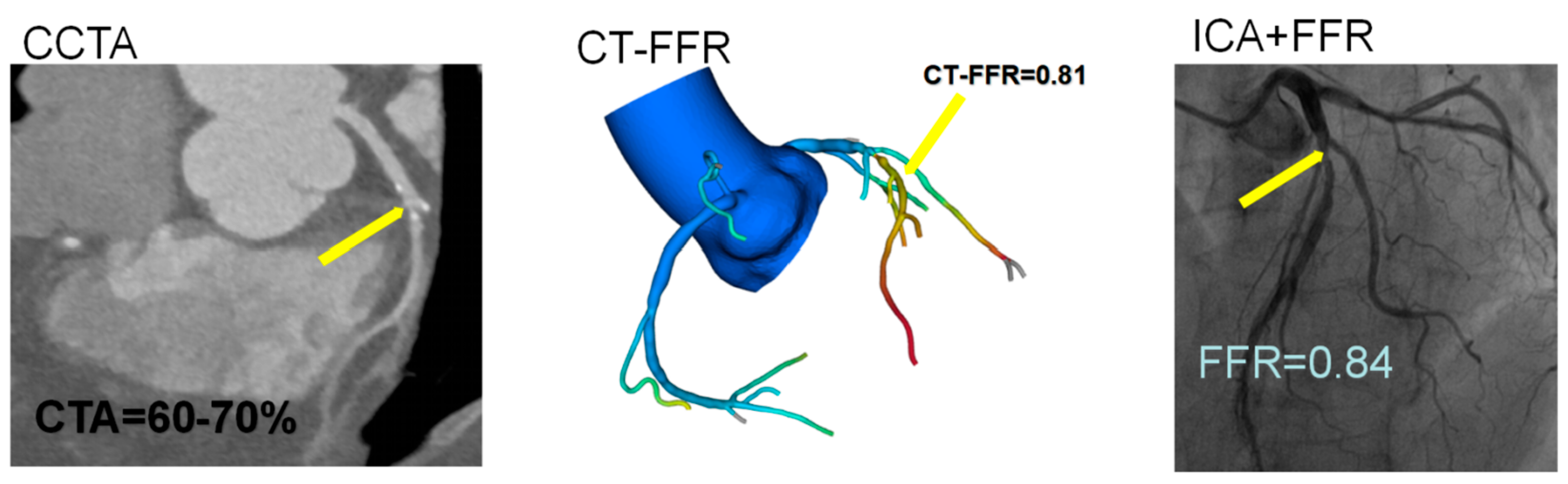
2.3. CT-FFR Analysis
2.4. Invasive Angiography and FFR Measurement
2.5. Echocardiographic Assessment of Left Ventricular Diastolic Function
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
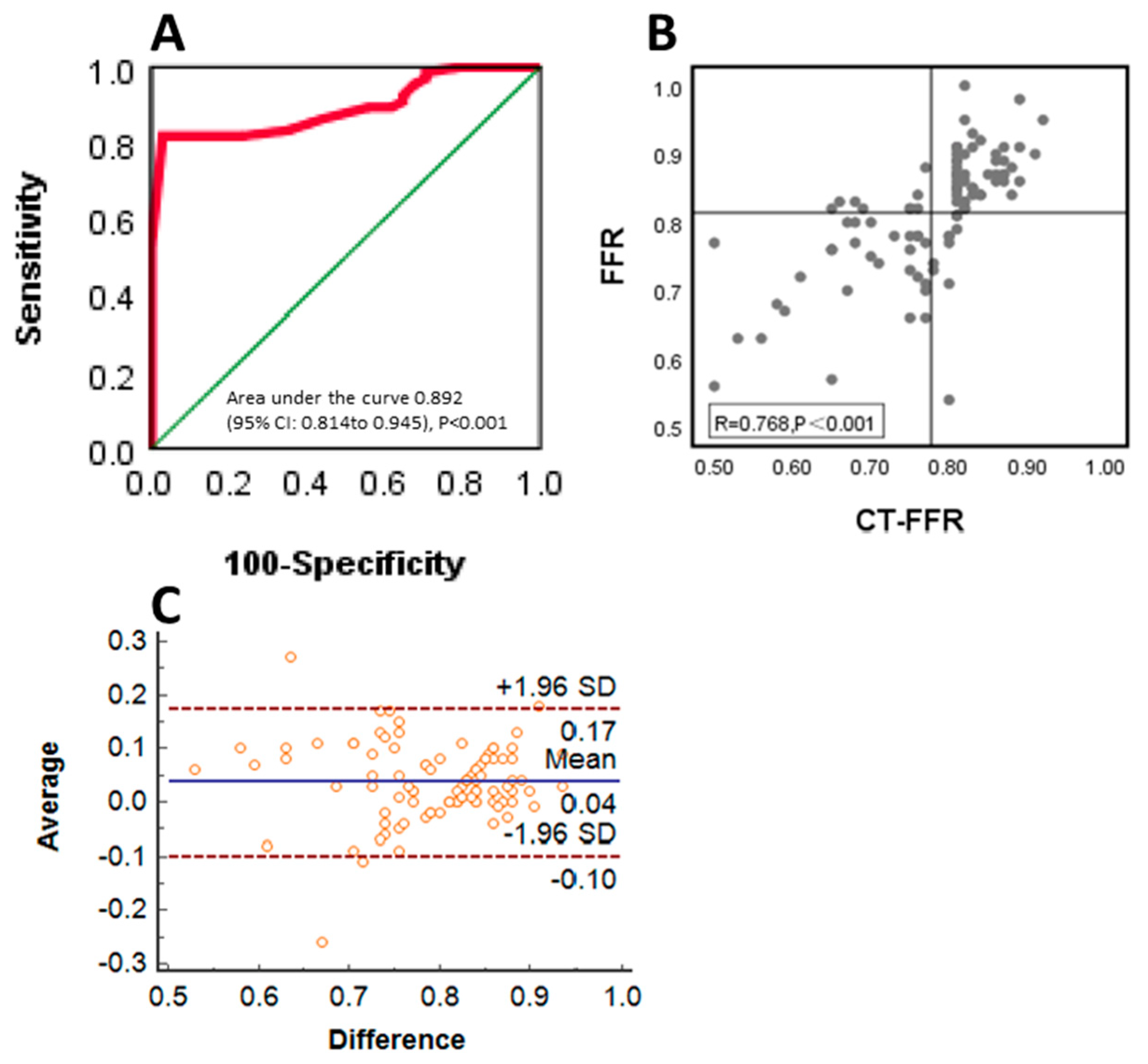
3.2. Diagnostic Performance of CT-FFR Compared with FFR
3.3. Diagnostic Performance and Correlation of CT-FFR to Invasive FFR between the Normal and Dysfunction Groups in Left Ventricular Diastolic Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bangalore, S.; Maron, D.J.; Hochman, J.S. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 37, 2541–2619. [Google Scholar]
- Pijls, N.H.; Fearon, W.F.; Tonino, P.A.; Siebert, U.; Ikeno, F.; Bornschein, B.; Veer, M.V.; Klauss, V.; Manoharan, G.; Engstrøm, T.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J. Am. Coll. Cardiol. 2010, 3, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bruyne, B.; Pijls, N.H.; Kalesan, B.; Barbato, E.; Tonino, P.A.; Piroth, Z.; Jagic, N.; Möbius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 2012, 11, 991–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, B.-K.; Erglis, A.; Doh, J.-H.; Daniels, D.V.; Jegere, S.; Kim, H.-S.; Dunning, A.; DeFrance, T.; Lansky, A.; Leipsic, J.; et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J. Am. Coll. Cardiol. 2011, 19, 1989–1997. [Google Scholar]
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 12, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, R.; Matsumoto, S.; Alani, A.; Li, D.; Kitslaar, P.H.; Broersen, A.; Koo, B.K.; Min, J.K.; Budoff, M.J. Diagnostic performance of transluminal attenuation gradient and fractional flow reserve by coronary computed tomographic angiography (FFR(CT)) compared to invasive FFR: A sub-group analysis from the DISCOVER-FLOW and DeFACTO studies. Int. J. Cardiovasc. Imaging 2015, 6, 1251–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celeng, C.; Leiner, T.; Maurovich-Horvat, P.; Merkely, B.; de Jong, P.; Dankbaar, J.W.; van Es, H.W.; Ghoshhajra, B.B.; Hoffmann, U.; Takx, R.A. Anatomical and Functional Computed Tomography for Diagnosing Hemodynamically Significant Coronary Artery Disease: A Meta-Analysis. JACC Cardiovasc. Imaging 2019, 7 Pt 2, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Kawaji, T.; Shiomi, H.; Morishita, H.; Morimoto, T.; Taylor, C.A.; Kanao, S.; Koizumi, K.; Kozawa, S.; Morihiro, K.; Watanabe, H.; et al. Feasibility and diagnostic performance of fractional flow reserve measurement derived from coronary computed tomography angiography in real clinical practice. Int. J. Cardiovasc. Imaging 2017, 2, 271–281. [Google Scholar] [CrossRef]
- Daud, A.; Xu, D.; Revelo, M.P.; Shah, Z.; Drakos, S.G.; Dranow, E.; Stoddard, G.; Kfoury, A.G.; Hammond, M.E.H.; Nativi-Nicolau, J.; et al. Microvascular Loss and Diastolic Dysfunction in Severe Symptomatic Cardiac Allograft Vasculopathy. Circ. Heart Fail. 2018, 8, e004759. [Google Scholar] [CrossRef]
- Vasiljevic, Z.; Krljanac, G.; Zdravkovic, M.; Lasica, R.; Trifunovic, D.; Asanin, M. Coronary Microcirculation in Heart Failure with Preserved Systolic Function. Curr. Pharm. Des. 2018, 25, 2960–2966. [Google Scholar] [CrossRef]
- Bogaert, J.; Maes, A.; Van de Werf, F.; Bosmans, H.; Herregods, M.C.; Nuyts, J.; Desmet, W.; Mortelmans, L.; Marchal, G.; Rademakers, F.E. Functional recovery of subepicardial myocardial tissue in transmural myocardial infarction after successful reperfusion: An important contribution to the improvement of regional and global left ventricular function. Circulation 1999, 1, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Coenen, A.; Rossi, A.; Lubbers, M.M.; Kurata, A.; Kono, A.K.; Chelu, R.G.; Segreto, S.; Dijkshoorn, M.L.; Wragg, A.; van Geuns, R.J.M.; et al. Integrating CT Myocardial Perfusion and CT-FFR in the Work-Up of Coronary Artery Disease. JACC Cardiovasc. Imaging 2017, 7, 760–770. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Y.; Tang, Z.; Li, T.; Jiang, X.; Ji, F.; Zhou, Y.; Ge, J.; Li, Z.; Zhao, Y.; et al. Diagnostic Accuracy of Computed Tomography-Based Fractional Flow Reserve with a Novel Coarse-to-Fine Subpixel Algorithm in Detecting Hemodynamically Significant Stenosis: A Prospective Multicenter Study. J. Am. Coll. Cardiol. 2022, 79, 1243. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 4, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Shaw, L.J.; Veledar, E.; Berman, D.S.; Hayes, S.W.; Friedman, J.; Slomka, P.; Germano, G.; Maron, D.J.; Mancini, G.B.J.; Hartigan, P.M.; et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008, 10, 1283–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarins, C.K.; Taylor, C.A.; Min, J.K. Computed fractional flow reserve (FFTCT) derived from coronary CT angiography. J. Cardiovasc. Transl. Res. 2013, 5, 708–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearon, W.F.; Bornschein, B.; Tonino, P.A.L.; Gothe, R.M.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Bruyne, B.D. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation 2010, 24, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, R.; Park, H.B.; Berman, D.S.; Gransar, H.; Koo, B.K.; Erglis, A.; Lin, F.Y.; Dunning, A.M.; Budoff, M.J.; Malpeso, J.; et al. Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: Results from the DeFACTO study. Circ. Cardiovasc. Imaging 2013, 6, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Nørgaard, B.L.; Hjort, J.; Gaur, S.; Hansson, N.; Bøtker, H.E.; Leipsic, J.; Mathiassen, O.N.; Grove, E.L.; Pedersen, K.; Christiansen, E.H.; et al. Clinical Use of Coronary CTA-Derived FFR for Decision-Making in Stable CAD. JACC Cardiovasc. Imaging 2017, 5, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Shiono, Y.; Matsuo, H.; Kawasaki, T.; Amano, T.; Kitabata, H.; Kubo, T.; Morino, Y.; Yoda, S.; Sakamoto, T.; Ito, H.; et al. Clinical Impact of Coronary Computed Tomography Angiography-Derived Fractional Flow Reserve on Japanese Population in the ADVANCE Registry. Circ. J. 2019, 6, 1293–1301. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, P.M.; Kolossváry, M.; Karády, J.; Ball, P.A.; Kelly, S.; Fitzsimons, D.; Spence, M.S.; Celeng, C.; Horváth, T.; Szilveszter, B.; et al. Experience With an On-Site Coronary Computed Tomography-Derived Fractional Flow Reserve Algorithm for the Assessment of Intermediate Coronary Stenoses. Am. J. Cardiol. 2018, 1, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Van Hamersvelt, R.W.; Voskuil, M.; de Jong, P.A.; Willemink, M.J.; Išgum, I.; Leiner, T. Diagnostic Performance of On-Site Coronary CT Angiography-derived Fractional Flow Reserve Based on Patient-specific Lumped Parameter Models. Radiol. Cardiothorac. Imaging 2019, 4, e190036. [Google Scholar] [CrossRef]
- Smiseth, O.A. Assessment of ventricular diastolic function. Can. J. Cardiol. 2001, 11, 1167–1176. [Google Scholar]
- Rydberg, E.; Willenheimer, R.; Erhardt, L. The prevalence of impaired left ventricular diastolic filling is related to the extent of coronary atherosclerosis in patients with stable coronary artery disease. Coron. Artery Dis. 2002, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ristow, B.; Na, B.; Ali, S.; Schiller, N.B.; Whooley, M.A. Prevalence and prognosis of asymptomatic left ventricular diastolic dysfunction in ambulatory patients with coronary heart disease. Am. J. Cardiol. 2007, 12, 1643–1647. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.B.; Lin, A.; Kwan, C.; Sippel, J.; Younger, J.F.; Hammett, C.; Thomas, L.; Atherton, J.J. Determinants of Diastolic Dysfunction Following Myocardial Infarction: Evidence for Causation Beyond Infarct Size. Heart Lung Circ. 2020, 12, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.I.; Payne, N.; Phillips, T.; D’Hooge, J.; Fraser, A.G. Strain rate imaging after dynamic stress provides objective evidence of persistent regional myocardial dysfunction in ischaemic myocardium: Regional stunning identified? Heart 2005, 2, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Chang, S.-A.; Sohn, D.-W.; Kim, D.-H.; Kim, Y.-J.; Oh, B.-H.; Park, Y.-B. Persistent regional diastolic dysfunction after myocardial ischemia and the effect of statin treatment: Assessment with two-dimensional radial strain rate. Echocardiography 2010, 3, 244–252. [Google Scholar] [CrossRef]
- He, Y.; Northrup, H.; Le, H.; Cheung, A.K.; Berceli, S.A.; Shiu, Y.T. Medical Image-Based Computational Fluid Dynamics and Fluid-Structure Interaction Analysis in Vascular Diseases. Front. Bioeng. Biotechnol. 2022, 10, 855791. [Google Scholar] [CrossRef]
- Mariotti, A.; Boccadifuoco, A.; Celi, S.; Salvetti, M.V. Hemodynamics and stresses in numerical simulations of the thoracic aorta: Stochastic sensitivity analysis to inlet flow-rate waveform. Comput. Fluids 2023, 230, 105123. [Google Scholar] [CrossRef]
- Taylor, C.A.; Fonte, T.A.; Min, J.K. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: Scientific basis. J. Am. Coll. Cardiol. 2013, 22, 2233–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahir, H.; Livesay, J.; Fogelson, B.; Baljepally, R. Association of Echocardiographic Diastolic Dysfunction with Discordance of Invasive Intracoronary Pressure Indices. J. Clin. Med. 2021, 16, 3670. [Google Scholar] [CrossRef] [PubMed]
- Kawata, T.; Daimon, M.; Miyazaki, S.; Ichikawa, R.; Maruyama, M.; Chiang, S.J.; Ito, C.; Sato, F.; Watada, H.; Daida, H. Coronary microvascular function is independently associated with left ventricular filling pressure in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2015, 14, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Basic Characteristics | All Patients | Dysfunction Group | Normal Group | p Value |
|---|---|---|---|---|
| No. of patients | 90 | 56 | 34 | - |
| No. of vessels | 100 | 39 | 61 | - |
| Age (years) | 64.1 ± 9.64 | 66 ± 10.6 | 60.94 ± 6.6 | 0.006 |
| Man, n (%) | 61 (67.8) | 36 (64.3) | 25 (73.5) | 0.363 |
| BMI (kg/m2) | 23.6 (22.3–25.3) | 23.7 (22.8–25.7) | 23.5 (22–24.7) | 0.296 |
| TC (mmol/L) | 4.1 (3.5–4.8) | 4.1 (3.5–4.5) | 4.1 (3.4–5.2) | 0.484 |
| TG (mmol/L) | 1.23 (0.84–1.83) | 1.23 (0.85–1.88) | 1.28 (0.83–1.73) | 0.970 |
| LDL-C (mmol/L) | 2.53 (2.1–3.19) | 2.52 (2.16–2.86) | 2.53 (2.02–3.55) | 0.671 |
| HDL-C (mmol/L) | 1.01 (0.89–1.21) | 1.00 (0.91–1.17) | 1.06 (0.86–1.24) | 0.758 |
| Cr (umol/L) | 76.2 (64.1–90.8) | 78.7 (67–94.1) | 73.4 (61.3–87.2) | 0.193 |
| Pertinent medical history, n (%) | ||||
| Hypertension | 52 (57.8) | 38 (67.9) | 14 (41.2) | 0.013 |
| Hyperlipidemia | 32 (35.6) | 23 (41.1) | 9 (26) | 0.161 |
| Family history of CAD | 2 (2.2) | 1 (1.8) | 1 (2.9) | 0.404 |
| smoker | 18 (20) | 8 (14.3) | 10 (29.4) | 0.082 |
| Diabetes | 16 (17.8) | 12 (21.4) | 4 (11.8) | 0.380 |
| Cerebral infarction | 7 (7.8) | 6 (10.7) | 1 (2.9) | 0.353 |
| Echocardiographic Parameters | ||||
| LVEF (%) | 64.9 ± 6.1 | 64.2 ± 6.6 | 66.2 ± 5.1 | 0.064 |
| E/e′ | 11.8 ± 3.9 | 13.3 ± 4.04 | 9.1 ± 1.6 | 0.001 |
| E/A | 0.85 ± 0.29 | 0.85 ± 0.3 | 0.86 ± 0.2 | 0.9 |
| e′ | 6.0 ± 1.5 | 5.4 ± 1.1 | 7.1 ± 1.59 | 0.001 |
| IVST (mm) | 10.0 ± 1.1 | 10.2 ± 1.1 | 9.65 ± 1.08 | 0.038 |
| LVEDD (mm) | 45.7 ± 5.4 | 45.5 ± 5.8 | 46.2 ± 7.1 | 0.274 |
| LVESD (mm) | 28.4 ± 5.1 | 28.4 ± 5.9 | 28.5 ± 3.5 | 0.484 |
| LVPW (mm) | 9.8 ± 1.2 | 10 ± 1.1 | 9.5 ± 1.2 | 0.046 |
| Vessel location, n (%) | ||||
| LAD | 70 (70) | 44 (72.1) | 26 (66.7) | 0.816 |
| LCX | 12 (12) | 8 (13.1) | 4 (10.2) | 0.983 |
| RCA | 18 (18) | 9 (14.8) | 9 (23.1) | 0.232 |
| FFR | 0.81 ± 0.87 | 0.81 ± 0.85 | 0.82 ± 0.90 | 0.586 |
| CT-FFR | 0.77 ± 0.88 | 0.77 ± 0.92 | 0.78 ± 0.81 | 0.533 |
| Analysis Basis | Results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modality | TP | TN | FP | FN | Sen. | Spec. | Acc. | PPV | NPV | AUC | ||
| Total | Per-vessel | CT-FFR | 28 | 54 | 12 | 6 | 82.3 | 81.8 | 82 | 70 | 90 | 0.89 |
| normal group | Per-vessel | CT-FFR | 11 | 23 | 3 | 2 | 84.6 | 88.5 | 87.2 | 78.6 | 92 | 0.920 |
| dysfunction group | Per-vessel | CT-FFR | 17 | 31 | 9 | 4 | 81 | 77.5 | 78.7 | 65.4 | 88.6 | 0.871 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Wu, T.; Mu, J.; Zhang, P.; Wang, X.; Liang, T.; Weng, Y.; Luo, J.; Yu, H. Influence of Left Ventricular Diastolic Dysfunction on the Diagnostic Performance of Coronary Computed Tomography Angiography-Derived Fractional Flow Reserve. J. Clin. Med. 2023, 12, 1724. https://doi.org/10.3390/jcm12051724
Xie Z, Wu T, Mu J, Zhang P, Wang X, Liang T, Weng Y, Luo J, Yu H. Influence of Left Ventricular Diastolic Dysfunction on the Diagnostic Performance of Coronary Computed Tomography Angiography-Derived Fractional Flow Reserve. Journal of Clinical Medicine. 2023; 12(5):1724. https://doi.org/10.3390/jcm12051724
Chicago/Turabian StyleXie, Zhixin, Tianlong Wu, Jing Mu, Ping Zhang, Xuan Wang, Tao Liang, Yihan Weng, Jianfang Luo, and Huimin Yu. 2023. "Influence of Left Ventricular Diastolic Dysfunction on the Diagnostic Performance of Coronary Computed Tomography Angiography-Derived Fractional Flow Reserve" Journal of Clinical Medicine 12, no. 5: 1724. https://doi.org/10.3390/jcm12051724




