Combined LTP Sublingual and Oral Immunotherapy in LTP Syndrome: Efficacy and Safety
Abstract
:1. Introduction
2. Methods
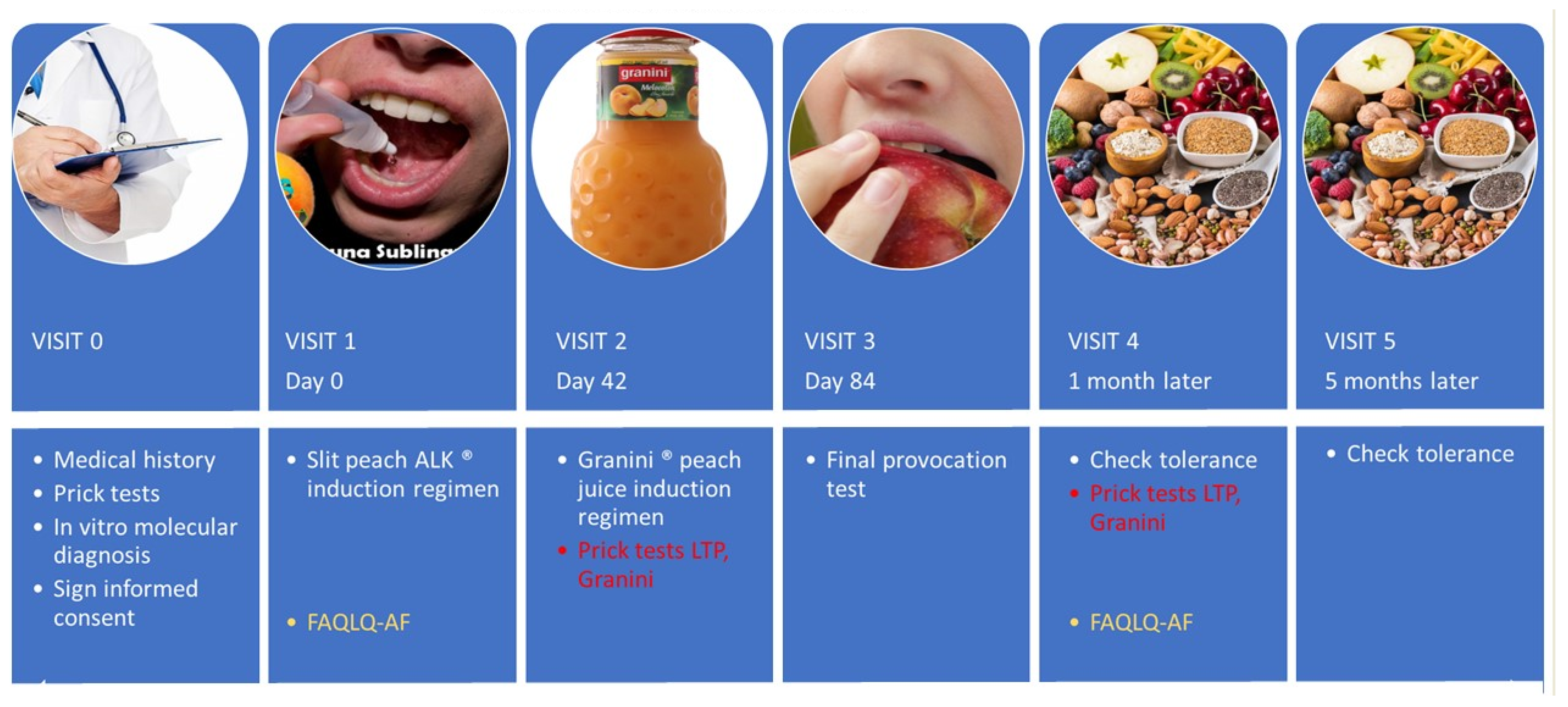
2.1. Study Population
2.2. Study Protocol
2.3. Sublingual Immunotherapy
2.4. Oral Immunotherapy with Granini® Peach Juice
2.5. Final Provocation Test (FPT)
2.6. Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Treated Patients
3.2. SLIT and OIT
3.3. Final Provocations and Follow-Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| nsLTPs | Nonspecific lipid transfer proteins |
| NSAIDs | non steroidal antiinflamatory drugs |
| SLIT | Sublingual immunotherapy |
| OIT | oral immunotherapy |
| OAS | oral allergy syndrome |
| EAACI | European Academy of Allergy and Clinical Immunology |
| WAO | world allergy organization |
| SPT | skin prick test |
| FAQLQ-AF | Food allergy quality of life questionnaire—adult form |
| FPT | Final provocation test |
References
- Nucera, E.; Mezzacappa, S.; Aruanno, A.; Pecora, V.; Rizzi, A.; Ricci, A.G.; Ferraironi, M.; Buonomo, A.; Schiavino, D. Hypersensitivity to major panallergens in a population of 120 patients. Adv. Dermatol. Allergol. 2015, 32, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. Off Publ. Eur. Soc. Pediatr. Allergy Immunol. 2016, 27 (Suppl. 23), 1–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salcedo, G.; Sanchez-Monge, R.; Díaz-Perales, A.; García-Casado, G.; Barber, D. Plant non-specific lipid transfer proteins as food and pollen allergens. Clin. Exp. Allergy 2004, 34, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J.; Bartra, J.; Ebo, D.G.; Faber, M.A.; Fernández-Rivas, M.; Gomez, F.; Luengo, O.; Till, S.J.; Asero, R.; Barber, D.; et al. The diagnosis and management of allergic reactions in patients sensitized to non-specific lipid transfer proteins. Allergy 2021, 76, 2433–2446. [Google Scholar] [CrossRef]
- Skypala, I.J.; Asero, R.; Barber, D.; Cecchi, L.; Diaz Perales, A.; Hoffmann-Sommergruber, K.; Pastorello, E.A.; Swoboda, I.; Bartra, J.; Ebo, D.G.; et al. Non-specific lipid-transfer proteins: Allergen structure and function, cross-reactivity, sensitization, and epidemiology. Clin. Transl. Allergy 2021, 11, e12010. [Google Scholar] [CrossRef]
- Egger, M.; Hauser, M.; Mari, A.; Ferreira, F.; Gadermaier, G. The Role of Lipid Transfer Proteins in Allergic Diseases. Curr. Allergy Asthma Rep. 2010, 10, 326–335. [Google Scholar] [CrossRef]
- Flokstra-de Blok, B.M.J.; Van Der Meulen, G.N.; DunnGalvin, A.; Vlieg-Boerstra, B.J.; Oude Elberink, J.N.G.; Duiverman, E.J.; Hourihane, J.O.B.; Dubois, A.E. Development and validation of the Food Allergy Quality of Life Questionnaire–Adult Form. Allergy 2009, 64, 1209–1217. [Google Scholar] [CrossRef]
- Kulis, M.D.; Patil, S.U.; Wambre, E.; Vickery, B.P. Immune mechanisms of oral immunotherapy. J. Allergy Clin. Immunol. 2017, 141, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Cuppari, C.; Leonardi, S.; Manti, S.; Filippelli, M.; Alterio, T.; Spicuzza, L.; Rigoli, L.; Arrigo, T.; Lougaris, V.; Salpietro, C. Allergen immunotherapy, routes of administration and cytokine networks: An update. Immunotherapy 2014, 6, 775–786. [Google Scholar] [CrossRef]
- A Schworer, S.; Kim, E.H. Sublingual immunotherapy for food allergy and its future directions. Immunotherapy 2020, 12, 921–931. [Google Scholar] [CrossRef]
- Betancor, D.; Gomez-Lopez, A.; Villalobos-Vilda, C.; Nuñez-Borque, E.; Fernández-Bravo, S.; Gozalo, M.D.L.H.; Pastor-Vargas, C.; Esteban, V.; Cuesta-Herranz, J. LTP Allergy Follow-Up Study: Development of Allergy to New Plant Foods 10 Years Later. Nutrients 2021, 13, 2165. [Google Scholar] [CrossRef]
- Enrique, E.; Pineda, F.; Malek, T.; Bartra, J.; Basagaña, M.; Tella, R.; Castelló, J.V.; Alonso, R.; de Mateo, J.A.; Cerdá-Trias, T.; et al. Sublingual immunotherapy for hazelnut food allergy: A randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J. Allergy Clin. Immunol. 2005, 116, 1073–1079. [Google Scholar] [CrossRef]
- Burks, A.W.; Wood, R.A.; Jones, S.M.; Sicherer, S.H.; Fleischer, D.M.; Scurlock, A.M.; Vickery, B.; Liu, A.H.; Henning, A.K.; Lindblad, R.; et al. Sublingual immunotherapy for peanut allergy: Long-term follow-up of a randomized multicenter trial. J. Allergy Clin. Immunol. 2015, 135, 1240–1248. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Rivas, M.; Fernández, S.G.; Nadal, J.A.; De Durana, M.D.A.D.; García, B.E.; Gonzalez-Mancebo, E.; Martin, S.; Barber, D.; Rico, P.; Tabar, A.I. Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy 2009, 64, 876–883. [Google Scholar] [CrossRef]
- Gomez, F.; Bogas, G.; Gonzalez, M.; Campo, P.; Salas, M.; Diaz-Perales, A.; Rodriguez, M.J.; Prieto, A.; Barber, D.; Blanca, M.; et al. The clinical and immunological effects of Pru p 3 sublingual immunotherapy on peach and peanut allergy in patients with systemic reactions. Clin. Exp. Allergy 2017, 47, 339–350. [Google Scholar] [CrossRef]
- Beitia, J.M.; Vega Castro, A.; Cárdenas, R.; Peña-Arellano, M.I. Pru p 3 Sublingual Immunotherapy in Patients with Lipid Transfer Protein Syndrome: Is It Worth? Int. Arch. Allergy Immunol. 2021, 182, 447–454. [Google Scholar] [CrossRef]
- Pajno, G.B.; Caminiti, L.; Chiera, F.; Crisafulli, G.; Salzano, G.; Arasi, S.; Passalacqua, G. Safety profile of oral immunotherapy with cow‘s milk and hen egg: A 10-year experience in controlled trials. Allergy Asthma Proc. 2016, 37, 400–403. [Google Scholar] [CrossRef]
- Navarro, B.; Alarcón, E.; Claver, Á.; Pascal, M.; Díaz-Perales, A.; Cisteró-Bahima, A. Oral immunotherapy with peach juice in patients allergic to LTPs. Allergy Asthma Clin. Immunol. 2019, 15, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef] [Green Version]
- Skin Tests Used in Type I Allergy Testing Position Paper. Sub-Committee on Skin Tests of the European Academy of Allergology and Clinical Immunology. Allergy 1989, 44, 11–59. [Google Scholar]
- Bindslev-Jensen, C.; Ballmer-Weber, B.K.; Bengtsson, U.; Blanco, C.; Ebner, C.; Hourihane, J.; Knulst, A.C.; Moneret-Vautrin, D.A.; Nekam, K.; Niggemann, B.; et al. Standardization of food challenges in patients with immediate reactions to foods-position paper from the European Academy of Allergology and Clinical Immunology. Allergy 2004, 59, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Wolters, P.; Ostermann, T.; Hofmann, S.C. Lipid transfer protein allergy: Characterization and comparison to birch-related food allergy. JDDG J. Dtsch. Dermatol. Ges. 2022, 20, 1430–1438. [Google Scholar] [CrossRef]
- Cardona, V.; Luengo, O.; Garriga, T.; Labrador-Horrillo, M.; Cunill, A.S.; Izquierdo, A.; Soto, L.; Guilarte, M. Co-factor-enhanced food allergy. Allergy 2012, 67, 1316–1318. [Google Scholar] [CrossRef] [PubMed]
- Ruano-Zaragoza, M.; Casas-Saucedo, R.; De la Cruz Martinez, C.A.; Araujo-Sanchez, G.; Gelis, S.; González, M.F.; San Bartolomé, C.; Pascal, M.; Jiménez-Rodriguez, T.W.; Gonzalez-Delgado, P.; et al. Advances in the understanding of the cofactor effect in lipid transfer protein food allergy: From phenotype description to clinical management. Allergy 2022, 77, 1924–1926. [Google Scholar] [CrossRef] [PubMed]
- Novembre, E.; Mori, F.; Contestabile, S.; Rossi, M.E.; Pucci, N. Correlation of anti-pru p 3 IgE levels with severity of peach allergy reactions in children. Ann. Allergy Asthma Immunol. 2012, 108, 271–274. [Google Scholar] [CrossRef]
- Asero, R.; Piantanida, M.; Pravettoni, V. Allergy to LTP: To eat or not to eat sensitizing foods? A follow-up study. Eur. Ann. Allergy Clin. Immunol. 2018, 50, 156. [Google Scholar] [CrossRef]



| N | 45 |
|---|---|
| Age, years (mean (SD)) | 32.29 (12.6) |
| Gender (F/M) | 27/18 |
| Family history of atopy n (%) | 38 (84.4%) |
| Personal history of atopy n (%) | 32 (71.1%) |
| Associated pollen allergy n (%) | 34 (75.6%) |
| Rhinoconjuntivitis n (%) | 35 (77.8%) |
| Asthma n (%) | 22 (48.9%) |
| Food groups involved n (%) | |
| Several Nuts | 1 (2.2%) |
| Several Fruits | 2 (4.4%) |
| Two groups of plant foods | 12 (26.7%) |
| Three groups of plant foods | 14 (31.1%) |
| ≥three groups of plant foods | 16 (35.6%) |
| Food that caused the most severe reaction n (%) | |
| Nuts | 14 (31.1%) |
| Peach/Rosaceae | 12 (26.7%) |
| Legumes | 4 (8.9%) |
| Another fruits | 4 (8.9%) |
| Grains, seeds, spices | 3 (6.7%) |
| Vegetables | 2(4.4%) |
| Plant foods of several of this groups | 6 (13.3%) |
| Cofactors | 11 (24.4%) |
| Exercise | 9 (20%) |
| NSAIDs | 2 (4.4%) |
| Symptoms | |
| Cutaneous symptoms | 43 (95.6%) |
| Urticaria-angioedema | 24 (53.3%) |
| Urticaria | 8 (17.7%) |
| Exantema | 11 (24.4%) |
| OAS | 38 (84.4%) |
| Asthma | 17.8% |
| Digestive symptoms | 13.4% |
| Anaphylaxis | 34 (75.6%) |
| Grade II | 11(32.4%) |
| Grade III | 8 (23.5%) |
| Grade IV | 15 (44.1%) |
| Microarray | 42/45 |
| ISAC | 14/45 |
| ALEX | 28/45 |
| SPT Pru p3 mm,( mean (SD)) | 7.3 (SD 3) |
| SPT Granini® (mm, mean (SD)) | 5.5 (SD 2.1) |
| LTP sensitization n (%) | 45 (100%) |
| Profilin co-sensitization | 5 (11.6%) |
| sIgE Pru p 3 kU/L, (mean (SD)) | |
| ISAC | 4.8 (9.8) |
| ALEX | 6.45 (7.2) |
| FAQLQ-AF score | 4.47 |
| PEACH ALK® SLIT | Well Tolerated | Mild Reactions (OAS) | Moderate Reactions | Severe Reactions |
|---|---|---|---|---|
| N = 45 | 37 (82.2%) | 5 (11.1%) | 3 (6%) | 0 |
| GRANINI® PEACH OIT | ||||
| N = 45 | 39 (86.6%) | 5 (11.1%) | 1 (2%) | 0 |
| FPT | ||||
| N = 45 | 39 (86.6%) | 3 (6%) | 2 (4%) | 1 (2%) |
| Almonds | Almonds | Lentils | ||
| Apple with peel | Apple with peel | |||
| Peanut |
| SPT | Baseline | Visit 4 | Sig |
|---|---|---|---|
| Pru p3, mean (SD) | 7.3 (3) | 6.9 (2.2) | NS * |
| Granini®, mean (SD) | 5.5 (2.1) | 5.1 ( 2.5) | NS * |
| FAQLQ-AF score | 4.47 | 2.7 | p < 0.01 * |
| SPT | Negative FPT | Positive FPT | |
| SPT Pru p3 | 7.4 (2.9) | 8.5 (3.3) | NS ** |
| SPT Granini® | 5.4 (1.9) | 6.8 (2.6) | NS ** |
| sIgE Pru p3 | 7.6 (11.7) | 4.6 (4.9) | NS ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin Iglesias, M.A.; Garcia Rodriguez, R.; Palacios Cañas, A.; Meneses Sotomayor, J.V.; Clar Castello, M.; Feo Brito, F. Combined LTP Sublingual and Oral Immunotherapy in LTP Syndrome: Efficacy and Safety. J. Clin. Med. 2023, 12, 1823. https://doi.org/10.3390/jcm12051823
Martin Iglesias MA, Garcia Rodriguez R, Palacios Cañas A, Meneses Sotomayor JV, Clar Castello M, Feo Brito F. Combined LTP Sublingual and Oral Immunotherapy in LTP Syndrome: Efficacy and Safety. Journal of Clinical Medicine. 2023; 12(5):1823. https://doi.org/10.3390/jcm12051823
Chicago/Turabian StyleMartin Iglesias, Maria Aranzazu, Rosa Garcia Rodriguez, Alberto Palacios Cañas, Jaime Vinicio Meneses Sotomayor, Miriam Clar Castello, and Francisco Feo Brito. 2023. "Combined LTP Sublingual and Oral Immunotherapy in LTP Syndrome: Efficacy and Safety" Journal of Clinical Medicine 12, no. 5: 1823. https://doi.org/10.3390/jcm12051823
APA StyleMartin Iglesias, M. A., Garcia Rodriguez, R., Palacios Cañas, A., Meneses Sotomayor, J. V., Clar Castello, M., & Feo Brito, F. (2023). Combined LTP Sublingual and Oral Immunotherapy in LTP Syndrome: Efficacy and Safety. Journal of Clinical Medicine, 12(5), 1823. https://doi.org/10.3390/jcm12051823





