Infiltration of Conduction Tissue Is a Major Cause of Electrical Instability in Cardiac Amyloidosis
Abstract
1. Introduction
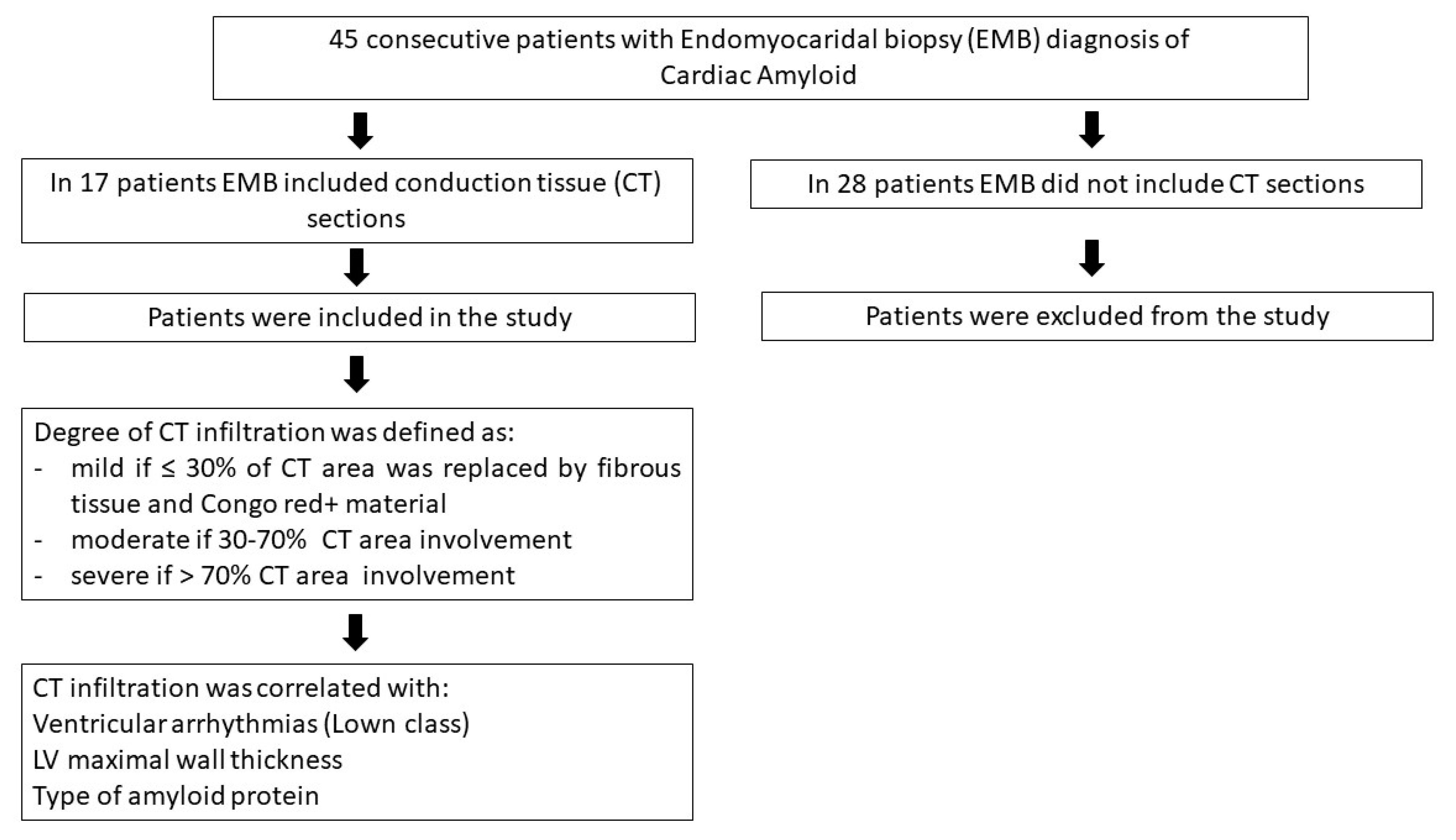
2. Materials and Methods
2.1. Patient Population
2.2. Cardiac Studies
2.3. CMR Image Acquisition and Analysis
2.4. Histology and Electron Microscopy
2.5. Statistics
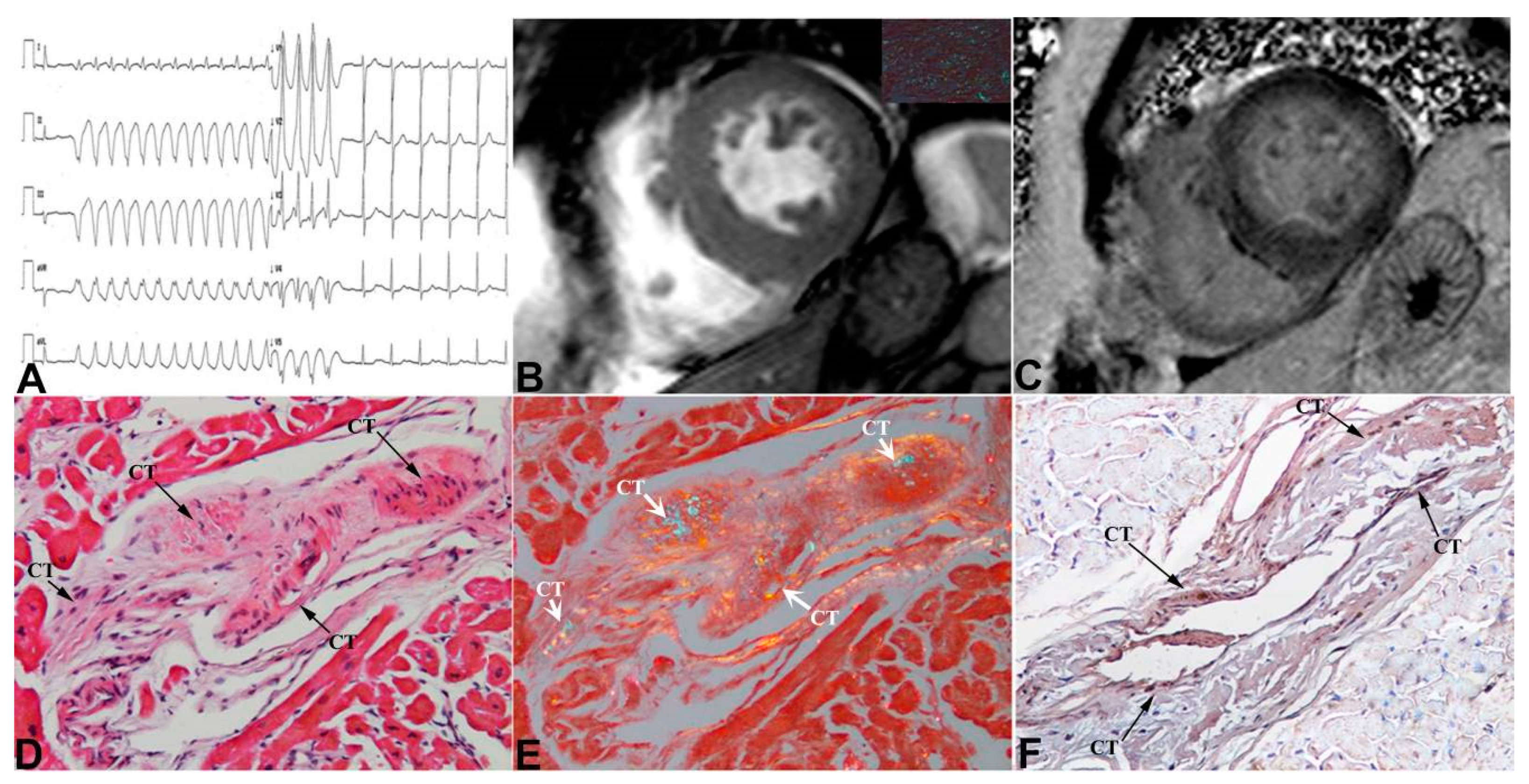
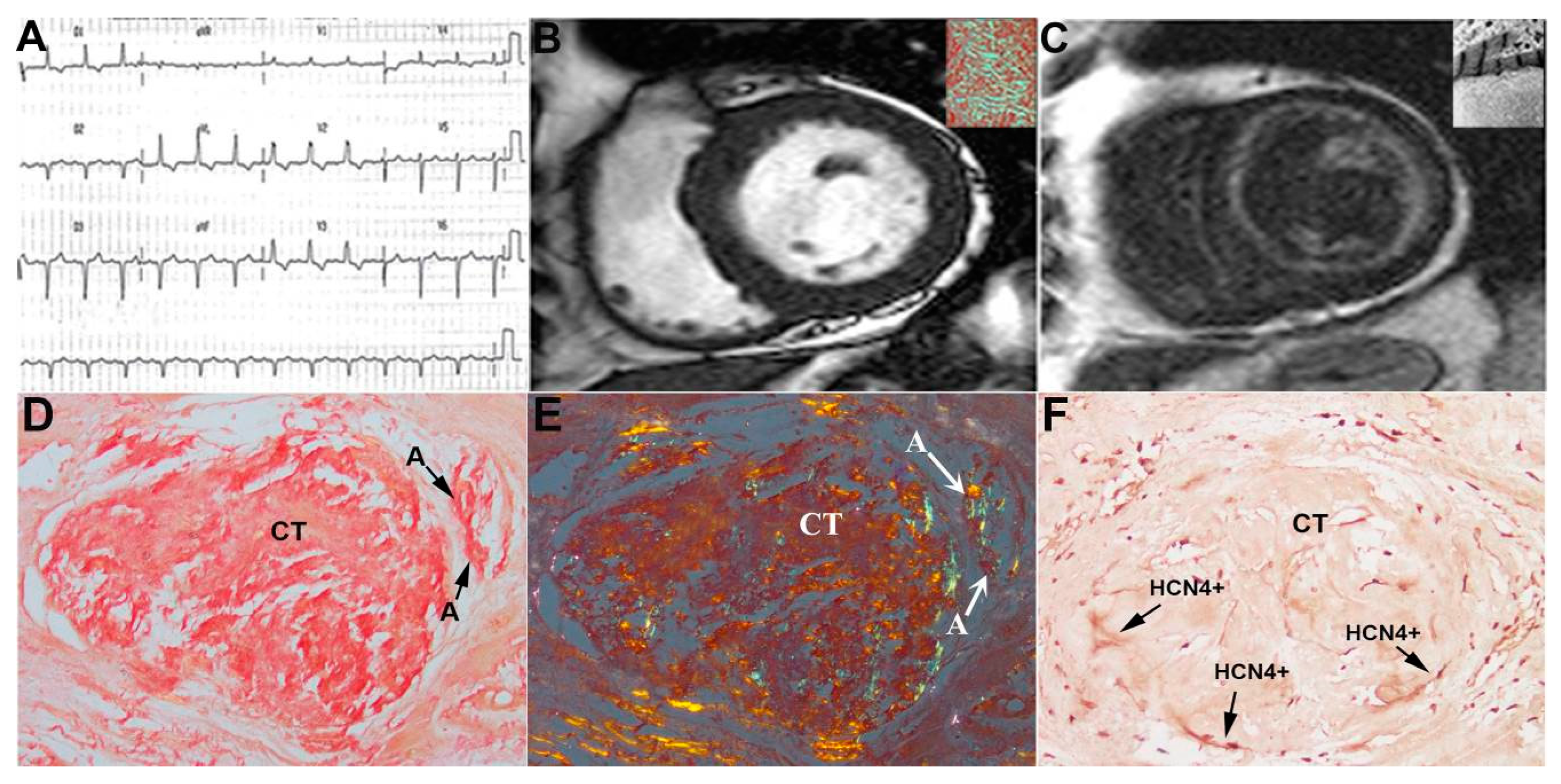
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendes de Sousa, M.; Vital, C.; Ostler, D.; Fernandes, R.; Pouget-Abadie, J.; Carles, C.; Saraiva, M.J. Apolipoprotein AI and Transthyretin as Components of Amyloid Fibrils in a Kindred with apoAI Leu178His Amyloidosis. Am. J. Pathol. 2000, 156, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Rowczenio, D.; Dogan, A.; Theis, J.D.; Vrana, J.A.; Lachmann, A.J.; Wechalekar, A.D.; Gilbertson, J.A.; Hunt, T.; Gibbs, S.D.; Sattianayagam, P.T.; et al. Amyloidogenicity and Clinical Phenotype Associated with Five Novel Mutations in Apolipoprotein A-I. Am. J. Pathol. 2011, 179, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Merlini, G.; Bellotti, V. Molecular Mechanisms of Amyloidosis. N. Engl. J. Med. 2003, 349, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Argirò, A.; Del Franco, A.; Mazzoni, C.; Allinovi, M.; Tomberli, A.; Tarquini, R.; Di Mario, C.; Perfetto, F.; Cappelli, F.; Zampieri, M. Arrhythmic Burden in Cardiac Amyloidosis: What We Know and What We Do Not. Biomedicines 2022, 10, 2888. [Google Scholar] [CrossRef] [PubMed]
- Porcari, A.; Rossi, M.; Cappelli, F.; Canepa, M.; Musumeci, B.; Cipriani, A.; Tini, G.; Barbati, G.; Varrà, G.G.; Morelli, C.; et al. Incidence and risk factors for pacemaker implantation in light-chain and transthyretin cardiac amyloidosis. Eur. J. Heart Fail 2022, 24, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, J.; Jaber, W.; Maurer, M.; Sperry, B.; Hanna, M.; Collier, P.; Patel, D.R.; Wazni, O.M.; Donnellan, E. Electrophysiological Manifestations of Cardiac Amyloidosis: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2021, 3, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Letizia, C.; Adamo, F.; Grande, C.; Verardo, R.; Chimenti, C. A-V block as presentation of cardiac amyloid: Prominent infiltration of conduction tissue revealed by endomyocardial biopsy. Amyloid 2017, 24, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Frustaci, A. Contribution and Risks of Left Ventricular Endomyocardial Biopsy in Patients with Cardiomyopathies. A Retrospective Study over a 28-Year Period. Circulation 2013, 128, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Morgante, E.; Russo, M.A.; Scopelliti, F.; Grande, C.; Verardo, R.; Franciosa, P.; Chimenti, C. Pathology and Function of Conduction Tissue in Fabry Disease Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2015, 8, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Verardo, R.; Grande, C.; Galea, N.; Piselli, P.; Carbone, I.; Alfarano, M.; Russo, M.A.; Chimenti, C. Immune-Mediated Myocarditis in Fabry Disease Cardiomyopathy. J. Am. Heart Assoc. 2018, 7, e009052. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Aimo, A.; Barison, A.; Emdin, M.; Porcari, A.; Linhart, A.; Keren, A.; Merlo, M.; Sinagra, G. Restrictive cardiomyopathy: Definition and diagnosis. Eur. Heart J. 2022, 43, 4679–4693. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J. Am. Coll. Cardiol. 2007, 50, 1914–1931. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail 2021, 23, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Aimo, A.; Serenelli, M.; Barison, A.; Vergaro, G.; Passino, C.; Panichella, G.; Sinagra, G.; Merlo, M.; Fontana, M.; et al. Critical Comparison of Documents from Scientific Societies on Cardiac Amyloidosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 1288–1303. [Google Scholar] [CrossRef]
- Bharati, S.; Lev, M.; Denes, P.; Modlinger, J.; Wyndham, C.; Bauernfeind, R.; Greenblatt, M.; Rosen, K.M. Infiltrative Cardiomyopathy with Conduction Disease and Ventricular Arrhythmia: Electrophysiologic and Pathologic Correlations. Am. J. Cardiol. 1960, 45, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Elliott, P.; Damy, T.; Nativi-Nicolau, J.; Berk, J.L.; Velazquez, E.J.; Boman, K.; Gundapaneni, B.; Patterson, T.A.; Schwartz, J.H.; et al. Efficacy of Tafamidis in Patients with Hereditary and Wild-Type Transthyretin Amyloid Cardiomyopathy: Further Analyses From ATTR-ACT. JACC Heart Fail 2021, 9, 115–123. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ikram, A.; Donnelly, J.P.; Sperry, B.W.; Samaras, C.; Valent, J.; Hanna, M. Diflunisal tolerability in transthyretin cardiac amyloidosis: A single center’s experience. Amyloid 2018, 25, 197–202. [Google Scholar] [CrossRef] [PubMed]

| Age/Sex | Amyloid Type | 2D-Echocardiogram Parameters | Conduction Tissue Infiltration Grading/% | Tachy- Arrhythmias | Brady- Arrhythmias | Therapy | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| LVMWT (mm) | LVEF (%) | Diastolic Dysfunction | V (Lown Class) | SV | ||||||
| 1 | 71/F | AL | 17 | 60 | Type II | Mild/22% | 1 | CHT | ||
| 2 | 58/F | AL | 17 | 58 | Type II | Mild/20% | 1 | CHT | ||
| 3 | 75/F | AL | 17 | 56 | Type II | Severe/80% | 3 | CHT | ||
| 4 | 69/M | AL | 15 | 46 | Type I | Severe/85% | 4A | 3° AV block | PM + CHT | |
| 5 | 57/M | AL | 15 | 60 | Type I | Severe/75% | 3 | CHT | ||
| 6 | 70/F | TTR | 18 | 64 | Type II | Mild/15% | 1 | |||
| 7 | 58/F | AL | 16 | 55 | Type II | Moderate/65% | 4A | Amiodarone + CHT | ||
| 8 | 61/F | AL | 21 | 50 | Type II | Severe/90% | 4A | AF | DC shock + Amiodarone + CHT | |
| 9 | 74/M | AL | 16 | 45 | Type II | Moderate/40% | 2 | CHT | ||
| 10 | 81/M | TTR | 22 | 35 | Type III | Severe/90% | 4A | AF | ICD + amiodarone | |
| 11 | 54/F | AL | 15 | 60 | Type III | Mild/25% | 1 | CHT | ||
| 12 | 52/F | AL | 12 | 50 | Type I | Severe/85% | 3 | 3-fascicular block | PM + CHT | |
| 13 | 60/M | AL | 17 | 55 | Type I | Moderate/55% | 2 | CHT | ||
| 14 | 68/M | TTR | 16 | 50 | Type III | Severe/80% | 4B | AT | Complete AV block | PM + amiodarone |
| 15 | 48/F | AL | 17 | 25 | Type III | Mild/20% | 1 | ICD + CHT | ||
| 16 | 63/M | TTR | 21 | 50 | Type III | Severe/75% | 3 | Atrial flutter | DC shock + flecainide + bisoprolol | |
| 17 | 72/M | AL | 17 | 55 | Type II | Severe/80% | 4B | Paroxystic AF | DC shock + amiodarone + CHT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frustaci, A.; Verardo, R.; Russo, M.A.; Caldarulo, M.; Alfarano, M.; Galea, N.; Miraldi, F.; Chimenti, C. Infiltration of Conduction Tissue Is a Major Cause of Electrical Instability in Cardiac Amyloidosis. J. Clin. Med. 2023, 12, 1798. https://doi.org/10.3390/jcm12051798
Frustaci A, Verardo R, Russo MA, Caldarulo M, Alfarano M, Galea N, Miraldi F, Chimenti C. Infiltration of Conduction Tissue Is a Major Cause of Electrical Instability in Cardiac Amyloidosis. Journal of Clinical Medicine. 2023; 12(5):1798. https://doi.org/10.3390/jcm12051798
Chicago/Turabian StyleFrustaci, Andrea, Romina Verardo, Matteo Antonio Russo, Marina Caldarulo, Maria Alfarano, Nicola Galea, Fabio Miraldi, and Cristina Chimenti. 2023. "Infiltration of Conduction Tissue Is a Major Cause of Electrical Instability in Cardiac Amyloidosis" Journal of Clinical Medicine 12, no. 5: 1798. https://doi.org/10.3390/jcm12051798
APA StyleFrustaci, A., Verardo, R., Russo, M. A., Caldarulo, M., Alfarano, M., Galea, N., Miraldi, F., & Chimenti, C. (2023). Infiltration of Conduction Tissue Is a Major Cause of Electrical Instability in Cardiac Amyloidosis. Journal of Clinical Medicine, 12(5), 1798. https://doi.org/10.3390/jcm12051798







