Association between the Seroprevalence of Antibodies against Seasonal Alphacoronaviruses and SARS-CoV-2 Humoral Immune Response, COVID-19 Severity, and Influenza Vaccination
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. Determination of Antibodies
2.3. Statistical Analysis
3. Results
3.1. Seroprevalence of IgG Antibodies against Seasonal Alphacoronaviruses
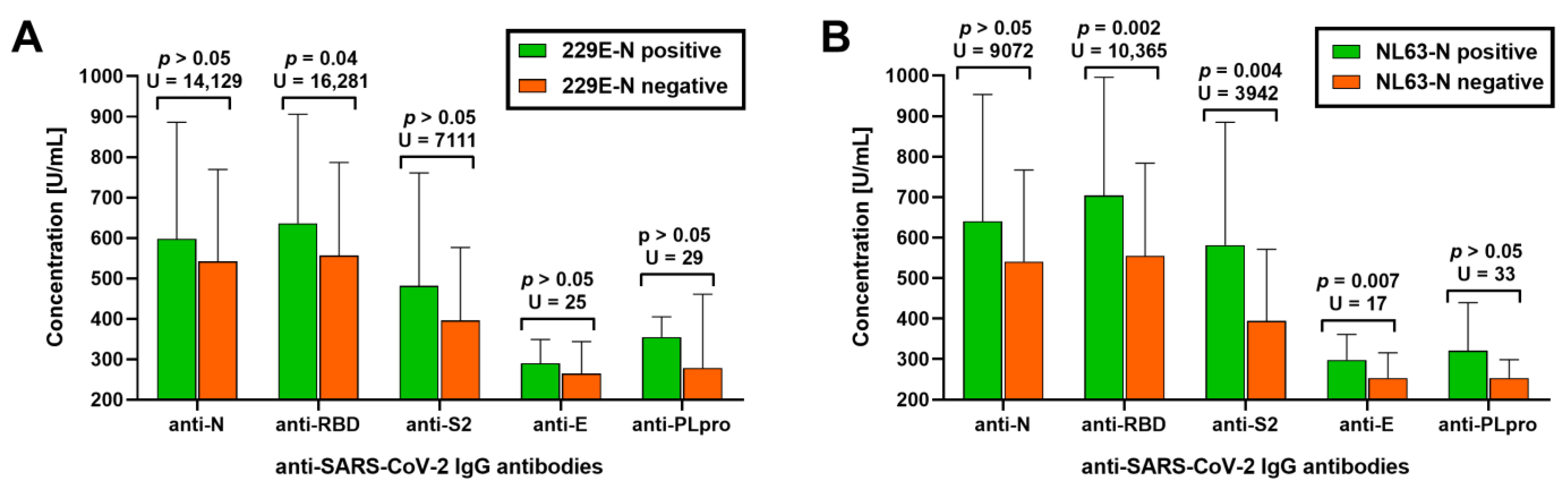
3.2. Association between Antibodies against Seasonal Alphacoronaviruses and Humoral Response to SARS-CoV-2 Infection
3.3. Factors Associated with the Prevalence of Antibodies against Seasonal Alphacoronaviruses
4. Discussion
4.1. Study Strengths
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talbot, H.K.; Shepherd, B.E.; Crowe, J.E., Jr.; Griffin, M.R.; Edwards, K.M.; Podsiad, A.B.; Tollefson, S.J.; Wright, P.F.; Williams, J.V. The Pediatric Burden of Human Coronaviruses Evaluated for Twenty Years. Pediatr. Infect. Dis. J. 2009, 28, 682–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendley, J.O.; Fishburne, H.B.; Gwaltney, J.M., Jr. Coronavirus Infections in Working Adults. Eight-Year Study with 229 E and OC 43. Am. Rev. Respir. Dis. 1972, 105, 805–811. [Google Scholar] [PubMed]
- Bastien, N.; Anderson, K.; Hart, L.; Van Caeseele, P.; Brandt, K.; Milley, D.; Hatchette, T.; Weiss, E.C.; Li, Y. Human Coronavirus NL63 Infection in Canada. J. Infect. Dis. 2005, 191, 503–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vabret, A.; Dina, J.; Gouarin, S.; Petitjean, J.; Corbet, S.; Freymuth, F. Detection of the New Human Coronavirus HKU1: A Report of 6 Cases. Clin. Infect. Dis. 2006, 42, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Tyrrell, D.A.J.; Cohen, S.; Schilarb, J.E. Signs and Symptoms in Common Colds. Epidemiol. Infect. 1993, 111, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Eboriadou, M.; Haidopoulou, K.; Xanthou, P.; Papa, A. Case ReportCoronaviruses OC43 and 229E Lower Respiratory Tract Co-Infections: A Clinical Report of Two Cases. Arch. Med. Sci. 2008, 4, 88–90. [Google Scholar]
- Pene, F.; Merlat, A.; Vabret, A.; Rozenberg, F.; Buzyn, A.; Dreyfus, F.; Cariou, A.; Freymuth, F.; Lebon, P. Coronavirus 229E-Related Pneumonia in Immunocompromised Patients. Clin. Infect. Dis. 2003, 37, 929–932. [Google Scholar] [CrossRef] [Green Version]
- Walsh, E.E.; Shin, J.H.; Falsey, A.R. Clinical Impact of Human Coronaviruses 229E and OC43 Infection in Diverse Adult Populations. J. Infect. Dis. 2013, 208, 1634–1642. [Google Scholar] [CrossRef] [Green Version]
- Gorse, G.J.; O’Connor, T.Z.; Hall, S.L.; Vitale, J.N.; Nichol, K.L. Human Coronavirus and Acute Respiratory Illness in Older Adults with Chronic Obstructive Pulmonary Disease. J. Infect. Dis. 2009, 199, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Smuts, H. Human Coronavirus NL63 Infections in Infants Hospitalised with Acute Respiratory Tract Infections in South Africa. Influenza Other Respi. Viruses 2008, 2, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risku, M.; Lappalainen, S.; Räsänen, S.; Vesikari, T. Detection of Human Coronaviruses in Children with Acute Gastroenteritis. J. Clin. Virol. 2010, 48, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Arbour, N.; Day, R.; Newcombe, J.; Talbot, P.J. Neuroinvasion by Human Respiratory Coronaviruses. J. Virol. 2000, 74, 8913–8921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.M.; Winn, A.; Dahl, R.M.; Kniss, K.L.; Silk, B.J.; Killerby, M.E. Seasonality of Common Human Coronaviruses, United States, 2014-2021. Emerg. Infect. Dis. 2022, 28, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Killerby, M.E.; Biggs, H.M.; Haynes, A.; Dahl, R.M.; Mustaquim, D.; Gerber, S.I.; Watson, J.T. Human Coronavirus Circulation in the United States 2014–2017. J. Clin. Virol. 2018, 101, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Nair, H. Global Seasonality of Human Seasonal Coronaviruses: A Clue for Postpandemic Circulating Season of Severe Acute Respiratory Syndrome Coronavirus 2? J. Infect. Dis. 2020, 222, 1090–1097. [Google Scholar] [CrossRef]
- Lepiller, Q.; Barth, H.; Lefebvre, F.; Herbrecht, R.; Lutz, P.; Kessler, R.; Fafi-Kremer, S.; Stoll-Keller, F. High Incidence but Low Burden of Coronaviruses and Preferential Associations between Respiratory Viruses. J. Clin. Microbiol. 2013, 51, 3039–3046. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.S.; Chan, K.H.; Chu, K.W.; Kwan, S.W.; Guan, Y.; Poon, L.L.M.; Peiris, J.S.M. Human Coronavirus NL63 Infection and Other Coronavirus Infections in Children Hospitalized with Acute Respiratory Disease in Hong Kong, China. Clin. Infect. Dis. 2005, 40, 1721–1729. [Google Scholar] [CrossRef] [Green Version]
- Gaunt, E.R.; Hardie, A.; Claas, E.C.J.; Simmonds, P.; Templeton, K.E. Epidemiology and Clinical Presentations of the Four Human Coronaviruses 229E, HKU1, NL63, and OC43 Detected over 3 Years Using a Novel Multiplex Real-Time PCR Method. J. Clin. Microbiol. 2010, 48, 2940–2947. [Google Scholar] [CrossRef] [Green Version]
- Kuypers, J.; Martin, E.T.; Heugel, J.; Wright, N.; Morrow, R.; Englund, J.A. Clinical Disease in Children Associated with Newly Described Coronavirus Subtypes. Pediatrics 2007, 119, e70–e76. [Google Scholar] [CrossRef]
- Uddin, S.M.I.; Englund, J.A.; Kuypers, J.Y.; Chu, H.Y.; Steinhoff, M.C.; Khatry, S.K.; LeClerq, S.C.; Tielsch, J.M.; Mullany, L.C.; Shrestha, L.; et al. Burden and Risk Factors for Coronavirus Infections in Infants in Rural Nepal. Clin. Infect. Dis. 2018, 67, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.; Lopez, P.; Weckx, L.; Borja-Tabora, C.; Ulloa-Gutierrez, R.; Lazcano-Ponce, E.; Kerdpanich, A.; Angel Rodriguez Weber, M.; Mascareñas de Los Santos, A.; Tinoco, J.-C.; et al. Respiratory Viruses and Influenza-like Illness: Epidemiology and Outcomes in Children Aged 6 Months to 10 Years in a Multi-Country Population Sample. J. Infect. 2017, 74, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Skevaki, C.L.; Tsialta, P.; Trochoutsou, A.I.; Logotheti, I.; Makrinioti, H.; Taka, S.; Lebessi, E.; Paraskakis, I.; Papadopoulos, N.G.; Tsolia, M.N. Associations between Viral and Bacterial Potential Pathogens in the Nasopharynx of Children with and without Respiratory Symptoms. Pediatr. Infect. Dis. J. 2015, 34, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Poutanen, S.M. Human Coronaviruses. In Principles and Practice of Pediatric Infectious Diseases; Long, S.S., Prober, C.G., Fischer, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1148–1152.e3. ISBN 9780323401814. [Google Scholar]
- Tamminen, K.; Salminen, M.; Blazevic, V. Seroprevalence and SARS-CoV-2 Cross-Reactivity of Endemic Coronavirus OC43 and 229E Antibodies in Finnish Children and Adults. Clin. Immunol. 2021, 229, 108782. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuoka, M.; Tabata, S.; Kitagawa, Y.; Nagura-Ikeda, M.; Kubota, K.; Fukada, A.; Takada, T.; Sato, M.; Noguchi, S.; et al. Cross-Reactive Humoral Immune Responses against Seasonal Human Coronaviruses in COVID-19 Patients with Different Disease Severities. Int. J. Infect. Dis. 2021, 111, 68–75. [Google Scholar] [PubMed]
- Dijkstra, J.M.; Hashimoto, K. Expected Immune Recognition of COVID-19 Virus by Memory from Earlier Infections with Common Coronaviruses in a Large Part of the World Population. F1000Research 2020, 9, 285. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wolf, J.; Brice, D.C.; Sun, Y.; Locke, M.; Cherry, S.; Castellaw, A.H.; Wehenkel, M.; Crawford, J.C.; Zarnitsyna, V.I.; et al. Pre-Existing Humoral Immunity to Human Common Cold Coronaviruses Negatively Impacts the Protective SARS-CoV-2 Antibody Response. Cell Host Microbe 2022, 30, 83–96.e4. [Google Scholar] [CrossRef]
- Sagar, M.; Reifler, K.; Rossi, M.; Miller, N.S.; Sinha, P.; White, L.F.; Mizgerd, J.P. Recent Endemic Coronavirus Infection Is Associated with Less-Severe COVID-19. J. Clin. Investig. 2021, 131, e143380. [Google Scholar] [CrossRef]
- Song, G.; He, W.-T.; Callaghan, S.; Anzanello, F.; Huang, D.; Ricketts, J.; Torres, J.L.; Beutler, N.; Peng, L.; Vargas, S.; et al. Cross-Reactive Serum and Memory B-Cell Responses to Spike Protein in SARS-CoV-2 and Endemic Coronavirus Infection. Nat. Commun. 2021, 12, 2938. [Google Scholar] [CrossRef]
- Amanat, F.; Clark, J.; Carreño, J.M.; Strohmeier, S.; Meade, P.; Bhavsar, D.; Muramatsu, H.; Sun, W.; Coughlan, L.; Pardi, N.; et al. Immunity to Seasonal Coronavirus Spike Proteins Does Not Protect from SARS-CoV-2 Challenge in a Mouse Model but Has No Detrimental Effect on Protection Mediated by COVID-19 MRNA Vaccination. bioRxiv 2023, e01664-22, Online ahead of print. [Google Scholar] [CrossRef]
- Lavell, A.H.A.; Sikkens, J.J.; Edridge, A.W.D.; van der Straten, K.; Sechan, F.; Oomen, M.; Buis, D.T.P.; Schinkel, M.; Burger, J.A.; Poniman, M.; et al. Recent Infection with HCoV-OC43 May Be Associated with Protection against SARS-CoV-2 Infection. iScience 2022, 25, 105105. [Google Scholar] [CrossRef]
- Dugas, M.; Grote-Westrick, T.; Merle, U.; Fontenay, M.; Kremer, A.E.; Hanses, F.; Vollenberg, R.; Lorentzen, E.; Tiwari-Heckler, S.; Duchemin, J.; et al. Lack of Antibodies against Seasonal Coronavirus OC43 Nucleocapsid Protein Identifies Patients at Risk of Critical COVID-19. J. Clin. Virol. 2021, 139, 104847. [Google Scholar] [CrossRef]
- Focosi, D.; Genoni, A.; Lucenteforte, E.; Tillati, S.; Tamborini, A.; Spezia, P.G.; Azzi, L.; Baj, A.; Maggi, F. Previous Humoral Immunity to the Endemic Seasonal Alphacoronaviruses NL63 and 229E Is Associated with Worse Clinical Outcome in COVID-19 and Suggests Original Antigenic Sin. Life 2021, 11, 298. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Kang, L.; Hu, Y.; Wang, L.; Zhong, J.; Chen, H.; Ren, L.; Gu, X.; Wang, G.; et al. Cross-Reactive Antibody against Human Coronavirus OC43 Spike Protein Correlates with Disease Severity in COVID-19 Patients: A Retrospective Study. Emerg. Microbes Infect. 2021, 10, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The Effects of the COVID-19 Pandemic on Community Respiratory Virus Activity. Nat. Rev. Microbiol. 2022, 21, 195–210. [Google Scholar] [CrossRef]
- García-García, E.; Rodríguez-Pérez, M.; Melón García, S.; Fernández Montes, R.; Suárez Castañón, C.; Amigo Bello, M.C.; Rodríguez Dehli, C.; Pérez-Méndez, C.; Alonso Álvarez, M.A.; Calle-Miguel, L. Change on the Circulation of Respiratory Viruses and Pediatric Healthcare Utilization during the COVID-19 Pandemic in Asturias, Northern Spain. Children 2022, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, M.A.; Pollara, C.; Gargiulo, F.; Giacomelli, M.; Caruso, A. Circulation of Respiratory Viruses in Hospitalized Adults before and during the COVID-19 Pandemic in Brescia, Italy: A Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 9525. [Google Scholar] [CrossRef] [PubMed]
- Smedberg, J.R.; DiBiase, L.M.; Hawken, S.E.; Allen, A.; Mohan, S.; Santos, C.; Smedberg, T.; Barzin, A.H.; Wohl, D.A.; Miller, M.B. Reduction and Persistence of Co-Circulating Respiratory Viruses during the SARS-CoV-2 Pandemic. Am. J. Infect. Control 2022, 50, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Conlon, A.; Ashur, C.; Washer, L.; Eagle, K.A.; Hofmann Bowman, M.A. Impact of the Influenza Vaccine on COVID-19 Infection Rates and Severity. Am. J. Infect. Control 2021, 49, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wang, H.; Sun, C.; Li, N.; Guo, X.; Song, Q.; Liang, Q.; Liang, M.; Ding, X.; Sun, Y. The Association between Previous Influenza Vaccination and COVID-19 Infection Risk and Severity: A Systematic Review and Meta-Analysis. Am. J. Prev. Med. 2022, 63, 121–130. [Google Scholar] [CrossRef]
- Flisiak, R.; Rzymski, P.; Zarębska-Michaluk, D.; Rogalska, M.; Rorat, M.; Czupryna, P.; Lorenc, B.; Ciechanowski, P.; Kozielewicz, D.; Piekarska, A.; et al. Demographic and Clinical Overview of Hospitalized COVID-19 Patients during the First 17 Months of the Pandemic in Poland. J. Clin. Med. 2021, 11, 117. [Google Scholar] [CrossRef]
- Hoang, V.-T.; Colson, P.; Levasseur, A.; Delerce, J.; Lagier, J.-C.; Parola, P.; Million, M.; Fournier, P.-E.; Raoult, D.; Gautret, P. Clinical Outcomes in Patients Infected with Different SARS-CoV-2 Variants at One Hospital during Three Phases of the COVID-19 Epidemic in Marseille, France. Infect. Genet. Evol. 2021, 95, 105092. [Google Scholar] [CrossRef]
- Hryhorowicz, S.; Ustaszewski, A.; Kaczmarek-Ryś, M.; Lis, E.; Witt, M.; Pławski, A.; Ziętkiewicz, E. European Context of the Diversity and Phylogenetic Position of SARS-CoV-2 Sequences from Polish COVID-19 Patients. J. Appl. Genet. 2021, 62, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Our World in Data 2020, 7, 345. [Google Scholar]
- WHO Recommendations on Influenza Vaccination during the 2019–2020 Winter Season. Available online: https://www.euro.who.int/__data/assets/pdf_file/0017/413270/Influenza-vaccine-recommendations-2019-2020_en.pdf (accessed on 11 February 2023).
- Rzymski, P.; Borkowski, L.; Drąg, M.; Flisiak, R.; Jemielity, J.; Krajewski, J.; Mastalerz-Migas, A.; Matyja, A.; Pyrć, K.; Simon, K.; et al. The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation. Vaccines 2021, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, E.; Sikora, D.; Poniedziałek, B.; Szymański, K.; Kondratiuk, K.; Żurawski, J.; Brydak, L.; Rzymski, P. IgG Autoantibodies against ACE2 in SARS-CoV-2 Infected Patients. J. Med. Virol. 2023, 95, e28273. [Google Scholar] [CrossRef] [PubMed]
- Masse, S.; Capai, L.; Villechenaud, N.; Blanchon, T.; Charrel, R.; Falchi, A. Epidemiology and Clinical Symptoms Related to Seasonal Coronavirus Identified in Patients with Acute Respiratory Infections Consulting in Primary Care over Six Influenza Seasons (2014–2020) in France. Viruses 2020, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.G.; Bazzi, L.A.; McDermott, A.B.; Follmann, D.; Esposito, D.; Hatcher, C.; Mateja, A.; Narpala, S.R.; O’Connell, S.E.; Martin, E.T.; et al. Coronavirus Occurrence in the Household Influenza Vaccine Evaluation (HIVE) Cohort of Michigan Households: Reinfection Frequency and Serologic Responses to Seasonal and Severe Acute Respiratory Syndrome Coronaviruses. J. Infect. Dis. 2021, 224, 49–59. [Google Scholar] [CrossRef]
- Mela, A.; Rdzanek, E.; Poniatowski, Ł.A.; Jaroszynski, J.; Kalicki, T.; Dutka, M.M.; Furman, M.; Czajka, A.; Furtak-Niczyporuk, M.; Wojciechowska, M.; et al. Epidemiological Features and Changes in the Occurrence of Infectious Diseases in Poland from 2015 to 2020 in the Context of the Emerging Novel SARS-CoV-2 (COVID-19) Pandemic. Oncol Clin Pract 2022, 18, 247–256. [Google Scholar] [CrossRef]
- Chan, K.H.; Cheng, V.C.C.; Woo, P.C.Y.; Lau, S.K.P.; Poon, L.L.M.; Guan, Y.; Seto, W.H.; Yuen, K.Y.; Peiris, J.S.M. Serological Responses in Patients with Severe Acute Respiratory Syndrome Coronavirus Infection and Cross-Reactivity with Human Coronaviruses 229E, OC43, and NL63. Clin. Diagn. Lab. Immunol. 2005, 12, 1317–1321. [Google Scholar] [CrossRef] [Green Version]
- Kubale, J.; Gleason, C.; Carreño, J.M.; Srivastava, K.; Singh, G.; PARIS Study Team; Gordon, A.; Krammer, F.; Simon, V. SARS-CoV-2 Spike-Binding Antibody Longevity and Protection from Reinfection with Antigenically Similar SARS-CoV-2 Variants. MBio 2022, e0178422. [Google Scholar] [CrossRef]
- Peluso, M.J.; Takahashi, S.; Hakim, J.; Kelly, J.D.; Torres, L.; Iyer, N.S.; Turcios, K.; Janson, O.; Munter, S.E.; Thanh, C.; et al. SARS-CoV-2 Antibody Magnitude and Detectability Are Driven by Disease Severity, Timing, and Assay. Sci. Adv. 2021, 7, eabh3409. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Hu, M.; Wen, L.; Wen, C.; Wang, Y.; Zhu, W.; Tai, S.; Jiang, Z.; Xiao, K.; et al. Antibody Seroconversion in Asymptomatic and Symptomatic Patients Infected with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Transl. Immunol. 2020, 9, e1182. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.R.; Islam, N.; Dambha-Miller, H. Association between Influenza Vaccination and Hospitalisation or All-Cause Mortality in People with COVID-19: A Retrospective Cohort Study. BMJ Open Respir. Res. 2021, 8, e000857. [Google Scholar] [CrossRef] [PubMed]
- Poniedziałek, B.; Hallmann, E.; Sikora, D.; Szymański, K.; Kondratiuk, K.; Żurawski, J.; Rzymski, P.; Brydak, L. Relationship between Humoral Response in COVID-19 and Seasonal Influenza Vaccination. Vaccines 2022, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Brydak, L.B.; Szymański, K.; Kondratiuk, K.; Poznańska, A.; Kolondra, A.; Hallmann, E. Importance of Influenza Anti-Hemagglutinin Antibodies during the SARS-CoV-2 Pandemic in the 2019/2020 Epidemic Season in Poland. Med. Sci. Monit. 2022, 28, e936495. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.-J.; et al. Induction of Trained Immunity by Influenza Vaccination—Impact on COVID-19. PLoS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef]
- Fink, G.; Orlova-Fink, N.; Schindler, T.; Grisi, S.; Ferrer, A.P.S.; Daubenberger, C.; Brentani, A. Inactivated Trivalent Influenza Vaccination Is Associated with Lower Mortality among Patients with COVID-19 in Brazil. BMJ Evid. Based Med. 2020, 26, 192–193. [Google Scholar] [CrossRef]
- Kraśnicka, J.; Krajewska-Kułak, E.; Klimaszewska, K.; Cybulski, M.; Guzowski, A.; Kowalewska, B.; Jankowiak, B.; Rolka, H.; Doroszkiewicz, H.; Kułak, W. Mandatory and Recommended Vaccinations in Poland in the Views of Parents. Hum. Vaccin. Immunother. 2018, 14, 2884–2893. [Google Scholar] [CrossRef] [Green Version]
- Nitsch-Osuch, A.; Gołębiak, I.; Wyszkowska, D.; Rosińska, R.; Kargul, L.; Szuba, B.; Tyszko, P.; Brydak, L.B. Influenza Vaccination Coverage among Polish Patients with Chronic Diseases. Adv. Exp. Med. Biol. 2017, 968, 19–34. [Google Scholar]
- Brydak, L.B.; Woźniak Kosek, A.; Nitsch-Osuch, A. Influenza Vaccines and Vaccinations in Poland—Past, Present and Future. Med. Sci. Monit. 2012, 18, RA166. [Google Scholar] [CrossRef] [Green Version]
- Treggiari, D.; Piubelli, C.; Formenti, F.; Silva, R.; Perandin, F. Resurgence of Respiratory Virus after Relaxation of COVID-19 Containment Measures: A Real-World Data Study from a Regional Hospital of Italy. Int. J. Microbiol. 2022, 2022, 4915678. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.A.; Sikazwe, C.T.; Minney-Smith, C.A.; Ernst, T.; Moore, H.C.; Nicol, M.P.; Smith, D.W.; Levy, A.; Blyth, C.C. An Unusual Resurgence of Human Metapneumovirus in Western Australia Following the Reduction of Non-Pharmaceutical Interventions to Prevent SARS-CoV-2 Transmission. Viruses 2022, 14, 2135. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Hartling, U.B.; Nielsen, J.; Vestergaard, L.S.; Dungu, K.H.S.; Nielsen, J.S.A.; Sellmer, A.; Matthesen, A.T.; Kristensen, K.; Holm, M. Hospital Admissions and Need for Mechanical Ventilation in Children with Respiratory Syncytial Virus before and during the COVID-19 Pandemic: A Danish Nationwide Cohort Study. Lancet Child Adolesc. Health 2023, 7, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Reeves, R.M.; Wang, X.; Bassat, Q.; Brooks, W.A.; Cohen, C.; Moore, D.P.; Nunes, M.; Rath, B.; Campbell, H.; et al. Global Patterns in Monthly Activity of Influenza Virus, Respiratory Syncytial Virus, Parainfluenza Virus, and Metapneumovirus: A Systematic Analysis. Lancet Glob. Health 2019, 7, e1031–e1045. [Google Scholar] [CrossRef] [Green Version]
- Drinka, P.J.; Gravenstein, S.; Krause, P.; Langer, E.H.; Barthels, L.; Dissing, M.; Shult, P.; Schilling, M. Non-Influenza Respiratory Viruses May Overlap and Obscure Influenza Activity. J. Am. Geriatr. Soc. 1999, 47, 1087–1093. [Google Scholar] [CrossRef]
- Franco, N.; Coletti, P.; Willem, L.; Angeli, L.; Lajot, A.; Abrams, S.; Beutels, P.; Faes, C.; Hens, N. Inferring Age-Specific Differences in Susceptibility to and Infectiousness upon SARS-CoV-2 Infection Based on Belgian Social Contact Data. PLoS Comput. Biol. 2022, 18, e1009965. [Google Scholar] [CrossRef]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.-J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of Antibody Responses up to 13 Months after SARS-CoV-2 Infection and Risk of Reinfection. EBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef]
- Dobrowolska, K.; Brzdęk, M.; Zarębska-Michaluk, D.; Rzymski, P.; Rogalska, M.; Moniuszko-Malinowska, A.; Szymanek-Pasternak, A.; Jaroszewicz, J.; Dutkiewicz, E.; Kowalska, J.; et al. Differences between the Course of SARS-CoV-2 Infections in the Periods of the Delta and Omicron Variants Dominance in Poland. Pol. Arch. Intern. Med. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Flisiak, R.; Rzymski, P.; Zarębska-Michaluk, D.; Ciechanowski, P.; Dobrowolska, K.; Rogalska, M.; Jaroszewicz, J.; Szymanek-Pasternak, A.; Rorat, M.; Kozielewicz, D.; et al. Variability in the Clinical Course of COVID-19 in a Retrospective Analysis of a Large Real-World Database. Viruses 2023, 15, 149. [Google Scholar] [CrossRef]
| Age, mean ± SD (min–max) | 37.2 ± 9.8 (18–80) |
| >50 years, % (n) | 9.8 (129) |
| Women/men, % (n) | 20.3 (266)/79.7 (1047) |
| COVID-19 severity | |
| 27.6 (363) |
| 66.2 (869) |
| 6.2 (81) |
| Influenza vaccination in the 2019/2020 season | |
| 50.1 (658) |
| 49.9 (655) |
| IgG anti-229E-N | IgG anti-NL63-N | |||
|---|---|---|---|---|
| Positive (n = 43) | Negative (n = 1270) | Positive (n = 31) | Negative (n = 1282) | |
| anti-N | 86.0 | 67.3 | 83.9 | 67.6 |
| p = 0.01 (χ2 = 6.7) | p = 0.05 (χ2 = 3.7) | |||
| anti-RBD | 93.0 | 77.9 | 100.0 | 77.8 |
| p = 0.02 (χ2 = 5.6) | p = 0.003 (χ2 = 8.8) | |||
| anti-S2 | 72.1 | 41.3 | 74.2 | 41.5 |
| p < 0.001 (χ2 = 16.2) | p < 0.001 (χ2 = 13.3) | |||
| anti-E | 34.9 | 0.55 | 45.2 | 0.62 |
| p < 0.001 (χ2 = 297.6) | p < 0.001 (χ2 = 364.4) | |||
| anti-PLpro | 37.2 | 0.47 | 48.4 | 0.54 |
| p < 0.001 (χ2 = 340.2) | p < 0.001 (χ2 = 420.5) | |||
| IgG anti-229E-N | IgG anti-NL63-N | |||
|---|---|---|---|---|
| Positive (n = 43) | Negative (n = 1270) | Positive (n = 31) | Negative (n = 1282) | |
| Age (mean ± SD) | 37.6 ± 9.5 | 37.2 ± 9.8 | 35.8 ± 9.9 | 37.2 ± 9.8 |
| p > 0.05 (U = 26,450) | p > 0.05 (U = 18,022) | |||
| Women/men (%) | 20.9/79.1 | 20.3/79.7 | 25.8/74.2 | 20.2/79.8 |
| p > 0.05 (χ2 = 0.01) | p > 0.05 (χ2 = 0.6) | |||
| Vaccinated against influenza/unvaccinated (%) | 27.9/72.1 | 50.9/49.1 | 51.6/48.4 | 50.1/49.9 |
| p = 0.003 (χ2 = 8.8) | p > 0.05 (χ2 = 0.03) | |||
COVID-19 severity
| ||||
| 48.8 | 26.9 | 48.4 | 27.1 | |
| 48.8 | 66.8 | 48.4 | 66.6 | |
| 2.3 | 6.3 | 3.2 | 6.2 | |
| p = 0.006 (χ2 = 10.3) | p = 6.9 (χ2 = 6.9) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brydak, L.; Sikora, D.; Poniedziałek, B.; Hallmann, E.; Szymański, K.; Kondratiuk, K.; Rzymski, P. Association between the Seroprevalence of Antibodies against Seasonal Alphacoronaviruses and SARS-CoV-2 Humoral Immune Response, COVID-19 Severity, and Influenza Vaccination. J. Clin. Med. 2023, 12, 1733. https://doi.org/10.3390/jcm12051733
Brydak L, Sikora D, Poniedziałek B, Hallmann E, Szymański K, Kondratiuk K, Rzymski P. Association between the Seroprevalence of Antibodies against Seasonal Alphacoronaviruses and SARS-CoV-2 Humoral Immune Response, COVID-19 Severity, and Influenza Vaccination. Journal of Clinical Medicine. 2023; 12(5):1733. https://doi.org/10.3390/jcm12051733
Chicago/Turabian StyleBrydak, Lidia, Dominika Sikora, Barbara Poniedziałek, Ewelina Hallmann, Karol Szymański, Katarzyna Kondratiuk, and Piotr Rzymski. 2023. "Association between the Seroprevalence of Antibodies against Seasonal Alphacoronaviruses and SARS-CoV-2 Humoral Immune Response, COVID-19 Severity, and Influenza Vaccination" Journal of Clinical Medicine 12, no. 5: 1733. https://doi.org/10.3390/jcm12051733







