Deprescribing Strategies for Opioids and Benzodiazepines with Emphasis on Concurrent Use: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategies
2.2. Study Selection, Data Extraction, and Data Synthesis
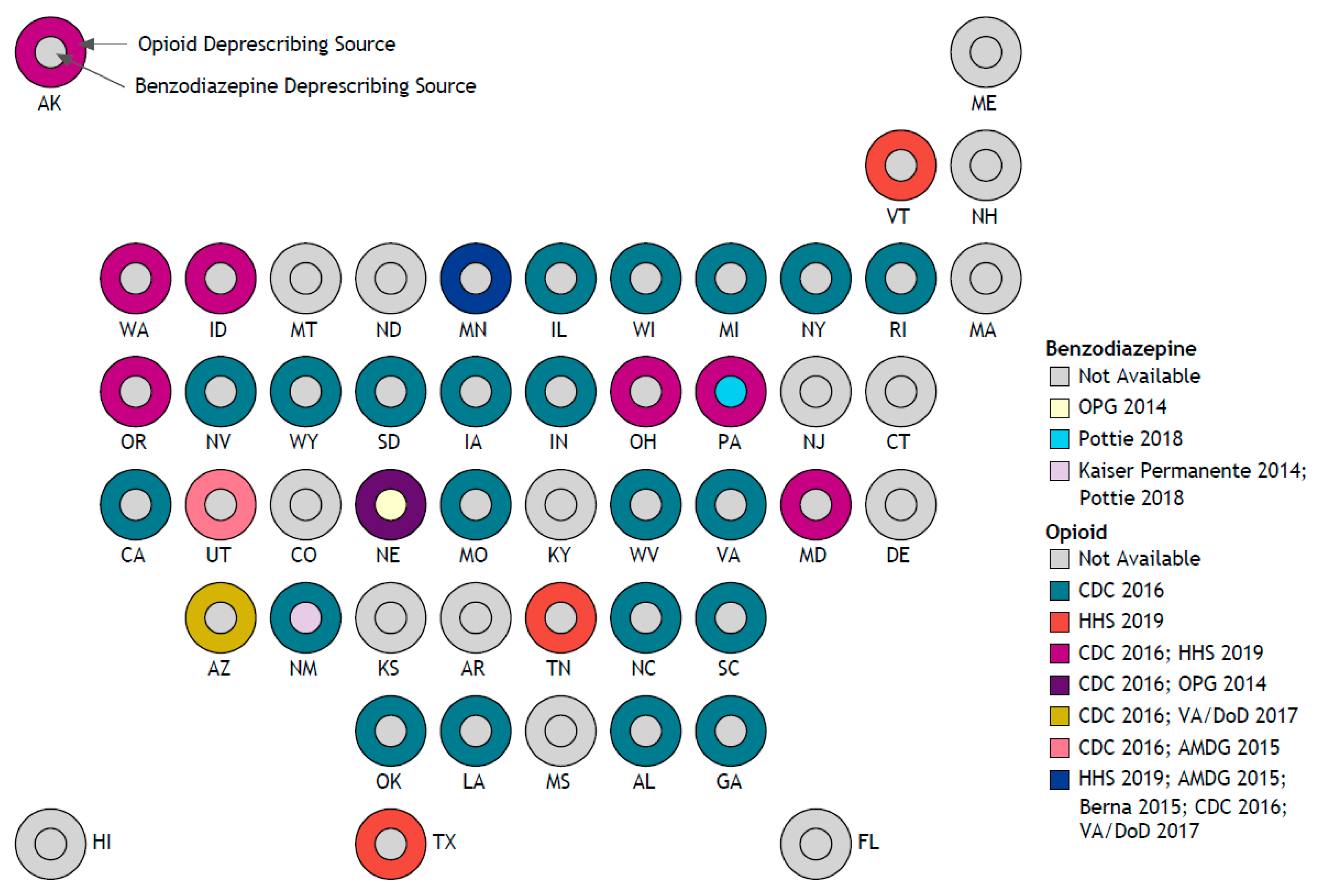
3. Results
3.1. Original Research Studies
3.2. Guidelines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Centers for Disease Control and Prevention. U.S. Overdose Deaths in 2021 Increased Half as Much as in 2020—But Are Still Up 15%. Available online: https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm (accessed on 19 July 2022).
- Centers for Disease Control and Prevention. Prescription Opioid Overdose Death Maps. Available online: https://www.cdc.gov/drugoverdose/deaths/prescription/maps.html (accessed on 28 July 2022).
- Jones, C.M.; McAninch, J.K. Emergency Department Visits and Overdose Deaths From Combined Use of Opioids and Benzodiazepines. Am. J. Prev. Med. 2015, 49, 493–501. [Google Scholar] [CrossRef]
- Park, T.W.; Saitz, R.; Ganoczy, D.; Ilgen, M.A.; Bohnert, A.S. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: Case-cohort study. BMJ 2015, 350, h2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, T.; Mamdani, M.M.; Dhalla, I.A.; Paterson, J.M.; Juurlink, D.N. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch. Intern. Med. 2011, 171, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; O’Donnell, J.; Gladden, R.M.; McGlone, L.; Chowdhury, F. Trends in Nonfatal and Fatal Overdoses Involving Benzodiazepines—38 States and the District of Columbia, 2019–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.; Funk, M.J.; Proescholdbell, S.; Hirsch, A.; Ribisl, K.M.; Marshall, S. Cohort Study of the Impact of High-Dose Opioid Analgesics on Overdose Mortality. Pain Med. 2016, 17, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Garg, R.K.; Fulton-Kehoe, D.; Franklin, G.M. Patterns of Opioid Use and Risk of Opioid Overdose Death among Medicaid Patients. Med. Care 2017, 55, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; He, M.; Brooks, M.M.; Zhang, Y. Exposure-Response Association Between Concurrent Opioid and Benzodiazepine Use and Risk of Opioid-Related Overdose in Medicare Part D Beneficiaries. JAMA Netw. Open 2018, 1, e180919. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, E.J.; Goldberg, S.B.; Malte, C.A.; Saxon, A.J. New Coprescription of Opioids and Benzodiazepines and Mortality Among Veterans Affairs Patients With Posttraumatic Stress Disorder. J. Clin. Psychiatry 2019, 80, 18m12689. [Google Scholar] [CrossRef]
- Calcaterra, S.L.; Severtson, S.G.; Bau, G.E.; Margolin, Z.R.; Bucher-Bartelson, B.; Green, J.L.; Dart, R.C. Trends in intentional abuse or misuse of benzodiazepines and opioid analgesics and the associated mortality reported to poison centers across the United States from 2000 to 2014. Clin. Toxicol. 2018, 56, 1107–1114. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA Warns about Serious Risks and Death When Combining Opioid Pain or Cough Medicines with Benzodiazepines; Requires Its Strongest Warning. Available online: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM518672.pdf (accessed on 4 September 2017).
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef] [Green Version]
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Jeffery, M.M.; Hooten, W.M.; Jena, A.B.; Ross, J.S.; Shah, N.D.; Karaca-Mandic, P. Rates of Physician Coprescribing of Opioids and Benzodiazepines after the Release of the Centers for Disease Control and Prevention Guidelines in 2016. JAMA Netw. Open 2019, 2, e198325. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Identifies Harm Reported from Sudden Discontinuation of Opioid Pain Medicines and Requires Label Changes to Guide Prescribers on Gradual, Individualized Tapering. Available online: https://www.fda.gov/media/122935/download (accessed on 16 January 2022).
- U.S. Food and Drug Administration. FDA Requiring Boxed Warning Updated to Improve Safe Use of Benzodiazepine Drug Class Includes Potential for Abuse, Addiction, and Other Serious Risks. Available online: https://www.fda.gov/media/142368/download (accessed on 16 January 2022).
- Scott, I.A.; Hilmer, S.N.; Reeve, E.; Potter, K.; Le Couteur, D.; Rigby, D.; Gnjidic, D.; Del Mar, C.B.; Roughead, E.E.; Page, A.; et al. Reducing inappropriate polypharmacy: The process of deprescribing. JAMA Intern. Med. 2015, 175, 827–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinman, M.; Reeve, E. Deprescribing. UpToDate. 2022. Available online: https://www.uptodate.com/contents/deprescribing (accessed on 12 July 2022).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Canadian Deprescribing Network. Available online: https://www.deprescribingnetwork.ca/canadian-deprescribing-network (accessed on 20 July 2022).
- US Deprescribing Research Network. US Deprescribing Research Network. Available online: https://deprescribingresearch.org/ (accessed on 16 September 2022).
- Statista. Largest Health Insurance Companies in the U.S. in 2021, by Revenue. Available online: https://www.statista.com/statistics/985501/largest-health-insurance-companies-in-us-by-revenue/ (accessed on 28 January 2022).
- Definitive Healthcare. Top 10 Largest Health Systems in the U.S. Available online: https://www.definitivehc.com/blog/top-10-largest-health-systems (accessed on 11 November 2021).
- Lee, J.Y.; Farrell, B.; Holbrook, A.M. Deprescribing benzodiazepine receptor agonists taken for insomnia: A review and key messages from practice guidelines. Pol. Arch. Intern. Med. 2019, 129, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Pollmann, A.S.; Murphy, A.L.; Bergman, J.C.; Gardner, D.M. Deprescribing benzodiazepines and Z-drugs in community-dwelling adults: A scoping review. BMC Pharmacol. Toxicol. 2015, 16, 19. [Google Scholar] [CrossRef] [Green Version]
- Nickerson, K.; Lieschke, G.; Rajappa, H.; Smith, A.; Inder, K.J. A scoping review of outpatient interventions to support the reduction of prescription opioid medication for chronic non cancer pain. J. Clin. Nurs. 2022, 31, 3368–3389. [Google Scholar] [CrossRef] [PubMed]
- de Kleijn, L.; Pedersen, J.R.; Rijkels-Otters, H.; Chiarotto, A.; Koes, B. Opioid reduction for patients with chronic pain in primary care: Systematic review. Br. J. Gen. Pract. 2022, 72, e293–e300. [Google Scholar] [CrossRef]
- Davis, M.P.; Digwood, G.; Mehta, Z.; McPherson, M.L. Tapering opioids: A comprehensive qualitative review. Ann. Palliat. Med. 2020, 9, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, S.; Maher, C.G.; Ferreira, G.E.; Hamilton, M.; Jansen, J.; McLachlan, A.J.; Underwood, M.; Lin, C.C. Correction to: Deprescribing Opioids in Chronic Noncancer Pain: Systematic Review of Randomised Trials. Drugs 2020, 80, 1577–1578. [Google Scholar] [CrossRef]
- Cunningham, J.L.; Evans, M.M.; King, S.M.; Gehin, J.M.; Loukianova, L.L. Opioid Tapering in Fibromyalgia Patients: Experience from an Interdisciplinary Pain Rehabilitation Program. Pain Med. 2016, 17, 1676–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliam, W.P.; Craner, J.R.; Cunningham, J.L.; Evans, M.M.; Luedtke, C.A.; Morrison, E.J.; Sperry, J.A.; Loukianova, L.L. Longitudinal Treatment Outcomes for an Interdisciplinary Pain Rehabilitation Program: Comparisons of Subjective and Objective Outcomes on the Basis of Opioid Use Status. J. Pain 2018, 19, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Zaman, T.; Rife, T.L.; Batki, S.L.; Pennington, D.L. An electronic intervention to improve safety for pain patients co-prescribed chronic opioids and benzodiazepines. Subst. Abus. 2018, 39, 441–448. [Google Scholar] [CrossRef]
- Oregon Pain Guidance of Southern Oregon. Opioid Prescribing Guidelines: A Provider and Community Resource. Available online: https://www.virginiapremier.com/wp-content/uploads/OPG_Guidelines.pdf (accessed on 12 July 2022).
- Nebraska Department of Health and Human Services. Nebraska Pain Management Guidance Document: A Provider and Community Resource. Available online: https://dhhs.ne.gov/Guidance%20Docs/Pain%20Management%20Pain%20Guidance%20Document.pdf#search=opioid%20prescribing (accessed on 12 July 2022).
- Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. Available online: https://www.agencymeddirectors.wa.gov/files/2015amdgopioidguideline.pdf (accessed on 12 July 2022).
- Minnesota Department of Human Services. Tapering and Discontinuing Opioid Use. Available online: https://mn.gov/dhs/opip/opioid-guidelines/tapering-opioids/ (accessed on 28 July 2022).
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022. MMWR Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Veterans Affairs/Department of Defense. VA/DoD Clinical Practice Guideline for the Use of Opioids in the Management of Chronic Pain. Available online: https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf (accessed on 12 July 2022).
- Oregon Health Authority Public Health Division. Oregon Opioid Tapering Guidelines. Available online: https://www.oregon.gov/omb/Topics-of-Interest/Documents/Oregon-Opioid-Tapering-Guidelines.pdf (accessed on 12 July 2022).
- Arizona Department of Health Services. Arizona Opioid Prescribing Guidelines. Available online: https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opioid-prescribing-guidelines.pdf (accessed on 12 July 2022).
- Cigna. Patient-Centered Safe Opioid Tapering Resource Guide. Available online: https://chk.static.cigna.com/assets/chcp/pdf/resourceLibrary/prescription/opioid-taper-resources.pdf (accessed on 12 July 2022).
- United Healthcare Services Inc. Opioid Tapering Recommendations. Available online: https://www.bhoptions.com/-/media/Files/HPN/pdf/Provider-Services/Opioid-Tapering-Recommendations.ashx?la=en (accessed on 12 July 2022).
- U.S. Department of Health and Human Services. HHS Guide for Clinicians on the Appropriate Dosage Reduction or Discontinuation of Long-Term Opioid Analgesics. Available online: https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf (accessed on 12 July 2022).
- Sunshine Health. Recommendations for Discontinuing and Tapering Opioids. 2022. Available online: https://www.sunshinehealth.com/content/dam/centene/Sunshine/pdfs/SH-PRO-Opioid%20Tapering%20_083120_DIGITAL.pdf (accessed on 12 July 2022).
- Mendoza, M.; Russell, H.A. Is it time to taper that opioid? (And how best to do it). J. Fam. Pract. 2019, 68, 324–331. [Google Scholar]
- Lumish, R.; Goga, J.K.; Brandt, N.J. Optimizing Pain Management Through Opioid Deprescribing. J. Gerontol. Nurs. 2018, 44, 9–14. [Google Scholar] [CrossRef]
- Murphy, L.; Babaei-Rad, R.; Buna, D.; Isaac, P.; Murphy, A.; Ng, K.; Regier, L.; Steenhof, N.; Zhang, M.; Sproule, B. Guidance on opioid tapering in the context of chronic pain: Evidence, practical advice and frequently asked questions. Can. Pharm. J. 2018, 151, 114–120. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Veterans Affairs. Opioid Taper Decision Tool: A VA Clinician’s Guide. Available online: https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/Pain_Opioid_Taper_Tool_IB_10_939_P96820.pdf (accessed on 12 July 2022).
- Berna, C.; Kulich, R.J.; Rathmell, J.P. Tapering Long-term Opioid Therapy in Chronic Noncancer Pain: Evidence and Recommendations for Everyday Practice. Mayo Clin. Proc. 2015, 90, 828–842. [Google Scholar] [CrossRef] [Green Version]
- Kaiser Permanente. Benzodiazepine and Z-Drug Safety Guideline. Available online: https://wa.kaiserpermanente.org/static/pdf/public/guidelines/benzo-zdrug.pdf (accessed on 12 July 2022).
- Payne, R.A.; Joshi, K.G. Helping patients through a benzodiazepine taper. Curr. Psychiatry 2019, 18, 9–10. [Google Scholar]
- Presbyterian Healthcare Services. Benzodiazepines: Tapering and Prescribing. Available online: https://onbaseext.phs.org/PEL/DisplayDocument?ContentID=pel_00943236 (accessed on 12 July 2022).
- Pottie, K.; Thompson, W.; Davies, S.; Grenier, J.; Sadowski, C.A.; Welch, V.; Holbrook, A.; Boyd, C.; Swenson, R.; Ma, A.; et al. Deprescribing benzodiazepine receptor agonists: Evidence-based clinical practice guideline. Can. Fam. Physician 2018, 64, 339–351. [Google Scholar]
- Choosing Wisely Canada. A Toolkit for Reducing Inappropriate Use of Benzodiazepines and Sedative-Hypnotics among Older Adults in Hospitals. Available online: https://choosingwiselycanada.org/wp-content/uploads/2017/07/CWC_BSH_Hospital_Toolkit_v1.3_2017-07-12.pdf (accessed on 12 July 2022).
- Choosing Wisely Canada. A Toolkit for Reducing Inappropriate Use of Benzodiazepines and Sedative-Hypnotics among Older Adults in Primary Care. Available online: https://choosingwiselycanada.org/wp-content/uploads/2017/12/CWC-Toolkit-BenzoPrimaryCare-V3.pdf (accessed on 12 July 2022).
- deprescribing.org. Benzodiazepine Receptor Agonist Deprescribing Algorithm. Available online: https://deprescribing.org/wp-content/uploads/2019/03/deprescribing_algorithms2019_BZRA_vf-locked.pdf (accessed on 12 July 2022).
- CalOptima. Deprescribing Benzodiazepines in Older Adults. Available online: https://www.caloptima.org/~/media/Files/CalOptimaOrg/508/Providers/Pharmacy/Medi-Cal/Updates/2021-08_DeprescribingBenzodiazepines_508.ashx (accessed on 12 July 2022).
- Pruskowski, J.; Rosielle, D.A.; Pontiff, L.; Reitschuler-Cross, E. Deprescribing and Tapering Benzodiazepines #355. J. Palliat. Med. 2018, 21, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, C.I.; Lembke, A. Tapering Patients Off of Benzodiazepines. Am. Fam. Physician 2017, 96, 606–610. [Google Scholar] [PubMed]
- U.S. Department of Veterans Affairs. Effective Treatments for PTSD: Helping Patients Taper from Benzodiazepines. Available online: https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/Academic_Detailing_Educational_Material_Catalog/59_PTSD_NCPTSD_Provider_Helping_Patients_Taper_BZD.pdf (accessed on 12 July 2022).
- Bélanger, L.; Belleville, G.; Morin, C.M. Management of Hypnotic Discontinuation in Chronic Insomnia. Sleep Med. Clin. 2009, 4, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Woodward, M.C. Deprescribing: Achieving Better Health Outcomes for Older People Through Reducing Medications. J. Pharm. Pract. Res. 2003, 33, 323–328. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm. Rep. 2016, 65, 1–49. [Google Scholar] [CrossRef]
- Centers for Medicare & Medicaid Services. Part C and D Performance Data. Available online: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/PerformanceData (accessed on 20 December 2022).
- Lo-Ciganic, W.H.; Hincapie-Castillo, J.; Wang, T.; Ge, Y.; Jones, B.L.; Huang, J.L.; Chang, C.Y.; Wilson, D.L.; Lee, J.K.; Reisfield, G.M.; et al. Dosing profiles of concurrent opioid and benzodiazepine use associated with overdose risk among US Medicare beneficiaries: Group-based multi-trajectory models. Addiction 2022, 117, 1982–1997. [Google Scholar] [CrossRef] [PubMed]
- Linsky, A.; Simon, S.R.; Marcello, T.B.; Bokhour, B. Clinical provider perceptions of proactive medication discontinuation. Am. J. Manag. Care 2015, 21, 277–283. [Google Scholar]
- Darnall, B.D.; Ziadni, M.S.; Stieg, R.L.; Mackey, I.G.; Kao, M.C.; Flood, P. Patient-Centered Prescription Opioid Tapering in Community Outpatients with Chronic Pain. JAMA Intern. Med. 2018, 178, 707–708. [Google Scholar] [CrossRef] [Green Version]
- Goodman, M.W.; Guck, T.P.; Teply, R.M. Dialing back opioids for chronic pain one conversation at a time. J. Fam. Pract. 2018, 67, 753–757. [Google Scholar]
- Sullivan, M.D.; Turner, J.A.; DiLodovico, C.; D’Appollonio, A.; Stephens, K.; Chan, Y.F. Prescription Opioid Taper Support for Outpatients With Chronic Pain: A Randomized Controlled Trial. J. Pain 2017, 18, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Kurita, G.P.; Hojsted, J.; Sjogren, P. Tapering off long-term opioid therapy in chronic non-cancer pain patients: A randomized clinical trial. Eur. J. Pain 2018, 22, 1528–1543. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Akbar, M.; Weinsheimer, N.; Gantz, S.; Schiltenwolf, M. Longitudinal observation of changes in pain sensitivity during opioid tapering in patients with chronic low-back pain. Pain Med. Malden Mass. 2011, 12, 1720–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, C.H.; Martin, J.L.; Alessi, C.; Dzierzewski, J.M.; Cook, I.A.; Moore, A.; Grinberg, A.; Zeidler, M.; Kierlin, L. Hypnotic Discontinuation Using a Blinded (Masked) Tapering Approach: A Case Series. Front. Psychiatry 2019, 10, 717. [Google Scholar] [CrossRef]
- Gorenstein, E.E.; Kleber, M.S.; Mohlman, J.; Dejesus, M.; Gorman, J.M.; Papp, L.A. Cognitive-behavioral therapy for management of anxiety and medication taper in older adults. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2005, 13, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Hadley, S.J.; Mandel, F.S.; Schweizer, E. Switching from long-term benzodiazepine therapy to pregabalin in patients with generalized anxiety disorder: A double-blind, placebo-controlled trial. J. Psychopharmacol. 2012, 26, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Rickels, K.; DeMartinis, N.; Garcia-Espana, F.; Greenblatt, D.J.; Mandos, L.A.; Rynn, M. Imipramine and buspirone in treatment of patients with generalized anxiety disorder who are discontinuing long-term benzodiazepine therapy. Am. J. Psychiatry 2000, 157, 1973–1979. [Google Scholar] [CrossRef]
- Rosenbaum, J.F.; Moroz, G.; Bowden, C.L. Clonazepam in the treatment of panic disorder with or without agoraphobia: A dose-response study of efficacy, safety, and discontinuance. Clonazepam Panic Disorder Dose-Response Study Group. J. Clin. Psychopharmacol. 1997, 17, 390–400. [Google Scholar] [CrossRef]
- Roy-Byrne, P.; Russo, J.; Pollack, M.; Stewart, R.; Bystrisky, A.; Bell, J.; Rosenbaum, J.; Corrigan, M.H.; Stolk, J.; Rush, A.J.; et al. Personality and symptom sensitivity predictors of alprazolam withdrawal in panic disorder. Psychol. Med. 2003, 33, 511–518. [Google Scholar] [CrossRef]
- Rynn, M.; Garcia-Espana, F.; Greenblatt, D.J.; Mandos, L.A.; Schweizer, E.; Rickels, K. Imipramine and buspirone in patients with panic disorder who are discontinuing long-term benzodiazepine therapy. J. Clin. Psychopharmacol. 2003, 23, 505–508. [Google Scholar] [CrossRef]
- Schweizer, E.; Case, W.G.; Garciaespana, F.; Greenblatt, D.J.; Rickels, K. Progesterone coadministration in patients discontinuing long-term benzodiazepine therapy—Effects on withdrawal severity and taper outcome. Psychopharmacology 1995, 117, 424–429. [Google Scholar] [CrossRef]
- Baillargeon, L.; Landreville, P.; Verreault, R.; Beauchemin, J.P.; Gregoire, J.P.; Morin, C.M. Discontinuation of benzodiazepines among older insomniac adults treated with cognitive-behavioural therapy combined with gradual tapering: A randomized trial. Can. Med. Assoc. J. 2003, 169, 1015–1020. [Google Scholar]
- Belanger, L.; Morin, C.M.; Bastien, C.; Ladouceur, R. Self-efficacy and compliance with benzodiazepine taper in older adults with chronic insomnia. Health Psychol. 2005, 24, 281–287. [Google Scholar] [CrossRef]
- Belleville, G.; Guay, C.; Guay, B.; Morin, C.M. Hypnotic taper with or without self-help treatment of insomnia: A randomized clinical trial. J. Consult. Clin. Psychol. 2007, 75, 325–335. [Google Scholar] [CrossRef]
- Dellechiaie, R.; Pancheri, P.; Casacchia, M.; Stratta, P.; Kotzalidis, G.D.; Zibellini, M. Assessment of the efficacy of buspirone in patients affected by generalized anxiety disorder, shifting to buspirone from prior treatment with lorazepam—A placebo-controlled, double-blind-study. J. Clin. Psychopharmacol. 1995, 15, 12–19. [Google Scholar] [CrossRef]
- Gosselin, P.; Ladouceur, R.; Morin, C.M.; Dugas, M.J.; Baillargeon, L. Benzodiazepine discontinuation among adults with GAD: A randomized trial of cognitive-behavioral therapy. J. Consult. Clin. Psychol. 2006, 74, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Colecchi, C.A.; Ling, W.D.; Sood, R.K. Cognitive behavior therapy to facilitate benzodiazepine discontinuation among hypnotic-dependent patients with insomnia. Behav. Ther. 1995, 26, 733–745. [Google Scholar] [CrossRef]
- Morin, C.M.; Bastien, C.; Guay, B.; Radouco-Thomas, M.; Leblanc, J.; Vallieres, A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am. J. Psychiat. 2004, 161, 332–342. [Google Scholar] [CrossRef]
- Morin, C.M.; Belanger, L.; Bastien, C.; Vallieres, A. Long-term outcome after discontinuation of benzodiazepines for insomnia: A survival analysis of relapse. Behav. Res. Ther. 2005, 43, 1–14. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.; Marchand, A.; Brousseau, L.; Aardema, F.; Mainguy, N.; Landry, P.; Savard, P.; Léveillé, C.; Lafrance, V.; Boivin, S.; et al. Cognitive-behavioural, pharmacological and psychosocial predictors of outcome during tapered discontinuation of benzodiazepine. Clin. Psychol. Psychother. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Tannenbaum, C.; Martin, P.; Tamblyn, R.; Benedetti, A.; Ahmed, S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: The EMPOWER cluster randomized trial. JAMA Intern. Med. 2014, 174, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Baandrup, L.; Glenthoj, B.Y.; Jennum, P.J. Objective and subjective sleep quality: Melatonin versus placebo add-on treatment in patients with schizophrenia or bipolar disorder withdrawing from long-term benzodiazepine use. Psychiatry Res. 2016, 240, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Curran, H.V.; Collins, R.; Fletcher, S.; Kee, S.C.; Woods, B.; Iliffe, S. Older adults and withdrawal from benzodiazepine hypnotics in general practice: Effects on cognitive function, sleep, mood and quality of life. Psychol. Med. 2003, 33, 1223–1237. [Google Scholar] [CrossRef] [Green Version]
- Mercier-Guyon, C.; Chabannes, J.P.; Saviuc, P. The role of captodiamine in the withdrawal from long-term benzodiazepine treatment. Curr. Med. Res. Opin. 2004, 20, 1347–1355. [Google Scholar] [CrossRef]
- Oude Voshaar, R.C.; Gorgels, W.; Mol, A.J.J.; Van Balkom, A.; Van de Lisdonk, E.H.; Breteler, M.H.M. Tapering off long-term benzodiazepine use with or without group cognitive-behavioural therapy: Three-condition, randomised controlled trial. Br. J. Psychiatry 2003, 182, 498–504. [Google Scholar] [CrossRef] [Green Version]
- Rubio, G.; Bobes, J.; Cervera, G.; Teran, A.; Perez, M.; Lopez-Gomez, V.; Rejas, J. Effects of Pregabalin on Subjective Sleep Disturbance Symptoms during Withdrawal from Long-Term Benzodiazepine Use. Eur. Addict. Res. 2011, 17, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Vicens, C.; Fiol, F.; Llobera, J.; Campoamor, F.; Mateu, C.; Alegret, S.; Socias, I. Withdrawal from long-term benzodiazepine use: Randomised trial in family practice. Br. J. Gen. Pract. 2006, 56, 958–963. [Google Scholar] [PubMed]
- Vicens, C.; Bejarano, F.; Sempere, E.; Mateu, C.; Fiol, F.; Socias, I.; Aragonès, E.; Palop, V.; Beltran, J.L.; Piñol, J.L.; et al. Comparative efficacy of two interventions to discontinue long-term benzodiazepine use: Cluster randomised controlled trial in primary care. Br. J. Psychiatry J. Ment. Sci. 2014, 204, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorma, H.; Naukkarinen, H.; Sarna, S.; Kuoppasalmi, K. Treatment of out-patients with complicated benzodiazepine dependence: Comparison of two approaches. Addict 2002, 97, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Zitman, F.G.; Couvee, J.E. Chronic benzodiazepine use in general practice patients with depression: An evaluation of controlled treatment and taper-off—Report on behalf of the Dutch Chronic Benzodiazepine Working Group. Br. J. Psychiatry 2001, 178, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Cardinali, D.P.; Gvozdenovich, E.; Kaplan, M.R.; Fainstein, I.; Shifis, H.A.; Pérez Lloret, S.; Albornoz, L.; Negri, A. A double blind-placebo controlled study on melatonin efficacy to reduce anxiolytic benzodiazepine use in the elderly. Neuro Endocrinol. Lett. 2002, 23, 55–60. [Google Scholar]
- Kitajima, R.; Miyamoto, S.; Tenjin, T.; Ojima, K.; Ogino, S.; Miyake, N.; Fujiwara, K.; Funamoto, Y.; Arai, J.; Tsukahara, S.; et al. Effects of tapering of long-term benzodiazepines on cognitive function in patients with schizophrenia receiving a second-generation antipsychotic. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Takeuchi, T.; Nomura, K.; Teramoto, T.; Yano, E. Clinical application of paroxetine for tapering benzodiazepine use in non-major-depressive outpatients visiting an internal medicine clinic. Psychiatry Clin. Neurosci. 2006, 60, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Tseng, C.H.; Lai, Y.S.; Hsu, S.C. Self-efficacy enhancement can facilitate hypnotic tapering in patients with primary insomnia. Sleep Biol. Rhythm. 2015, 13, 242–251. [Google Scholar] [CrossRef]

| Source | Target Population | Deprescribing Rate | Organizations Using the Guideline | Elements in the Guidelines/Protocols (Y = Yes; N = No): (a) Risk of Concurrent Use; (b) Criteria for Considering Tapering Opioid/Benzodiazepine Therapy; (c) Shared Decision-Making with Pts; (d) Withdrawal Management; (e) Nonpharmacological Approaches; (f) Alternative Medication Therapies. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Opioids (N = 16) | (a) | (b) | (c) | (d) | (e) | (f) | |||
| CDC, 2022 [38] | Pts with CNCP | 1. OPI use ≥1 year: reduce dose 10% or less per month. 2. OPI use for shorter durations: reduce initial dose 10% or less per week until ~30%, followed by 10% per week. | * | Y | Y | Y | Y | Y | Y |
| VA/DoD, 2022 [39] | Pts with CNCP | Gradual and individualized taper: reduce dose 5–20% every 4 weeks or longer. | Y | Y | Y | Y | Y | Y | |
| Minnesota Department of Human Service, 2021 [37] | Pts with CNCP | Individualized taper based on patient tolerance: 1. Slower: reduce dose 5–10% per month to start but may need to reduce rate to less than 5% per month over 2–3 months as tolerated. 2. Faster: reduce initial dose 10% per week or longer; not preferred but used when risk of continuing therapy outweighs risk of rapid taper or when part of treatment program for short time period. 3. May taper BZD first for pts with high daily MME and intermittent BZD. | Y | Y | Y | Y | Y | Y | |
| Oregon Health Authority, 2020 [40] | Pts on OPIs | Individualized: generally, reduce dose 5–10% per month. | Y | Y | Y | Y | Y | Y | |
| Arizona Department of Health Services, 2018 [41] | Pts on LTOT | Individualized taper based on risk assessment: 1. Slowest (over years): reduce dose 2–10% every 4–8 weeks with pauses in taper as needed. 2. Slow (over months to years): reduce dose 5–20% every 4 weeks with pauses in taper as needed. 3. Faster (over weeks): reduce dose 10–20% every week. 4. Rapid (over days): reduce first dose 20–50%, then 10–20% every day. | Y | Y | Y | Y | Y | Y | |
| Cigna, 2019 [42] | Pts on LTOT | Individualized taper based on pt health history, preferences, and risk factors, e.g., reduce dose 5–10% per month. | Y | Y | Y | Y | Y | Y | |
| FDA, 2019 [16] | Pts physically dependent on OPIs | Gradual and individualized taper: 1. No more than 10–25% reduction every 2–4 weeks. 2. More rapid taper: pts on opioids for shorter time periods. | UnitedHealthcare [43] | N | Y | Y | Y | Y | N |
| HHS, 2019 [44] | Pts on LTOT for chronic pain | 1. Common: reduce dose 5–20% every 4 weeks. 2. Gradual: reduce dose 10% per month or slower. 3. Rapid: reduce dose 10% per week up to 30% of the original dose and then 10% per week. | Sunshine Health [45] | Y | Y | Y | Y | Y | Y |
| Mendoza, 2019 [46] | Pts with CNCP | 1. Rapid: reduce dose 20% per week or abrupt discontinuation. 2. Slow: reduce dose 5–20% every 2–4 weeks. | Y | Y | Y | Y | Y | Y | |
| Lumish, 2018 [47] | Pts ≥65 years with CNCP | Reduce dose 5–20% every 4 weeks. | Y | Y | Y | Y | Y | Y | |
| Murphy, 2018 [48] | Pts with CNCP | Reduce dose 5–10% every 2–4 weeks. | Y | Y | Y | Y | Y | Y | |
| Nebraska Department of Health and Human Services, 2017 [35] | Pts on OPIs | 1. Long-acting OPIs: reduce dose 5–10% per week. 2. Short-acting OPIs: reduce dose 5–15% per week. 3. After reaching 1/4–1/2 of the initial dose, may slow dose reduction rate for pts cooperative with therapy. 4. Taper opioid first to reduce risk of overdose in pts on both OPI and BZD. | Y | Y | Y | Y | Y | Y | |
| VA PBM, 2016 [49] | Pts on OPIs | 1. Slowest: reduce dose 2–10% every 4–8 weeks with pauses in taper as needed (e.g., pts taking high doses of long-acting opioids for many years). 2. Slower: reduce dose 5–20% every 4 weeks. 3. Faster: reduce dose 10–20% per week. 4. Rapid: reduce dose 20–50% of first dose, then 10–20% every day. | United Healthcare [43] | Y | Y | Y | Y | Y | Y |
| AMDG, 2015 [36] | Pts with CNCP | 1. Taper off opioid first and then benzodiazepine with a deprescribing rate based on safety profile. 2. Slow: reduce dose by ≤10% per week for pts with no acute safety concerns from a mental/physical health perspective. 3. Rapid: discontinue over 2–3 weeks if pts having severe adverse outcomes (e.g., overdose or SUD). 4. Immediate: discontinuation if diversion/nonmedical use. | Sunshine Health [45] | Y | Y | Y | Y | Y | Y |
| Berna, 2015 [50] | Pts with CNCP | Reduce dose 10% every 5–7 days until reaching 30% of the initial dose, then reduce 10% per week. | United Healthcare [43] | N | Y | Y | Y | Y | Y |
| Oregon Pain Guidance, 2014 [34] | Pts with CNCP | 1. Long-acting OPIs: reduce dose 5–10% per week. 2. Short-acting OPIs: reduce dose 5–15% per week. 3. Slow down rate toward the end of taper. Once reaching 25–50% of initial dose, slow to 5% per week. 4. Taper BZD followed by OPI if both drugs involved. | Sunshine Health [45] | Y | Y | Y | Y | Y | Y |
| Benzodiazepines (N = 11) | |||||||||
| Kaiser Permanente, 2022 [51] | Pts on chronic BZD therapy | Individualized taper based on indication: 1. Slow (reduce dose 10% every 2–4 weeks): function not improved or tolerance developed with long-term Rx. 2. Moderate (reduce dose 10% per week): risks greater than benefit or increased risk with comorbidities. 3. Rapid (reduce dose 25% per week): substance abuse, significant risk due to unstable clinical condition or recent overdose or misuse or diversion of medication. | Y | Y | Y | Y | Y | Y | |
| FDA, 2020 [17] | Pts on BZDs | 1. Gradual and individualized taper. 2. When experiencing withdrawal symptoms, may pause the taper or raise BZD to previous dose. Once stable, proceed with more gradual taper. | Y | Y | Y | Y | Y | N | |
| Payne, 2019 [52] | Pts on BZDs | 1. Reduce dose 10–12.5% every 1–2 weeks over 2–12 months. 2. Reduce dose 10–25% every 2 weeks over 4–8 weeks. | N | Y | Y | Y | Y | N | |
| Presbyterian Healthcare Services, 2019 [53] | Pts ≥18 years on BZD or Z-drug therapy | Taper duration (considering prior use duration) 1. Prior use < 3 months: over 1 week 2. Prior use 3 months–1 year: over 1 month 3. Prior use > 1 year: over 3 months Taper dosage 1. Typical 3-month duration, reduce from 100% to 50% of initial dose during the first 4 weeks, then reduce from 50% to 0% during remaining 2 months. 2. More rapid (25% per week) may be appropriate for pts having increased risk of respiratory depression or misusing/diverting Rx. | Y | Y | Y | Y | Y | Y | |
| Pottie, 2018 [54] | Pts ≥65 years on BZRAs Pts 18–64 years on BZRAs > 4 weeks | Reduce dose 25% every 2 weeks, then 12.5% near end with duration of taper dependent upon patient tolerance, dependence, and potential for withdrawal effects. | Choosing Wisely Canada [55,56] deprescribing.org [57] CalOptima [58] | N | Y | Y | Y | Y | Y |
| Pruskowski, 2018 [59] | “Older” pts in palliative care on BZD | Reduce over 8–12 weeks total, reduce baseline dose 10–25% every 2–3 weeks based on BZD terminal half-life. | Y | Y | Y | Y | Y | Y | |
| Ogbonna, 2017 [60] | Pts on BZDs daily > 1 month | Reduce initial dose 5–25%, then 5–25% every 1–4 weeks as tolerated; reduce supratherapeutic dose 25–30% as tolerated, followed by 5–10% daily, weekly, or monthly as appropriate. Complex cases may require stabilization at 50% dose reduction for several months prior to resuming taper. | CalOptima [58] | Y | Y | Y | Y | Y | Y |
| VA PTSD, 2015 [61] | Pts with PTSD | Reduce dose 50% the first 2–4 weeks then maintain dose for 1–2 months. Further reduce dose 25% every 2 weeks. | Y | Y | Y | Y | Y | Y | |
| Oregon Pain Guidance, 2014 [34] | Pts on BZDs | 1. Slow: Reduce initial dose 25–50%, then 5–10% per week with follow up; total dose reduction of long-acting drug 5–10% per week in divided doses. When 25–50% of starting dose is reached, slowly taper further to 5% or less per week. 2. Rapid: Pre-medicate for 2 weeks prior with carbamazepine 200 mg every morning and 400 mg every bedtime or valproate 500 mg twice daily. Continue these medications 4 weeks after BZD is discontinued. Discontinue the current BZD and switch to diazepam 2 mg twice daily × 2 days, followed by 2 mg daily × 2 days, then stop. For high doses, may begin with 5 mg twice daily × 2 days and then continue as described. 3. Taper BZD followed by OPI if both drugs involved. | Sunshine Health [45] | Y | Y | Y | Y | Y | Y |
| Belanger, 2009 [62] | Pts with chronic insomnia | 1. Reduce dose by 25% every 1–2 weeks to smallest minimal dose. 2. Switch short-acting BZD to longer-acting BZD. Reduce initial dose 25% by 2nd week, 50% by 4th week, 100% by 10th week. | Y | Y | Y | Y | Y | Y | |
| Woodward, 2003 [63] | Pts ≥ 65 years | 1. Short-acting: cease or wean if not needed. 2. Long-acting: reduce dose 10–15% per week. 3. Both 1 and 2: combine with sleep hygiene/psychotherapy. | Y | Y | Y | Y | Y | Y | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wilson, D.L.; Fernandes, D.; Adkins, L.E.; Bantad, A.; Copacia, C.; Dharma, N.; Huang, P.-L.; Joseph, A.; Park, T.W.; et al. Deprescribing Strategies for Opioids and Benzodiazepines with Emphasis on Concurrent Use: A Scoping Review. J. Clin. Med. 2023, 12, 1788. https://doi.org/10.3390/jcm12051788
Wang Y, Wilson DL, Fernandes D, Adkins LE, Bantad A, Copacia C, Dharma N, Huang P-L, Joseph A, Park TW, et al. Deprescribing Strategies for Opioids and Benzodiazepines with Emphasis on Concurrent Use: A Scoping Review. Journal of Clinical Medicine. 2023; 12(5):1788. https://doi.org/10.3390/jcm12051788
Chicago/Turabian StyleWang, Yanning, Debbie L. Wilson, Deanna Fernandes, Lauren E. Adkins, Ashley Bantad, Clint Copacia, Nilay Dharma, Pei-Lin Huang, Amanda Joseph, Tae Woo Park, and et al. 2023. "Deprescribing Strategies for Opioids and Benzodiazepines with Emphasis on Concurrent Use: A Scoping Review" Journal of Clinical Medicine 12, no. 5: 1788. https://doi.org/10.3390/jcm12051788






