Sustained ACE2 Expression by Probiotic Improves Integrity of Intestinal Lymphatics and Retinopathy in Type 1 Diabetic Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Experimental Animals and LP-ACE2 Treatment
2.3. Assessment of Retinal Functions and Acellular Capillaries Quantification
2.4. Circulating Lipid Profile Assay
2.5. Immunofluorescence Staining of Intestinal Lacteal, Gut Barriers, and Retina
2.6. RNA Isolation and qRT-PCR
2.7. Statistical Analysis
3. Results
3.1. LP-ACE2 Treatment Attenuates Diabetes-Induced Lacteal Defects and Gut Barrier Dysfunction in Akita Mice
3.2. Effect of Oral Administration of LP-ACE2 on Circulating Lipid Levels
3.3. LP-ACE2 Restored Reverse Cholesterol Transport Protein (ABCG1/ABCA1) in the Retina of Akita Mice
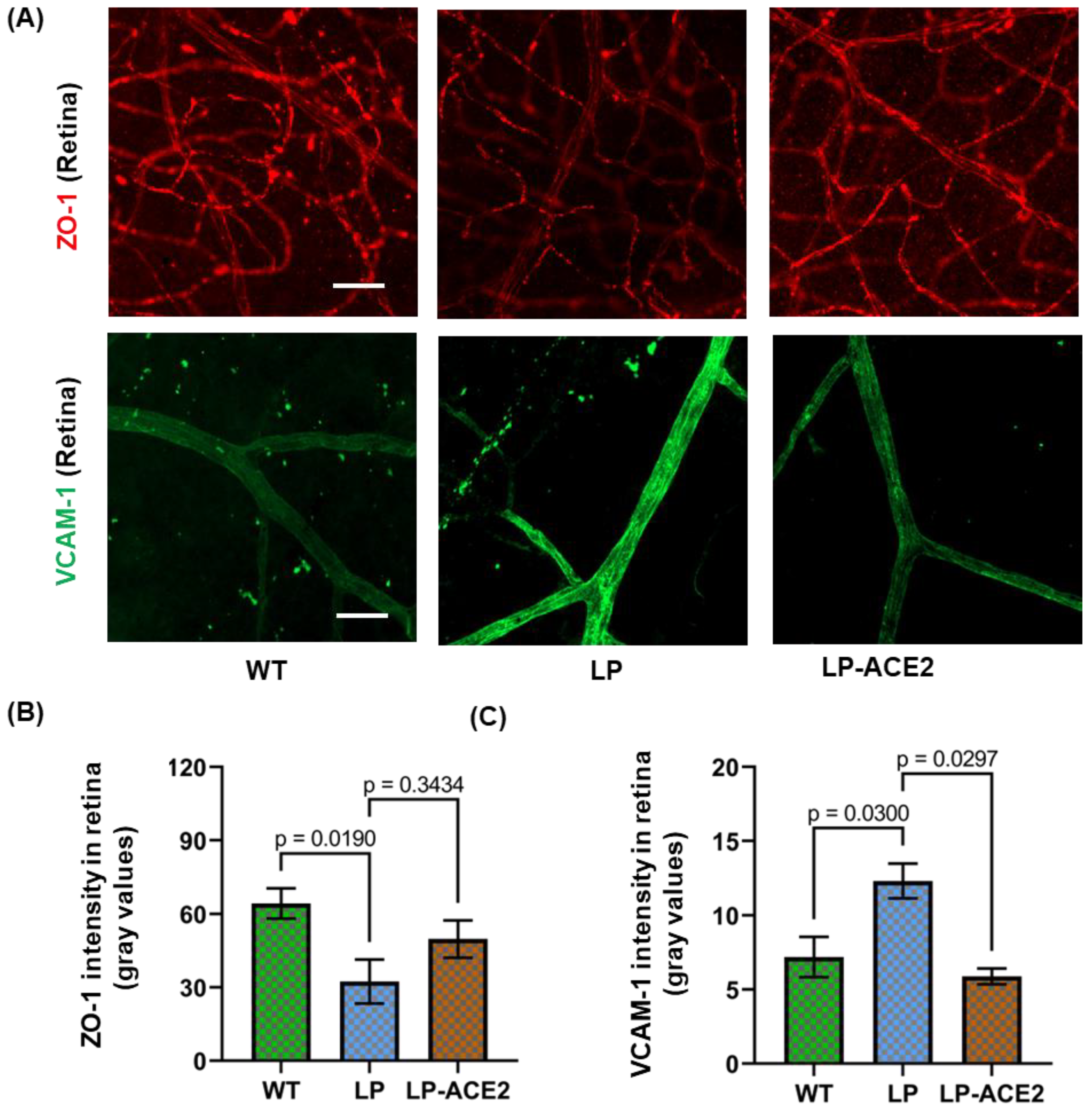
3.4. LP-ACE2 Inhibits Diabetes-Induced Blood-Retinal Barrier (BRB) Dysfunction and Microglial Inflammation in Akita Mice
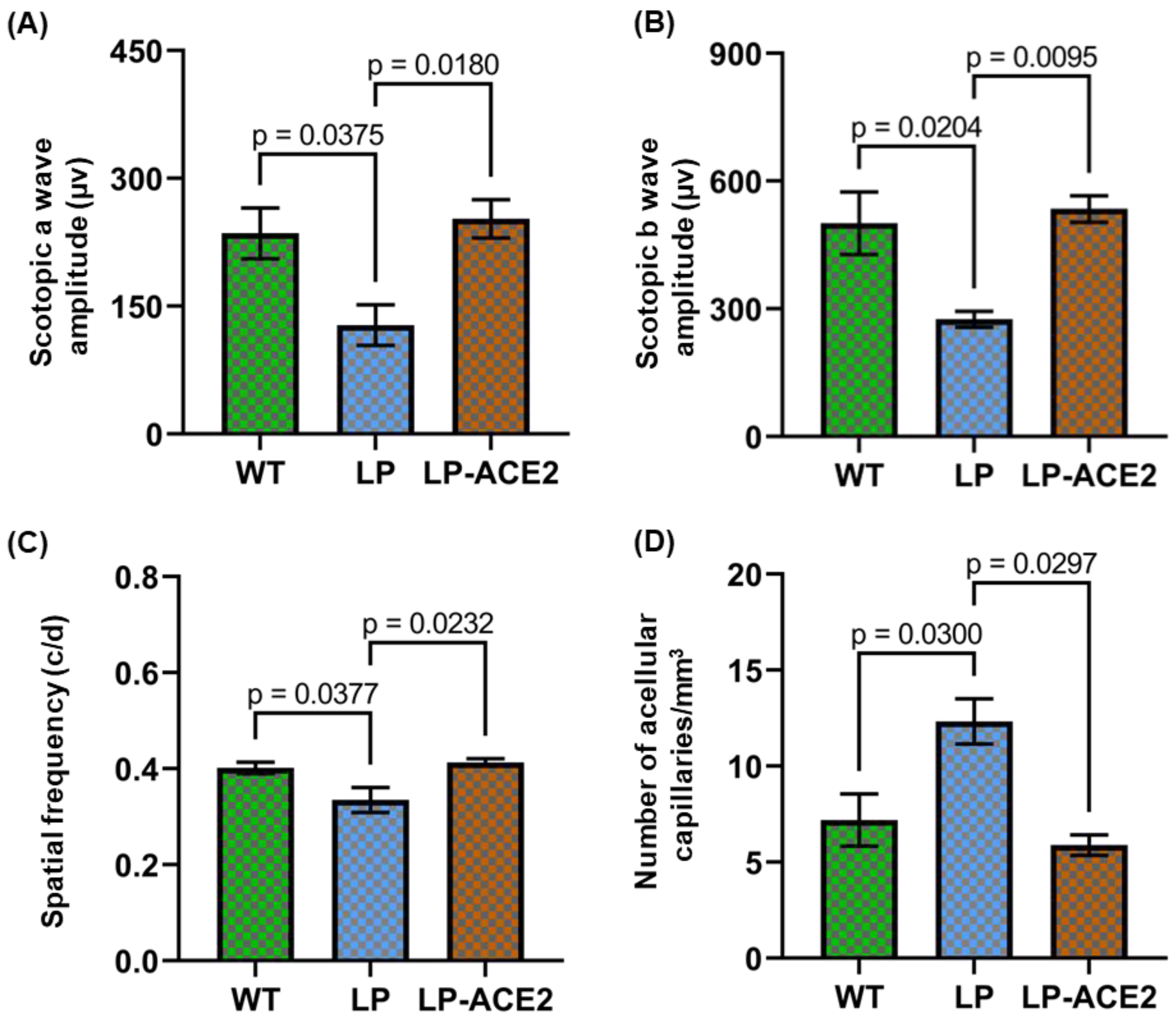
3.5. Effect of LP-ACE2 Treatment Improves Retinal Function and Reduces the Number of Acellular Capillaries in the Akita Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Redondo, M.J.; Steck, A.K.; Pugliese, A. Genetics of type 1 diabetes. Pediatr Diabetes 2018, 19, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Busik, J.V. Lipid metabolism dysregulation in diabetic retinopathy. J. Lipid Res. 2021, 62, 100017. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J. Am. Soc. Nephrol. 2017, 28, 1040–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, D.J. Clinical relevance of local Renin Angiotensin systems. Front. Endocrinol. 2014, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Nehme, A.; Zouein, F.A.; Zayeri, Z.D.; Zibara, K. An Update on the Tissue Renin Angiotensin System and Its Role in Physiology and Pathology. J. Cardiovasc. Dev. Dis. 2019, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, V.P.; Barbosa, M.A.; de Castro, U.G.M.; Zacarias, A.C.; Bezerra, F.S.; de Sa, R.G.; de Lima, W.G.; Dos Santos, R.A.S.; Alzamora, A.C. Antioxidant Effects of Oral Ang-(1-7) Restore Insulin Pathway and RAS Components Ameliorating Cardiometabolic Disturbances in Rats. Oxidative Med. Cell. Longev. 2019, 2019, 5868935. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, J.M., 2nd; Hu, P.; Caballero, S.; Moldovan, L.; Verma, A.; Oudit, G.Y.; Li, Q.; Grant, M.B. Adeno-Associated Virus Overexpression of Angiotensin-Converting Enzyme-2 Reverses Diabetic Retinopathy in Type 1 Diabetes in Mice. Am. J. Pathol. 2016, 186, 1688–1700. [Google Scholar] [CrossRef] [Green Version]
- Jarajapu, Y.P.R.; Bhatwadekar, A.D.; Caballero, S.; Hazra, S.; Shenoy, V.; Medina, R.; Kent, D.; Stitt, A.W.; Thut, C.; Finney, E.M.; et al. Activation of the ACE2/angiotensin-(1-7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes 2013, 62, 1258–1269. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Shan, Z.; Lei, B.; Yuan, L.; Liu, X.; Nakagawa, T.; Grant, M.B.; Lewin, A.S.; Hauswirth, W.W.; Raizada, M.K.; et al. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Mol. Ther. 2012, 20, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Beli, E.; Li Calzi, S.; Quigley, J.L.; Miller, R.C.; Moldovan, L.; Feng, D.; Salazar, T.E.; Hazra, S.; Al-Sabah, J.; et al. Loss of Angiotensin-Converting Enzyme 2 Exacerbates Diabetic Retinopathy by Promoting Bone Marrow Dysfunction. Stem Cells 2018, 36, 1430–1440. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Prasad, R.; Feng, D.; Beli, E.; Li Calzi, S.; Longhini, A.L.F.; Lamendella, R.; Floyd, J.L.; Dupont, M.; Noothi, S.K.; et al. Bone Marrow-Derived Cells Restore Functional Integrity of the Gut Epithelial and Vascular Barriers in a Model of Diabetes and ACE2 Deficiency. Circ. Res. 2019, 125, 969–988. [Google Scholar] [CrossRef]
- Prasad, R.; Floyd, J.L.; Dupont, M.; Harbour, A.; Adu-Agyeiwaah, Y.; Asare-Bediako, B.; Chakraborty, D.; Kichler, K.; Rohella, A.; Li Calzi, S.; et al. Maintenance of Enteral ACE2 Prevents Diabetic Retinopathy in Type 1 Diabetes. Circ. Res. 2023, 132, e1–e21. [Google Scholar] [CrossRef]
- Fyhrquist, F.; Saijonmaa, O. Renin-angiotensin system revisited. J. Intern. Med. 2008, 264, 224–236. [Google Scholar] [CrossRef]
- Patel, V.B.; Zhong, J.C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Penninger, J.M.; Grant, M.B.; Sung, J.J.Y. The Role of Angiotensin Converting Enzyme 2 in Modulating Gut Microbiota, Intestinal Inflammation, and Coronavirus Infection. Gastroenterology 2021, 160, 39–46. [Google Scholar] [CrossRef]
- Rodrigues Prestes, T.R.; Rocha, N.P.; Miranda, A.S.; Teixeira, A.L.; Simoes, E.S.A.C. The Anti-Inflammatory Potential of ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Evidence from Basic and Clinical Research. Curr. Drug Targets 2017, 18, 1301–1313. [Google Scholar] [CrossRef]
- Shirbhate, E.; Pandey, J.; Patel, V.K.; Kamal, M.; Jawaid, T.; Gorain, B.; Kesharwani, P.; Rajak, H. Understanding the role of ACE-2 receptor in pathogenesis of COVID-19 disease: A potential approach for therapeutic intervention. Pharmacol. Rep. 2021, 73, 1539–1550. [Google Scholar] [CrossRef]
- Beacon, T.H.; Delcuve, G.P.; Davie, J.R. Epigenetic regulation of ACE2, the receptor of the SARS-CoV-2 virus(1). Genome 2021, 64, 386–399. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Ohto-Nakanishi, T.; Penninger, J.M. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ. J. 2010, 74, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Elmorsi, R. The therapeutic potential of targeting ACE2 in COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9744–9747. [Google Scholar] [CrossRef]
- Kohan, A.B.; Yoder, S.M.; Tso, P. Using the lymphatics to study nutrient absorption and the secretion of gastrointestinal hormones. Physiol. Behav. 2011, 105, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backhed, F.; Crawford, P.A.; O’Donnell, D.; Gordon, J.I. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc. Natl. Acad. Sci. USA 2007, 104, 606–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, N.L.; Srinivasan, R.S.; Dillard, M.E.; Johnson, N.C.; Witte, M.H.; Boyd, K.; Sleeman, M.W.; Oliver, G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 2005, 37, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Dongaonkar, R.M.; Laine, G.A.; Stewart, R.H.; Quick, C.M. Balance point characterization of interstitial fluid volume regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R6–R16. [Google Scholar] [CrossRef]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [Green Version]
- Dixon, J.B. Mechanisms of chylomicron uptake into lacteals. Ann. N. Y. Acad. Sci. 2010, 1207 (Suppl. S1), E52–E57. [Google Scholar] [CrossRef] [Green Version]
- Dixon, J.B. Lymphatic lipid transport: Sewer or subway? Trends Endocrinol. Metab. 2010, 21, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Asare-Bediako, B.; Noothi, S.K.; Li Calzi, S.; Athmanathan, B.; Vieira, C.P.; Adu-Agyeiwaah, Y.; Dupont, M.; Jones, B.A.; Wang, X.X.; Chakraborty, D.; et al. Characterizing the Retinal Phenotype in the High-Fat Diet and Western Diet Mouse Models of Prediabetes. Cells 2020, 9, 464. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Asare-Bediko, B.; Harbour, A.; Floyd, J.L.; Chakraborty, D.; Duan, Y.; Lamendella, R.; Wright, J.; Grant, M.B. Microbial Signatures in The Rodent Eyes With Retinal Dysfunction and Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2022, 63, 5. [Google Scholar] [CrossRef]
- Storti, F.; Raphael, G.; Griesser, V.; Klee, K.; Drawnel, F.; Willburger, C.; Scholz, R.; Langmann, T.; von Eckardstein, A.; Fingerle, J.; et al. Regulated efflux of photoreceptor outer segment-derived cholesterol by human RPE cells. Exp. Eye Res. 2017, 165, 65–77. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Duncan, K.G.; Hosseini, K.; Bailey, K.R.; Yang, H.; Lowe, R.J.; Matthes, M.T.; Kane, J.P.; LaVail, M.M.; Schwartz, D.M.; Duncan, J.L. Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. Br. J. Ophthalmol. 2009, 93, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.Z.; Le, Y.Z. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2160–2164. [Google Scholar] [CrossRef]
- Lazzara, F.; Longo, A.M.; Giurdanella, G.; Lupo, G.; Platania, C.B.M.; Rossi, S.; Drago, F.; Anfuso, C.D.; Bucolo, C. Vitamin D(3) preserves blood retinal barrier integrity in an in vitro model of diabetic retinopathy. Front. Pharmacol. 2022, 13, 971164. [Google Scholar] [CrossRef]
- Gustavsson, C.; Agardh, C.D.; Zetterqvist, A.V.; Nilsson, J.; Agardh, E.; Gomez, M.F. Vascular cellular adhesion molecule-1 (VCAM-1) expression in mice retinal vessels is affected by both hyperglycemia and hyperlipidemia. PLoS ONE 2010, 5, e12699. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.V.; Shaw, L.C.; Grant, M.B. Inflammation in the pathogenesis of microvascular complications in diabetes. Front. Endocrinol. 2012, 3, 170. [Google Scholar] [CrossRef] [Green Version]
- Cifarelli, V.; Eichmann, A. The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Norden, P.R.; Kume, T. The Role of Lymphatic Vascular Function in Metabolic Disorders. Front. Physiol. 2020, 11, 404. [Google Scholar] [CrossRef]
- Verma, A.; Xu, K.; Du, T.; Zhu, P.; Liang, Z.; Liao, S.; Zhang, J.; Raizada, M.K.; Grant, M.B.; Li, Q. Expression of Human ACE2 in Lactobacillus and Beneficial Effects in Diabetic Retinopathy in Mice. Mol. Ther. Methods Clin. Dev. 2019, 14, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Zemski Berry, K.A.; Gordon, W.C.; Murphy, R.C.; Bazan, N.G. Spatial organization of lipids in the human retina and optic nerve by MALDI imaging mass spectrometry. J. Lipid Res. 2014, 55, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Chen, C.T.; Cagnone, G.; Heckel, E.; Sun, Y.; Cakir, B.; Tomita, Y.; Huang, S.; Li, Q.; Britton, W.; et al. Dyslipidemia in retinal metabolic disorders. EMBO Mol. Med. 2019, 11, e10473. [Google Scholar] [CrossRef] [PubMed]
- Fliesler, S.J.; Anderson, R.E. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983, 22, 79–131. [Google Scholar] [CrossRef] [PubMed]
- Benarous, R.; Sasongko, M.B.; Qureshi, S.; Fenwick, E.; Dirani, M.; Wong, T.Y.; Lamoureux, E.L. Differential association of serum lipids with diabetic retinopathy and diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7464–7469. [Google Scholar] [CrossRef]
- Chew, E.Y.; Klein, M.L.; Ferris, F.L., 3rd; Remaley, N.A.; Murphy, R.P.; Chantry, K.; Hoogwerf, B.J.; Miller, D. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch. Ophthalmol. 1996, 114, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, N.; Sahni, A. Association of systemic risk factors with the severity of retinal hard exudates in a north Indian population with type 2 diabetes. J. Postgrad. Med. 2010, 56, 3–6. [Google Scholar] [CrossRef]
- Rema, M.; Srivastava, B.K.; Anitha, B.; Deepa, R.; Mohan, V. Association of serum lipids with diabetic retinopathy in urban South Indians—The Chennai Urban Rural Epidemiology Study (CURES) Eye Study—2. Diabet. Med. 2006, 23, 1029–1036. [Google Scholar] [CrossRef] [Green Version]
- Sasongko, M.B.; Wong, T.Y.; Nguyen, T.T.; Kawasaki, R.; Jenkins, A.; Shaw, J.; Wang, J.J. Serum apolipoprotein AI and B are stronger biomarkers of diabetic retinopathy than traditional lipids. Diabetes Care 2011, 34, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Lu, X.M.; Tuo, X.; Liu, J.Y.; Zhang, Y.C.; Song, L.N.; Cheng, Z.Q.; Yang, J.K.; Xin, Z. Angiotensin-converting enzyme 2 regulates endoplasmic reticulum stress and mitochondrial function to preserve skeletal muscle lipid metabolism. Lipids Health Dis. 2019, 18, 207. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, R.; Adu-Agyeiwaah, Y.; Floyd, J.L.; Asare-Bediako, B.; Li Calzi, S.; Chakraborty, D.; Harbour, A.; Rohella, A.; Busik, J.V.; Li, Q.; et al. Sustained ACE2 Expression by Probiotic Improves Integrity of Intestinal Lymphatics and Retinopathy in Type 1 Diabetic Model. J. Clin. Med. 2023, 12, 1771. https://doi.org/10.3390/jcm12051771
Prasad R, Adu-Agyeiwaah Y, Floyd JL, Asare-Bediako B, Li Calzi S, Chakraborty D, Harbour A, Rohella A, Busik JV, Li Q, et al. Sustained ACE2 Expression by Probiotic Improves Integrity of Intestinal Lymphatics and Retinopathy in Type 1 Diabetic Model. Journal of Clinical Medicine. 2023; 12(5):1771. https://doi.org/10.3390/jcm12051771
Chicago/Turabian StylePrasad, Ram, Yvonne Adu-Agyeiwaah, Jason L. Floyd, Bright Asare-Bediako, Sergio Li Calzi, Dibyendu Chakraborty, Angela Harbour, Aayush Rohella, Julia V. Busik, Qiuhong Li, and et al. 2023. "Sustained ACE2 Expression by Probiotic Improves Integrity of Intestinal Lymphatics and Retinopathy in Type 1 Diabetic Model" Journal of Clinical Medicine 12, no. 5: 1771. https://doi.org/10.3390/jcm12051771





