Impact of Enterococci vs. Staphylococci Induced Infective Endocarditis after Transcatheter Aortic Valve Implantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Definitions
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De, B.M.; De, P.R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D. TAVR Prognosis, Aging, and the Second TAVR Tsunami: Insights From France. J. Am. Coll. Cardiol. 2016, 68, 1648–1650. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Chamandi, C.; Rodriguez-Gabella, T.; Rodes-Cabau, J. Future of transcatheter aortic valve implantation—Evolving clinical indications. Nat. Rev. Cardiol. 2018, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lanz, J.; Kim, W.K.; Walther, T.; Burgdorf, C.; Mollmann, H.; Linke, A.; Redwood, S.; Thilo, C.; Hilker, M.; Joner, M.; et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: A randomised non-inferiority trial. Lancet 2019, 394, 1619–1628. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Del Val, D.; Linke, A.; Abdel-Wahab, M.; Latib, A.; Ihlemann, N.; Urena, M.; Won-Keun, K.; Husser, O.; Herrmann, H.C.; Nombela-Franco, L.; et al. Long-Term Outcomes After Infective Endocarditis After Transcatheter Aortic Valve Replacement. Circulation 2020, 142, 1497–1499. [Google Scholar] [CrossRef]
- Mangner, N.; Woitek, F.; Haussig, S.; Schlotter, F.; Stachel, G.; Hollriegel, R.; Wilde, J.; Lindner, A.; Holzhey, D.; Leontyev, S.; et al. Incidence, Predictors, and Outcome of Patients Developing Infective Endocarditis Following Transfemoral Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2907–2908. [Google Scholar] [CrossRef]
- Regueiro, A.; Linke, A.; Latib, A.; Ihlemann, N.; Urena, M.; Walther, T.; Husser, O.; Herrmann, H.C.; Nombela-Franco, L.; Cheema, A.N.; et al. Association Between Transcatheter Aortic Valve Replacement and Subsequent Infective Endocarditis and In-Hospital Death. JAMA 2016, 316, 1083–1092. [Google Scholar] [CrossRef] [Green Version]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar]
- Del Val, D.; Abdel-Wahab, M.; Linke, A.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Landt, M.; Auffret, V.; et al. Temporal Trends, Characteristics, and Outcomes of Infective Endocarditis after Transcatheter Aortic Valve Replacement. Clin. Infect. Dis. 2021, 73, e3750–e3758. [Google Scholar] [CrossRef]
- Del Val, D.; Abdel-Wahab, M.; Mangner, N.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Gasior, T.; Wojakowski, W.; et al. Stroke Complicating Infective Endocarditis After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2021, 77, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del, Z.F.; Dulgheru, R.; El, K.G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [PubMed]
- Roques, F.; Michel, P.; Goldstone, A.R.; Nashef, S.A. The logistic EuroSCORE. Eur. Heart J. 2003, 24, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J.; Genereux, P.; Piazza, N.; Van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 2012, 60, 1438–1454. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Hammond-Haley, M.; Hartley, A.; Al-Khayatt, B.M.; Delago, A.J.; Ghajar, A.; Ojha, U.; Marshall, D.C.; Salciccioli, J.D.; Prendergast, B.D.; Shalhoub, J. Trends in the incidence and mortality of infective endocarditis in high-income countries between 1990 and 2019. Int. J. Cardiol. 2023, 371, 441–451. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- Del Val, D.; Abdel-Wahab, M.; Mangner, N.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Gasior, T.; Wojakowski, W.; et al. Infective Endocarditis Caused by Staphylococcus aureus After Transcatheter Aortic Valve Replacement. Can. J. Cardiol. 2022, 38, 102–112. [Google Scholar] [CrossRef]
- Stortecky, S.; Heg, D.; Tueller, D.; Pilgrim, T.; Muller, O.; Noble, S.; Jeger, R.; Toggweiler, S.; Ferrari, E.; Taramasso, M.; et al. Infective Endocarditis After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 75, 3020–3030. [Google Scholar] [CrossRef]
- Wang, A.; Athan, E.; Pappas, P.A.; Fowler, V.G.; Olaison, L., Jr.; Pare, C.; Almirante, B.; Munoz, P.; Rizzi, M.; Naber, C.; et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007, 297, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Russo, A.; Venditti, M. Optimizing antibiotic therapy of bacteremia and endocarditis due to staphylococci and enterococci: New insights and evidence from the literature. J. Infect. Chemother. 2015, 21, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Widmer, D.; Widmer, A.F.; Jeger, R.; Dangel, M.; Stortecky, S.; Frei, R.; Conen, A. Prevalence of enterococcal groin colonization in patients undergoing cardiac interventions: Challenging antimicrobial prophylaxis with cephalosporins in patients undergoing transcatheter aortic valve replacement. J. Hosp. Infect. 2022, 129, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Conen, A.; Stortecky, S.; Moreillon, P.; Hannan, M.M.; Franzeck, F.C.; Jeger, R.; Widmer, A.F. A review of recommendations for infective endocarditis prevention in patients undergoing transcatheter aortic valve implantation. EuroIntervention 2021, 16, 1135–1140. [Google Scholar] [CrossRef]
- Panagides, V.; Del Val, D.; Abdel-Wahab, M.; Mangner, N.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Gasior, T.; et al. Perivalvular Extension of Infective Endocarditis after Transcatheter Aortic Valve Replacement. Clin. Infect. Dis. 2021, 75, 638–646. [Google Scholar] [CrossRef]
- Mangner, N.; Leontyev, S.; Woitek, F.J.; Kiefer, P.; Haussig, S.; Binner, C.; Mende, M.; Schlotter, F.; Stachel, G.; Hollriegel, R.; et al. Cardiac Surgery Compared With Antibiotics Only in Patients Developing Infective Endocarditis After Transcatheter Aortic Valve Replacement. J. Am. Heart. Assoc. 2018, 7, e010027. [Google Scholar] [CrossRef]
- Mangner, N.; Del Val, D.; Abdel-Wahab, M.; Crusius, L.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; Gasior, T.; et al. Surgical Treatment of Patients With Infective Endocarditis After Transcatheter Aortic Valve Implantation. J. Am. Coll. Cardiol. 2022, 79, 772–785. [Google Scholar] [CrossRef]
- Dickerman, S.A.; Abrutyn, E.; Barsic, B.; Bouza, E.; Cecchi, E.; Moreno, A.; Doco-Lecompte, T.; Eisen, D.P.; Fortes, C.Q.; Fowler, V.G., Jr.; et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: An analysis from the ICE Prospective Cohort Study (ICE-PCS). Am. Heart J. 2007, 154, 1086–1094. [Google Scholar] [CrossRef]
- Oberbach, A.; Schlichting, N.; Hagl, C.; Lehmann, S.; Kullnick, Y.; Friedrich, M.; Kohl, U.; Horn, F.; Kumbhari, V.; Loffler, B.; et al. Four decades of experience of prosthetic valve endocarditis reflect a high variety of diverse pathogens. Cardiovasc. Res. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Kaziród-Wolski, K.; Sielski, J.; Ciuraszkiewicz, K. Infective endocarditis in intensive cardiac care unit—Clinical and biochemical differences of blood-culture negative infective endocarditis. Pol. Merkur. Lekarski. 2017, 42, 21–25. [Google Scholar]
- Suardi, L.R.; de Alarcón, A.; García, M.V.; Ciezar, A.P.; Hidalgo Tenorio, C.; Martinez-Marcos, F.J.; Concejo-Martínez, E.; De la Torre Lima, J.; García, D.V.; Marquez, R.L.; et al. Blood culture-negative infective endocarditis: A worse outcome? Results from a large multicentre retrospective Spanish cohort study. Infect. Dis. 2021, 53, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Panagides, V.; Del Val, D.; Abdel-Wahab, M.; Crusius, L.; Durand, E.; Ihlemann, N.; Urena, M.; Pellegrini, C.; Giannini, F.; et al. Incidence, Clinical Characteristics, and Impact of Absent Echocardiographic Signs in Patients with Infective Endocarditis after Transcatheter Aortic Valve Implantation. Clin. Infect. Dis. 2022. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Diab, M.; Franz, M.; Hagel, S.; Guenther, A.; Struve, A.; Musleh, R.; Penzel, A.; Sponholz, C.; Lehmann, T.; Kuehn, H.; et al. Impact of an In-Hospital Endocarditis Team and a State-Wide Endocarditis Network on Perioperative Outcomes. J. Clin. Med. 2021, 10, 4734. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 122) | EC-IE (n = 53) | SC-IE (n = 69) | Unadjusted p-Value a | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years, median (IQR) | 80 (76–84) | 80 (78–84) | 81 (75–84) | 0.396 |
| Gender, male, n (%) | 72/122 (59.0) | 28/53 (52.8) | 44/69 (63.8) | 0.223 |
| Body mass index, kg/m2, median (IQR) | 27.3 (24.2–31.2) | 29.0 (25.4–32.6) | 25.8 (23.9–29.7) | 0.029 |
| Diabetes mellitus, n (%) | 51/122 (41.8) | 26/53 (49.1) | 25/69 (36.2) | 0.155 |
| Atrial fibrillation, n (%) | 57/122 (46.7) | 32/53 (60.4) | 25/69 (36.2) | 0.008 |
| Chronic kidney disease, n (%) | 75/122 (61.5) | 30/53 (56.6) | 45/69 (65.2) | 0.333 |
| COPD, n (%) | 34/122 (27.9) | 16/53 (30.2) | 18/69 (26.1) | 0.616 |
| Previous Stroke, n (%) | 10/122 (8.2) | 6/53 (11.3) | 4/69 (5.8) | 0.328 |
| Previous heart surgery, n (%) | 19/122 (15.6) | 9/53 (17.0) | 10/69 (14.5) | 0.707 |
| Previous infective endocarditis, n (%) | 1/122 (0.8) | 0/53 (0) | 1/69 (1.4) | 1.000 |
| Logistic EuroSCORE, % median (IQR) | 12.2 (7.2–21.5) n = 116 | 13.5 (7.9–24.1) n = 50 | 11.6 (6.5–19.1) n = 66 | 0.283 |
| Left ventricular ejection fraction, % median (IQR) | 56 (45–65) n = 120 | 59 (48–65) n = 52 | 55 (42–65) n = 68 | 0.135 |
| Mean transaortic gradient, median (IQR), mmHg | 43 (31–54) n = 112 | 46 (40–57) n = 46 | 40 (27–50) n = 66 | 0.001 |
| Mitral regurgitation ≥ 2, n (%) | 18/120 (15.0) | 7/52 (13.5) | 11/68 (16.2) | 0.680 |
| Periprocedural characteristics | ||||
| Implantation site | ||||
| Hybrid room, n (%) | 122/122 (100) | 53/53 (100) | 69/69 (100) | N/A |
| Orotracheal intubation, n (%) | 47/120 (39.2) | 16/52 (30.8) | 31/68 (45.6) | 0.099 |
| Antibiotic prophylaxis | ||||
| Cephalosporins alone, n (%) | 102/102 (100) | 45/45 (100) | 57/57 (100) | n.a. |
| Approach, n (%) | ||||
| Transfemoral | 116/122 (95.1) | 51/53 (96.2) | 65/69 (94.2) | 0.696 |
| Prosthesis type | ||||
| Balloon-expandable, n (%) | 32/122 (26.2) | 13/53 (24.5) | 19/69 (27.5) | 0.708 |
| Self-/Mechanically expanding, n (%) | 90/122 (73.8) | 40/53 (75.5) | 50/69 (72.5) | |
| In-hospital Outcomes (TAVI) | ||||
| Stroke, n (%) | 9/120 (7.5) | 2/52 (3.8) | 7/68 (10.3) | 0.296 |
| Major vascular complication, n (%) | 12/120 (10.0) | 4/52 (7.7) | 8/68 (11.8) | 0.461 |
| Major bleeding, n (%) | 13/120 (10.8) | 4/52 (7.7) | 9/68 (13.2) | 0.333 |
| Acute renal failure, n (%) | 25/120 (20.8) | 12/52 (23.1) | 13/68 (19.1) | 0.597 |
| Device success, n (%) | 112/122 (91.8) | 51/53 (96.2) | 61/69 (88.4) | 0.184 |
| New pacemaker implantation, n (%) | 33/120 (27.5) | 8/52 (15.4) | 25/68 (36.8) | 0.009 |
| Residual aortic regurgitation ≥ 2 at discharge, n (%) | 7/113 (6.2) | 2/49 (4.1) | 5/64 (7.8) | 0.697 |
| Mean residual transaortic gradient, median (IQR), mm Hg | 10 (7–14) n = 104 | 12 (7–15) n = 44 | 10 (7–13) n = 60 | 0.186 |
| Length of hospital stay, median (IQR), days | 14 (10–23) n = 120 | 14 (10–25) n = 52 | 13 (9–23) n = 68 | 0.332 |
| All Patients (n = 122) | EC-IE (n = 53) | SC-IE (n = 69) | Unadjusted p-Value a | |
|---|---|---|---|---|
| Time from TAVI, median (IQR), days | 110 (18–375) | 142 (24–416) | 85 (18–291) | 0.413 |
| Early IE (within 1 year), n (%) | 91/122 (74.6) | 37/53 (69.8) | 54/69 (78.3) | 0.288 |
| Late IE (>1 year), n (%) | 31/122 (25.4) | 16/53 (30.2) | 15/69 (21.7) | |
| Very early (within one month), n (%) | 39/122 (32.0) | 17/53 (32.1) | 22/69 (31.9) | 0.982 |
| Initial symptoms | ||||
| Fever, n (%) | 107/121 (88.4) | 45/52 (86.5) | 62/69 (89.9) | 0.572 |
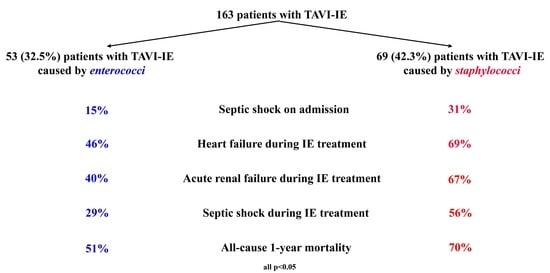
| Septic shock, n (%) | 29/120 (24.2) | 8/52 (15.4) | 21/68 (30.9) | 0.049 |
| New-onset heart failure, n (%) | 69/120 (57.5) | 27/52 (51.9) | 42/68 (61.8) | 0.280 |
| Neurological, n (%) | 27/121 (22.3) | 11/52 (21.2) | 16/69 (23.2) | 0.790 |
| Systemic embolism, n (%) | 26/121 (21.5) | 11/52 (21.2) | 15/69 (21.7) | 0.938 |
| Weight loss, n (%) | 8/73 (11.0) | 6/31 (19.4) | 2/42 (4.8) | 0.065 |
| Echocardiographic findings | ||||
| Vegetation, n (%) | 89/116 (76.7) | 40/52 (76.9) | 49/64 (76.6) | 0.964 |
| Vegetation size, mm | 8 (4–15) n = 46 | 9 (5–13) n = 22 | 7 (1–19) n = 24 | 0.895 |
| Perivalvular extension, n (%) | 19/122 (15.6) | 10/53 (18.9) | 9/69 (13.0) | 0.379 |
| Valve involved | ||||
| Isolated or involved THV | 63/122 (51.6) | 33/53 (62.3) | 30/69 (52.2) | 0.040 |
| Mitral valve | 19/122 (15.6) | 7/53 (13.2) | 12/69 (17.4) | 0.528 |
| Isolated PM | 7/122 (5.7) | 0/53 (0) | 7/69 (10.1) | 0.018 |
| New aortic regurgitation, n (%) | 7/122 (5.7) | 4/53 (7.5) | 3/69 (4.3) | 0.466 |
| New mitral regurgitation, n (%) | 23/122 (18.9) | 10/52 (18.9) | 13/69 (18.8) | 0.997 |
| Causative microorganisms | ||||
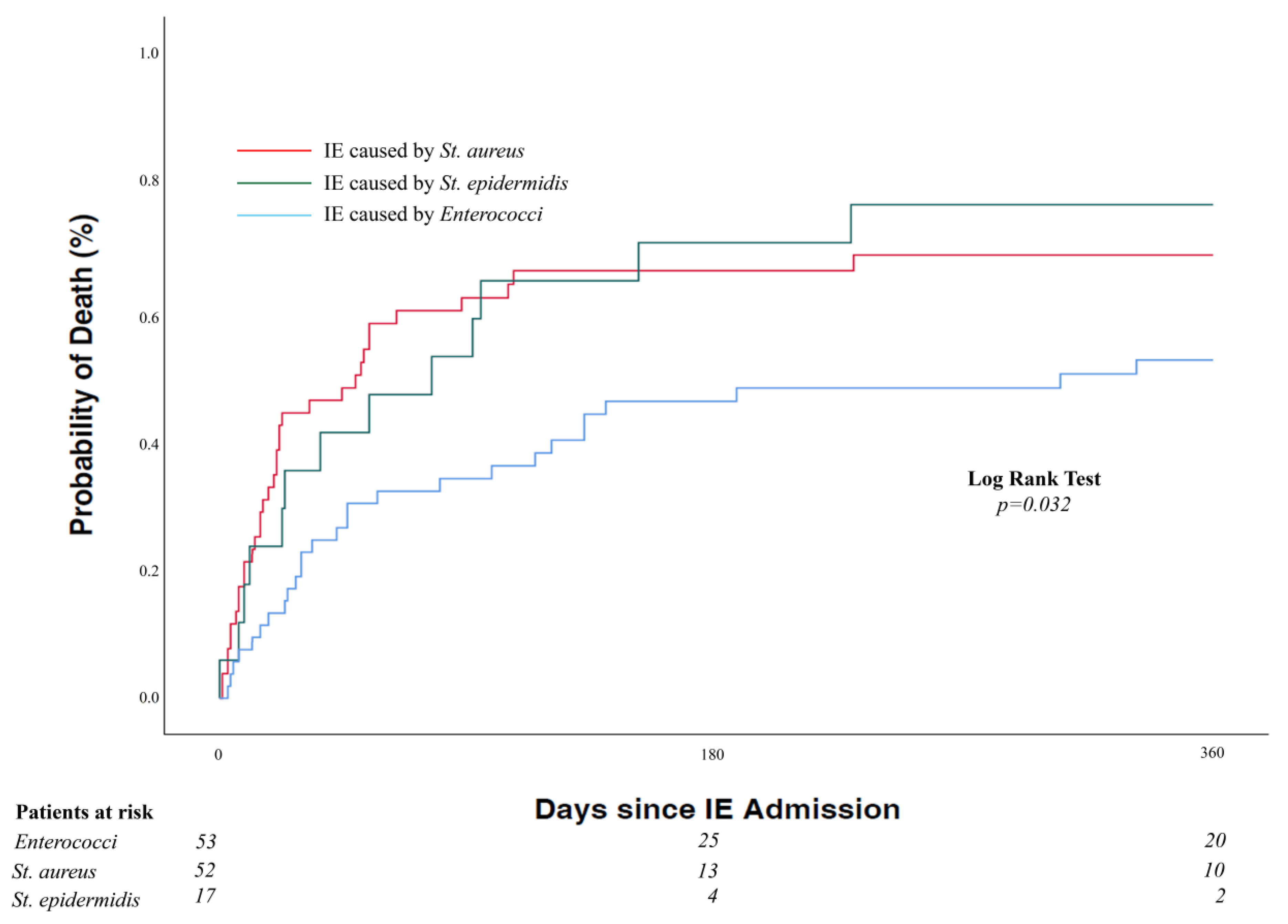
| Staphylococcus aureus, n (%) | 45/69 (65.2) | N/A | ||
| Methicillin-resistant | 7/69 (10.1) | N/A | ||
| Coagulase-negative staphylococci, n (%) | 17/69 (24.6) | N/A | ||
| Enterococci, n (%) | 53/53 (100) | N/A | ||
| Complications during IE hospitalization | ||||
| Any complication, n (%) | 100/122 (82.0) | 37/53 (69.8) | 63/69 (91.3) | 0.002 |
| Heart failure, n (%) | 71/120 (59.2) | 24/52 (46.2) | 47/68 (69.1) | 0.011 |
| Acute renal failure, n (%) | 65/118 (55.1) | 21/52 (40.4) | 44/66 (66.7) | 0.004 |
| Septic shock, n (%) | 53/120 (44.2) | 15/52 (28.8) | 38/68 (55.9) | 0.003 |
| Stroke, n (%) | 8/120 (6.7) | 2/52 (3.8) | 6/68 (8.8) | 0.463 |
| Systemic embolization, n (%) | 14/120 (11.7) | 7/52 (13.5) | 7/68 (10.3) | 0.592 |
| Persistent bacteremia, n (%) | 36/70 (51.4) | 12/30 (40.0) | 24/40 (60.0) | 0.098 |
| Management and Outcomes | ||||
| Antibiotic treatment alone, n (%) | 95/122 (78.7) | 42/53 (79.2) | 53/69 (76.8) | 0.748 |
| Antibiotic + Surgery during IE hospitalization, n (%) | 27/122 (22.1) | 11/53 (20.8) | 16/69 (23.2) | |
| Time to surgery, median (IQR), days | 10 (4–35) | 10 (5–48) | 11 (2–23) | 0.099 |
| Open heart surgery, n (%) | 22/122 (18.0) | 11/53 (20.8) | 11/69 (15.9) | 0.120 |
| Isolated pacemaker extraction, n (%) | 5/122 (4.1) | 0/53 (0) | 5/69 (7.2) | |
| Follow-up, median (IQR), days b | 394 (142–980) | 729 (222–1163) | 292 (104–778) | 0.044 |
| In-hospital mortality, n (%) | 57/120 (47.5) | 19/52 (36.5) | 38/68 (55.9) | 0.035 |
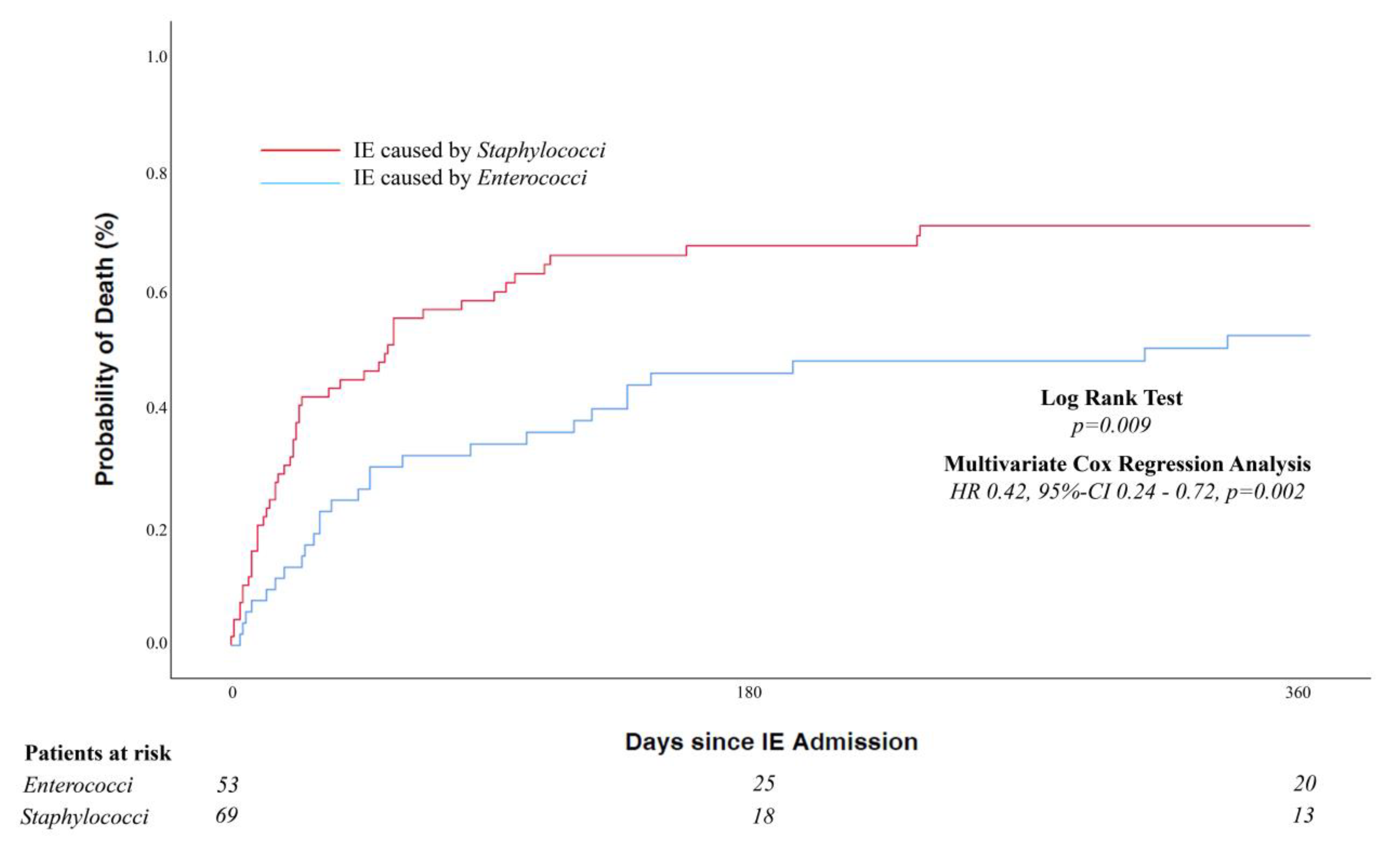
| 1-year mortality rate, % (95% CI) c | 61.5 (52.2–70.1) | 50.9 (36.8–64.9) | 69.6 (57.3–80.0) | 0.009 d |
| Overall mortality, n (%) | 87/122 (71.3) | 33/53 (62.3) | 54/69 (78.3) | 0.053 |
| Univariate Analysis OR (95% CI) | Unadjusted p Value | Multivariate Analysis OR (95% CI) | Adjusted p Value | |
|---|---|---|---|---|
| Baseline and TAVI features | ||||
| Age | 1.05 (0.99–1.12) | 0.124 | 1.03 (0.90–1.18) | 0.688 |
| Male gender | 0.64 (0.31–1.32) | 0.224 | 0.21 (0.04–1.00) | 0.050 |
| Body mass index > median 27.3 kg/m2 | 2.85 (1.35–5.99) | 0.006 | 4.90 (1.19–20.16) | 0.028 |
| Atrial fibrillation | 2.68 (1.28–5.61) | 0.009 | 9.56 (2.17–42.17) | 0.003 |
| Orotracheal intubation | 0.53 (0.25–1.13) | 0.100 | ||
| New pacemaker implantation | 0.31 (0.13–0.77) | 0.011 | 0.09 (0.01–0.65) | 0.018 |
| IE characteristics on admission | ||||
| Septic shock | 0.41 (0.16–1.01) | 0.053 | 0.12 (0.02–0.71) | 0.019 |
| Weight loss | 4.80 (0.90–25.67) | 0.067 | ||
| Structure involved | ||||
| Involved THV | 2.15 (1.03–4.46) | 0.041 | 1.86 (0.49–7.06) | 0.361 |
| Cardiac device involvement * | 0.54 (0.46–0.64) | 0.018 |
| Unadjusted Hazard Ratios | Unadjusted p-Value | Adjusted Hazard Ratios | Adjusted p-Value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Logistic EuroSCORE | 1.03 (1.01–1.06) | 0.018 | 0.94 (0.81–1.10) | 0.449 |
| Initial symptoms | ||||
| New onset heart failure a | 3.27 (1.40–7.62) | 0.006 | ||
| Septic shock a | 4.96 (2.09–11.77) | <0.001 | ||
| Neurological | 1.59 (0.67–3.78) | 0.296 | ||
| Echocardiography | ||||
| THV involvement | 1.06 (0.49–2.32) | 0.883 | ||
| Periannular involvement | 1.32 (0.76–2.31) | 0.328 | ||
| Complications during IE treatment | ||||
| Heart failure | 2.53 (1.14–5.62) | 0.022 | 2.26 (0.37–13.96) | 0.379 |
| Acute renal failure | 3.42 (1.53–7.64) | 0.003 | 3.94 (0.70–22.26) | 0.120 |
| Septic shock | 8.38 (3.67–19.15) | <0.001 | 6.58 (1.51–28.66) | 0.012 |
| Stroke | 3.51 (0.80–15.40) | 0.097 | ||
| Systemic embolization | 1.15 (0.34–3.86) | 0.823 | ||
| Persistent bacteremia | 3.51 (1.13–10.86) | 0.030 | 1.15 (0.26–5.05) | 0.849 |
| Treatment | ||||
| Cardiac Surgery | 1.28 (0.51–3.17) | 0.600 |
| Unadjusted Hazard Ratios | Unadjusted p-Value | Adjusted Hazard Ratios | Adjusted p-Value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Logistic EuroSCORE | 1.02 (0.99–1.04) | 0.149 | ||
| Initial symptoms | ||||
| New onset heart failure | 1.40 (0.77–2.56) | 0.269 | ||
| Septic shock | 1.27 (0.70–2.32) | 0.429 | ||
| Neurological | 1.70 (0.90–3.21) | 0.105 | ||
| Echocardiography | ||||
| THV involvement | 1.17 (0.67–2.08) | 0.574 | ||
| Periannular involvement | 1.08 (0.65–1.78) | 0.770 | ||
| Complications during IE hospitalization | ||||
| Heart failure | 1.40 (0.74–2.65) | 0.302 | ||
| Acute renal failure | 2.25 (1.14–4.47) | 0.020 | 1.42 (0.51–3.94) | 0.500 |
| Septic shock | 5.59 (2.83–11.02) | <0.001 | 2.27 (0.72–7.21) | 0.091 |
| Stroke | 1.34 (0.53–3.40) | 0.535 | ||
| Systemic embolization | 1.28 (0.54–3.02) | 0.574 | ||
| Persistent bacteremia | 6.59 (2.22–19.53) | 0.001 | 3.66 (1.03–13.02) | 0.045 |
| Treatment | ||||
| Cardiac Surgery | 0.69 (0.34–1.38) | 0.291 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasior, T.; Woitek, F.J.; Schroth, A.; Abdel-Wahab, M.; Crusius, L.; Haussig, S.; Kiefer, P.; Scislo, P.; Huczek, Z.; Dabrowski, M.; et al. Impact of Enterococci vs. Staphylococci Induced Infective Endocarditis after Transcatheter Aortic Valve Implantation. J. Clin. Med. 2023, 12, 1817. https://doi.org/10.3390/jcm12051817
Gasior T, Woitek FJ, Schroth A, Abdel-Wahab M, Crusius L, Haussig S, Kiefer P, Scislo P, Huczek Z, Dabrowski M, et al. Impact of Enterococci vs. Staphylococci Induced Infective Endocarditis after Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine. 2023; 12(5):1817. https://doi.org/10.3390/jcm12051817
Chicago/Turabian StyleGasior, Tomasz, Felix J. Woitek, Antonia Schroth, Mohamed Abdel-Wahab, Lisa Crusius, Stephan Haussig, Philipp Kiefer, Piotr Scislo, Zenon Huczek, Maciej Dabrowski, and et al. 2023. "Impact of Enterococci vs. Staphylococci Induced Infective Endocarditis after Transcatheter Aortic Valve Implantation" Journal of Clinical Medicine 12, no. 5: 1817. https://doi.org/10.3390/jcm12051817
APA StyleGasior, T., Woitek, F. J., Schroth, A., Abdel-Wahab, M., Crusius, L., Haussig, S., Kiefer, P., Scislo, P., Huczek, Z., Dabrowski, M., Witkowski, A., Olasinska-Wisniewska, A., Grygier, M., Protasiewicz, M., Hudziak, D., Kappert, U., Holzhey, D., Wojakowski, W., Linke, A., & Mangner, N. (2023). Impact of Enterococci vs. Staphylococci Induced Infective Endocarditis after Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine, 12(5), 1817. https://doi.org/10.3390/jcm12051817