COVID-19 Accelerated Cognitive Decline in Elderly Patients with Pre-Existing Dementia Followed up in an Outpatient Memory Care Facility
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Dementia Diagnosis, Cognitive Function, and ADL Assessment of Patients
2.3. Statistical Methods
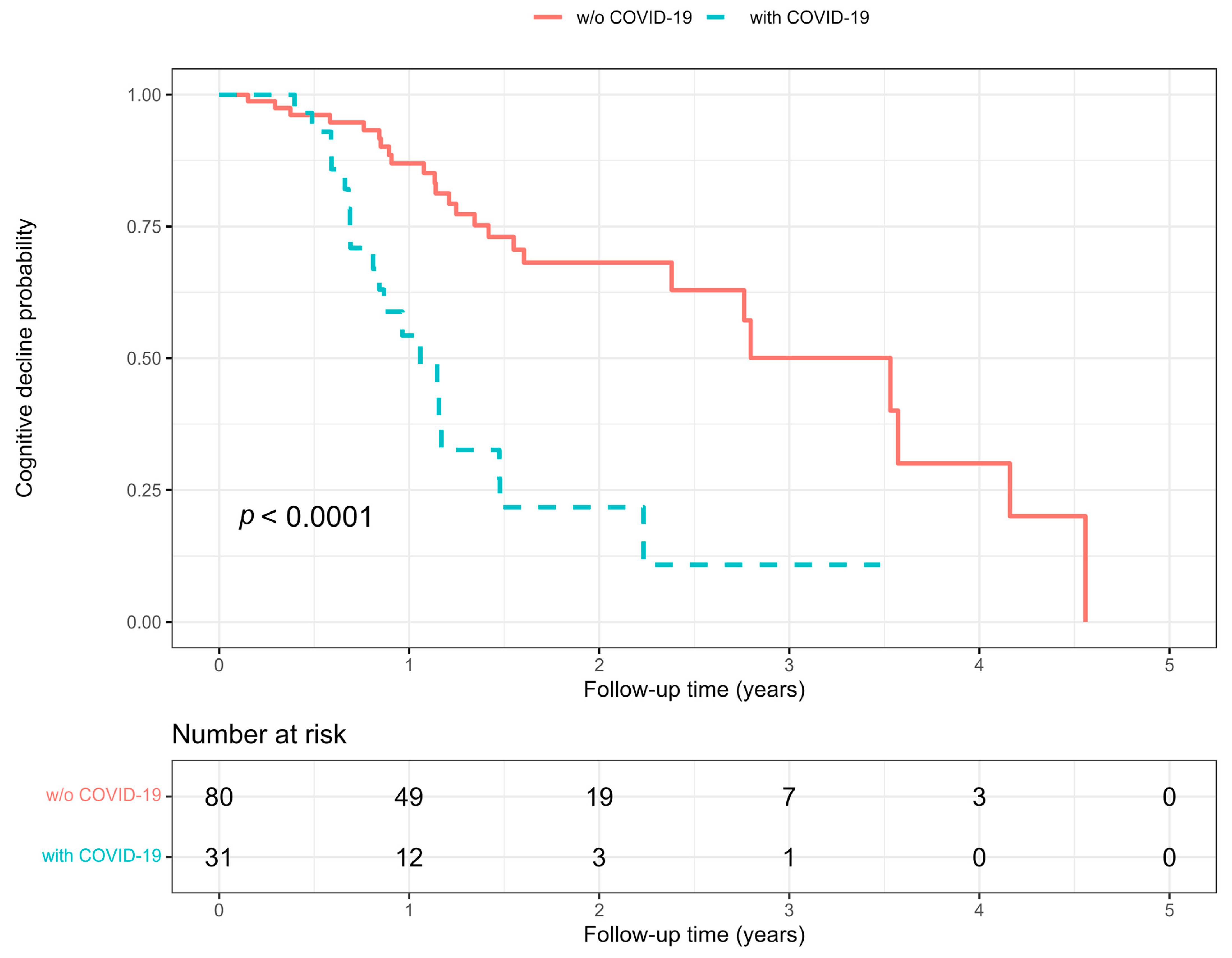
3. Results
3.1. Predictors of COVID-19 and Cognitive Decline
3.2. Effect of COVID-19 on the Yearly Change in MMSE Score and ADL Indexes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crivelli, L.; Palmer, K.; Calandri, I.; Guekht, A.; Beghi, E.; Carroll, W.; Frontera, J.; García-Azorín, D.; Westenberg, E.; Winkler, A.S.; et al. Changes in Cognitive Functioning after COVID-19: A Systematic Review and Meta-Analysis. Alzheimers Dement. J. Alzheimers Assoc. 2022, 18, 1047–1066. [Google Scholar] [CrossRef] [PubMed]
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Rozanski, G.; Breyman, E.; Nasr, L.; et al. Post-Acute COVID-19 Syndrome Negatively Impacts Physical Function, Cognitive Function, Health-Related Quality of Life, and Participation. Am. J. Phys. Med. Rehabil. 2022, 101, 48–52. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Zheng, J. COVID-19 and Alzheimer’s Disease: How One Crisis Worsens the Other. Transl. Neurodegener. 2021, 10, 15. [Google Scholar] [CrossRef]
- Holwerda, T.J.; Deeg, D.J.H.; Beekman, A.T.F.; van Tilburg, T.G.; Stek, M.L.; Jonker, C.; Schoevers, R.A. Feelings of Loneliness, but Not Social Isolation, Predict Dementia Onset: Results from the Amsterdam Study of the Elderly (AMSTEL). J. Neurol. Neurosurg. Psychiatry 2014, 85, 135–142. [Google Scholar] [CrossRef]
- Scarlata, S.; Cardaci, V.; Santangelo, C.; Matarese, M.; Cesari, M.; Antonelli Incalzi, R. Distancing Measures in COVID-19 Pandemic: Loneliness, More than Physical Isolation, Affects Health Status and Psycho-Cognitive Wellbeing in Elderly Patients with Chronic Obstructive Pulmonary Disease. COPD 2021, 18, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-C.; Liu, S.; Gan, J.; Ma, L.; Du, X.; Zhu, H.; Han, J.; Xu, J.; Wu, H.; Fei, M.; et al. The Impact of the COVID-19 Pandemic and Lockdown on Mild Cognitive Impairment, Alzheimer’s Disease and Dementia With Lewy Bodies in China: A 1-Year Follow-Up Study. Front. Psychiatry 2021, 12, 711658. [Google Scholar] [CrossRef]
- Soysal, P.; Smith, L.; Trott, M.; Alexopoulos, P.; Barbagallo, M.; Tan, S.G.; Koyanagi, A.; Shenkin, S.; Veronese, N. European Society of Geriatric Medicine Special Interest Group in Dementia and Systematic Reviews and Meta-Analyses The Effects of COVID-19 Lockdown on Neuropsychiatric Symptoms in Patients with Dementia or Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Psychogeriatrics 2022, 22, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, T.B.; Powell, L.; Emrani, S.; Wasserman, V.; Higgins, S.; Chopra, A.; Cavalieri, T.A.; Libon, D.J. Instrumental Activities of Daily Living, Neuropsychiatric Symptoms, and Neuropsychological Impairment in Mild Cognitive Impairment. J. Am. Osteopath. Assoc. 2019, 119, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Covinsky, K.E.; Palmer, R.M.; Fortinsky, R.H.; Counsell, S.R.; Stewart, A.L.; Kresevic, D.; Burant, C.J.; Landefeld, C.S. Loss of Independence in Activities of Daily Living in Older Adults Hospitalized with Medical Illnesses: Increased Vulnerability with Age. J. Am. Geriatr. Soc. 2003, 51, 451–458. [Google Scholar] [CrossRef]
- Ehlenbach, W.J.; Hough, C.L.; Crane, P.K.; Haneuse, S.J.P.A.; Carson, S.S.; Curtis, J.R.; Larson, E.B. Association between Acute Care and Critical Illness Hospitalization and Cognitive Function in Older Adults. JAMA 2010, 303, 763–770. [Google Scholar] [CrossRef]
- Andrei Appelt, P.; Taciana Sisconetto, A.; Baldo Sucupira, K.S.M.; de Moura Neto, E.; de Jesus Chagas, T.; Bazan, R.; Moura Cabral, A.; de Oliveira Andrade, A.; de Souza, L.A.P.S.; José Luvizutto, G. Changes in Electrical Brain Activity and Cognitive Functions Following Mild to Moderate COVID-19: A One-Year Prospective Study After Acute Infection. Clin. EEG Neurosci. 2022, 53, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Miners, S.; Kehoe, P.G.; Love, S. Cognitive Impact of COVID-19: Looking beyond the Short Term. Alzheimers Res. Ther. 2020, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- van Lith, T.J.; Sluis, W.M.; Wijers, N.T.; Meijer, F.J.; Kamphuis-van Ulzen, K.; de Bresser, J.; Dankbaar, J.W.; van den Heuvel, F.M.; Antoni, M.L.; Mulders-Manders, C.M.; et al. Prevalence, Risk Factors, and Long-Term Outcomes of Cerebral Ischemia in Hospitalized COVID-19 Patients—Study Rationale and Protocol of the CORONIS Study: A Multicentre Prospective Cohort Study. Eur. Stroke J. 2022, 7, 180–187. [Google Scholar] [CrossRef]
- Calagnan, E.; Gobbato, M.; Burba, I.; Del Zotto, S.; Toffolutti, F.; Serraino, D.; Tonutti, G. COVID-19 Infections in the Friuli Venezia Giulia Region (Northern Italy): A Population-Based Retrospective Analysis. Epidemiol. Prev. 2020, 44, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Caruso, P.; Giuffré, M.; Tiribelli, C. COVID-19 Lockdown Effect on Not Institutionalized Patients with Dementia and Caregivers. Healthcare 2021, 9, 893. [Google Scholar] [CrossRef]
- WHO. Laboratory Testing of 2019 Novel Coronavirus (2019-NCoV) in Suspected Human Cases: Interim Guidance; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Doody, R.S.; Massman, P.; Dunn, J.K. A Method for Estimating Progression Rates in Alzheimer Disease. Arch. Neurol. 2001, 58, 449–454. [Google Scholar] [CrossRef]
- Reisberg, B. Diagnostic Criteria in Dementia: A Comparison of Current Criteria, Research Challenges, and Implications for DSM-V. J. Geriatr. Psychiatry Neurol. 2006, 19, 137–146. [Google Scholar] [CrossRef]
- Hugo, J.; Ganguli, M. Dementia and Cognitive Impairment: Epidemiology, Diagnosis, and Treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Magni, E.; Binetti, G.; Bianchetti, A.; Rozzini, R.; Trabucchi, M. Mini-Mental State Examination: A Normative Study in Italian Elderly Population. Eur. J. Neurol. 1996, 3, 198–202. [Google Scholar] [CrossRef]
- Katz, S.; Downs, T.D.; Cash, H.R.; Grotz, R.C. Progress in Development of the Index of ADL. Gerontologist 1970, 10, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.; Hazlett, C.; Imai, K. Covariate Balancing Propensity Score for a Continuous Treatment: Application to the Efficacy of Political Advertisements. Ann. Appl. Stat. 2018, 12, 156–177. [Google Scholar] [CrossRef]
- Clark, C.M.; Sheppard, L.; Fillenbaum, G.G.; Galasko, D.; Morris, J.C.; Koss, E.; Mohs, R.; Heyman, A.; the CERAD Investigators. Variability in Annual Mini-Mental State Examination Score in Patients With Probable Alzheimer Disease: A Clinical Perspective of Data From the Consortium to Establish a Registry for Alzheimer’s Disease. Arch. Neurol. 1999, 56, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Wang, Y.-R.; Wang, Q.-H.; Chen, Y.; Chen, X.; Li, Y.; Cen, Y.; Xu, C.; Hu, T.; Liu, X.-D.; et al. Post-Infection Cognitive Impairments in a Cohort of Elderly Patients with COVID-19. Mol. Neurodegener. 2021, 16, 48. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Chen, Y.; Wang, Q.-H.; Wang, L.-R.; Jiang, L.; Yang, Y.; Chen, X.; Li, Y.; Cen, Y.; Xu, C.; et al. One-Year Trajectory of Cognitive Changes in Older Survivors of COVID-19 in Wuhan, China: A Longitudinal Cohort Study. JAMA Neurol. 2022, 79, 509–517. [Google Scholar] [CrossRef]
- Weihe, S.; Mortensen, C.B.; Haase, N.; Andersen, L.P.K.; Mohr, T.; Siegel, H.; Ibsen, M.; Jørgensen, V.R.L.; Buck, D.L.; Pedersen, H.B.S.; et al. Long-Term Cognitive and Functional Status in Danish ICU Patients with COVID-19. Acta Anaesthesiol. Scand. 2022, 66, 978–986. [Google Scholar] [CrossRef]
- Latronico, N.; Peli, E.; Calza, S.; Rodella, F.; Novelli, M.P.; Cella, A.; Marshall, J.; Needham, D.M.; Rasulo, F.A.; Piva, S.; et al. Physical, Cognitive and Mental Health Outcomes in 1-Year Survivors of COVID-19-Associated ARDS. Thorax 2022, 77, 300–303. [Google Scholar] [CrossRef]
- de Medeiros, M.M.D.; Carletti, T.M.; Magno, M.B.; Maia, L.C.; Cavalcanti, Y.W.; Rodrigues-Garcia, R.C.M. Does the Institutionalization Influence Elderly’s Quality of Life? A Systematic Review and Meta–Analysis. BMC Geriatr. 2020, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Iyamu, I.; Plottel, L.; Snow, M.E.; Zhang, W.; Havaei, F.; Puyat, J.; Sawatzky, R.; Salmon, A. Culture Change in Long-Term Care-Post COVID-19: Adapting to a New Reality Using Established Ideas and Systems. Can. J. Aging Rev. Can. Vieil. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Borges-Machado, F.; Barros, D.; Ribeiro, Ó.; Carvalho, J. The Effects of COVID-19 Home Confinement in Dementia Care: Physical and Cognitive Decline, Severe Neuropsychiatric Symptoms and Increased Caregiving Burden. Am. J. Alzheimers Dis. Other Demen. 2020, 35, 1533317520976720. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Liu, S.; Wu, H.; Chen, Z.; Fei, M.; Xu, J.; Dou, Y.; Wang, X.; Ji, Y. The Impact of the COVID-19 Pandemic on Alzheimer’s Disease and Other Dementias. Front. Psychiatry 2021, 12, 703481. [Google Scholar] [CrossRef] [PubMed]
- Vernuccio, L.; Sarà, D.; Inzerillo, F.; Catanese, G.; Catania, A.; Vesco, M.; Cacioppo, F.; Dominguez, L.J.; Veronese, N.; Barbagallo, M. Effect of COVID-19 Quarantine on Cognitive, Functional and Neuropsychiatric Symptoms in Patients with Mild Cognitive Impairment and Dementia. Aging Clin. Exp. Res. 2022, 34, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Rainero, I.; Bruni, A.C.; Marra, C.; Cagnin, A.; Bonanni, L.; Cupidi, C.; Laganà, V.; Rubino, E.; Vacca, A.; Di Lorenzo, R.; et al. The Impact of COVID-19 Quarantine on Patients With Dementia and Family Caregivers: A Nation-Wide Survey. Front. Aging Neurosci. 2020, 12, 625781. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 Is Associated with Changes in Brain Structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.; Kern, F.; Losada, P.M.; Agam, M.R.; Maat, C.A.; Schmartz, G.P.; Fehlmann, T.; Stein, J.A.; Schaum, N.; Lee, D.P.; et al. Dysregulation of Brain and Choroid Plexus Cell Types in Severe COVID-19. Nature 2021, 595, 565–571. [Google Scholar] [CrossRef]
- Creditor, M.C. Hazards of hospitalization of the elderly. Ann. Intern. Med. 1993, 118, 219–223. [Google Scholar] [CrossRef]
- Starr, J.M.; Whalley, L.J. Drug-induced dementia. Incidence, management and prevention. Drug Saf. 1994, 11, 310–317. [Google Scholar] [CrossRef]
- Krogseth, M.; Wyller, T.B.; Engedal, K.; Juliebø, V. Delirium is a risk factor for institutionalization and functional decline in older hip fracture patients. J. Psychosom. Res. 2014, 76, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Giostra, F.; Mirarchi, M.G.; Farina, G.; Paolillo, C.; Sepe, C.; Benedusi, F.; Bellone, A.; Ghiadoni, L.; Barbieri, G.; Santini, M.; et al. Impact of COVID-19 Pandemic and Lockdown on Emergency Room Access in Northern and Central Italy. Emerg. Care J. 2021, 17, 9705. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Hanlon, P.; Gray, S.R.; Welsh, P.; Gill, J.M.R.; Foster, H.; Katikireddi, S.V.; Lyall, D.; Mackay, D.F.; O’Donnell, C.A.; et al. Comparison of two different frailty measurements and risk of hospitalisation or death from COVID-19: Findings from UK Biobank. BMC Med. 2020, 18, 355. [Google Scholar] [CrossRef] [PubMed]



| All | Without COVID-19 | With COVID-19 | p | |
|---|---|---|---|---|
| Patients (n) | 111 | 80 | 31 | - |
| Baseline variables | ||||
| Age (years) | 82 ± 5 | 82 ± 5 | 83 ± 5 | 0.315 |
| Male sex (n (%)) | 35 (32) | 24 (30) | 11 (36) | 0.651 |
| Hypertension (n (%)) | 66 (60) | 44 (55) | 22 (71) | 0.138 |
| Diabetes (n (%)) | 27 (24) | 15 (19) | 12 (39) | 0.047 |
| Dyslipidemia (n (%)) | 41 (37) | 30 (38) | 11 (36) | 1.000 |
| Cerebrovascular disease (n (%)) | 11 (9.9) | 8 (10) | 3 (9.7) | 1.000 |
| Cardiovascular disease (n (%)) | 37 (33) | 26 (33) | 11 (36) | 0.824 |
| Chronic kidney disease (n (%)) | 10 (9.0) | 7 (8.8) | 3 (9.7) | 1.000 |
| Parkinson’s disease (n (%)) | 7 (6.3) | 7 (8.8) | 0 | 0.187 |
| Self-sufficient (n (%)) | 26 (23) | 22 (28) | 4 (13) | 0.136 |
| Institutionalized (n (%)) | 7 (6.3) | 2 (2.5) | 5 (16) | 0.018 |
| Dementia type (n (%)): | 0.729 | |||
| 28 (25) | 21 (26) | 7 (23) | |
| 25 (23) | 19 (24) | 6 (19) | |
| 37 (33) | 24 (30) | 13 (42) | |
| 21 (19) | 16 (20) | 5 (16) | |
| BADL index | 5.0 [3.0, 5.5] | 5.0 [3, 6] | 3.0 [2, 4] | 0.006 |
| IADL index | 2.0 [1.0, 4.0] | 3.0 [1, 4] | 1.0 [0, 3] | 0.020 |
| MMSE score | 19.1 ± 5.0 | 19.8 ± 4.3 | 17.5 ± 6.3 | 0.027 |
| Total drugs number | 4.0 [2.0, 6.0] | 3.5 [2.0, 6.0] | 5.0 [3.0, 6.0] | 0.107 |
| Memantine (n (%)) | 5 (4.5) | 4 (5.0) | 1 (3.2) | 1.000 |
| Anticholinergic drug (n (%)) | 17 (15) | 11 (14) | 6 (19) | 0.558 |
| Antipsychotic drug (n (%)) | 22 (20) | 15 (19) | 7 (23) | 0.791 |
| Antidepressant drug (n (%)) | 23 (21) | 16 (20) | 7 (23) | 0.797 |
| Benzodiazepines (n (%)) | 21 (19) | 16 (20) | 5 (16) | 0.790 |
| Follow-up variables | ||||
| Follow-up time (years) | 1.1 [0.7–1.7] | 1.2 [0.8, 1.9] | 0.9 [0.6, 1.2] | 0.013 |
| Change in MMSE score | −3.0 [−6.8, −1.4] | −2.7 [−5.8, −1.0] | −6.0 [−10, −3.2] | 0.002 |
| Significant cognitive decline (n (%)) | 44 (40) | 25 (31) | 19 (61) | 0.005 |
| Change in BADL index | −1.0 [−2.0, 0.0] | −1.0 [−2.0, 0.0] | −1.0 [−2.0, 0.0] | 0.848 |
| Change in IADL index | −1.0 [−2.5, 0.0] | −1.0 [−3.0, 0.0] | −1.0 [−2.0, 0.0] | 0.235 |
| Hospitalization for any cause (n (%)) | 6 (5.4) | 0 | 6 (19) | <0.001 |
| New institutionalization (n (%)) | 30 (27) | 16 (20) | 14 (45) | 0.016 |
| Without Cognitive Decline | With Cognitive Decline | p | |
|---|---|---|---|
| Patients (n) | 67 | 44 | - |
| Baseline variables | |||
| Age (years) | 82 ± 5 | 82 ± 6 | 0.734 |
| Male sex (n (%)) | 19 (28) | 16 (36) | 0.409 |
| Hypertension (n (%)) | 38 (57) | 28 (64) | 0.555 |
| Diabetes (n (%)) | 14 (21) | 13 (30) | 0.367 |
| Dyslipidemia (n (%)) | 26 (39) | 15 (34) | 0.690 |
| Cerebrovascular disease (n (%)) | 4 (6.0) | 7 (16) | 0.109 |
| Cardiovascular disease (n (%)) | 23 (34) | 14 (32) | 0.839 |
| Chronic kidney disease (n (%)) | 5 (7.5) | 5 (11) | 0.514 |
| Parkinson’s disease (n (%)) | 4 (6.0) | 3 (6.8) | 1.000 |
| Self-sufficient (n (%)) | 21 (31) | 5 (11) | 0.021 |
| Institutionalized (n (%)) | 3 (4.5) | 4 (9.1) | 0.432 |
| Dementia type (n (%)) | 0.808 | ||
| 18 (27) | 10 (23) | |
| 13 (19) | 12 (28) | |
| 23 (34) | 14 (32) | |
| 13 (19) | 8 (18) | |
| MMSE score | 18.8 ± 4.4 | 19.6 ± 5.7 | 0.390 |
| BADL index | 5.0 [3.0, 5.5] | 4.5 [3.0, 5.25] | 0.963 |
| IADL index | 2.0 [1.0, 5.0] | 2.0 [1.0, 4.0] | 0.552 |
| Total drugs number (n) | 4.0 [2.0, 6.0] | 4.0 [2.0, 6.0] | 0.340 |
| Memantine (n (%)) | 1 (1.5) | 4 (9.1) | 0.079 |
| Anticholinergic drug (n (%)) | 10 (15) | 7 (16) | 1.000 |
| Antipsychotic drug (n (%)) | 14 (21) | 8 (18) | 0.811 |
| Antidepressant drug (n (%)) | 14 (21) | 9 (21) | 1.000 |
| Benzodiazepine drug (n (%)) | 14 (21) | 7 (16) | 0.623 |
| Follow-up variables | |||
| COVID-19 (n (%)) | 12 (18) | 19 (43) | 0.005 |
| Follow-up time (years) | 1.1 [0.8, 1.8] | 1.1 [0.7, 1.5] | 0.602 |
| Change in MMSE score | −2.0 [−3.0, 0.0] | −8.2 [−10.1, −6.0] | <0.001 |
| Change in BADL index | 0.0 [−1.0, 0.0] | −1.0 [−2.25, 0.0] | 0.001 |
| Change in IADL index | −1.0 [−2.0, 0.0] | −1.0 [−3.0, −1.0] | 0.192 |
| Hospitalization for any cause (n (%)) | 1 (1.5) | 5 (11) | 0.035 |
| New institutionalization (n (%)) | 14 (21) | 16 (36) | 0.084 |
| COVID-19 | Cognitive Decline | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p | HR (95% CI) | p |
| Age (every 10 years) | 1.43 (0.73, 2.80) | 0.293 | 0.98 (0.56–1.71) | 0.936 |
| Male sex (yes/no) | 1.39 (0.66, 2.91) | 0.382 | 1.62 (0.86, 3.02) | 0.133 |
| Hypertension (yes/no) | 2.12 (0.97, 4.63) | 0.059 | 1.77 (0.94, 3.33) | 0.078 |
| Diabetes (yes/no) | 2.31 (1.11, 4.78) | 0.025 | 1.38 (0.71, 2.67) | 0.344 |
| Dyslipidemia (yes/no) | 0.99 (0.47, 2.09) | 0.988 | 1.07 (0.56, 2.03) | 0.841 |
| Cerebrovascular disease (yes/no) | 1.00 (0.30, 3.30) | 0.994 | 2.28 (0.99, 5.25) | 0.053 |
| Cardiovascular disease (yes/no) | 1.29 (0.62, 2.69) | 0.503 | 1.17 (0.61, 2.23) | 0.635 |
| Chronic kidney disease (yes/no) | 1.06 (032, 3.52) | 0.919 | 1.62 (0.63, 4.17) | 0.319 |
| Parkinson’s disease (yes/no) | - | - | 0.98 (0.30, 3.22) | 0.973 |
| Self-sufficient (yes/no) | 0.46 (0.16, 1.32) | 0.148 | 0.42 (0.17, 1.08) | 0.073 |
| Institutionalized (yes/no) | 2.86 (1.10, 7.48) | 0.032 | 1.69 (0.60, 4.79) | 0.320 |
| Dementia type (yes/no): | ||||
| 1.00 | - | 1.00 | - |
| 0.99 (0.33, 2.93) | 0.980 | 1.52 (0.65, 3.54) | 0.331 |
| 1.58 (0.63, 3.97) | 0.329 | 1.33 (0.58, 3.02) | 0.498 |
| 0.84 (0.26, 2.64) | 0.759 | 0.68 (0.26, 1.80) | 0.434 |
| BADL (every 1 point) | 0.66 (0.51, 0.85) | 0.001 | 0.79 (0.64, 0.99) | 0.039 |
| IADL (every 1 point) | 0.80 (0.67, 0.96) | 0.014 | 0.86 (0.75, 0.98) | 0.026 |
| MMSE score (every 1 point) | 0.92 (0.86, 0.99) | 0.024 | 0.98 (0.92, 1.04) | 0.548 |
| New institutionalization (yes/no) | - | - | 1.71 (0.91, 3.20) | 0.096 |
| Drug numbers (every 1 drug) | 1.15 (1.00, 1.32) | 0.054 | 1.13 (1.01, 1.27) | 0.038 |
| Memantine (yes/no) | 0.83 (0.11, 6.12) | 0.853 | 4.13 (1.42, 12.0) | 0.009 |
| Anticholinergic drug (yes/no) | 1.63 (0.66, 4.01) | 0.290 | 1.83 (0.79, 4.21) | 0.157 |
| Antipsychotic drug (yes/no) | 1.43 (0.61, 3.34) | 0.412 | 1.48 (0.67, 3.23) | 0.330 |
| Antidepressant drug (yes/no) | 1.25 (0.54, 2.92) | 0.604 | 1.30 (0.62, 2.76) | 0.487 |
| Anxiolytic drug (yes/no) | 0.78 (0.30, 2.04) | 0.616 | 0.67 (0.28, 1.60) | 0.368 |
| COVID-19 (yes/no) | - | - | 3.94 (2.09, 7.43) | <0.001 |
| Hospitalization for any cause (yes/no) | - | - | 4.10 (1.59, 10.6) | 0.003 |
| Dependent Variable | |||
|---|---|---|---|
| MMSE Score | Basic ADL | Instrumental ADL | |
| Independent Variable | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) |
| A | |||
| COVID-19 (yes/no) | −3.1 (−5.4, −0.9) ** | −0.9 (−1.5, −0.2) ** | −1.0 (−1.9, −0.2) * |
| Follow-up (year) | −1.7 (−2.3, −1.1) *** | −0.5 (−0.7, −0.4) *** | −0.8 (−1.0, −0.6) *** |
| COVID x Follow-up | −1.6 (−3.0, −0.2) * | −0.2 (−0.6, 0.2) | −0.1 (−0.6, 0.4) |
| B | |||
| COVID-19 (yes/no) | −3.4 (−5.7, −1.0) ** | −0.8 (−1.5, −0.1) * | −1.0 (−1.9, −0.05) * |
| Follow-up (year) | −1.7 (−2.2, −1.1) *** | −0.5 (−0.7, −0.4) *** | −0.8 (−1.0, −0.6) *** |
| COVID x Follow-up | −1.5 (−2.9, −0.06) * | −0.2 (−0.6, 0.2) | −0.1 (−0.6, 0.4) |
| C | |||
| COVID-19 (yes/no) | −1.6 (−3.9, 0.7) | −0.6 (−1.3, 0.1) | −0.7 (−1.7, 0.3) |
| Follow-up (year) | −1.7 (−2.2, −1.1) *** | −0.5 (−0.7, −0.4) *** | −0.8 (−1.0, −0.6) *** |
| COVID x Follow-up | −1.5 (−2.9, −0.08) * | −0.2 (−0.6, 0.2) | −0.1 (−0.6, 0.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merla, L.; Montesi, M.C.; Ticali, J.; Bais, B.; Cavarape, A.; Colussi, G. COVID-19 Accelerated Cognitive Decline in Elderly Patients with Pre-Existing Dementia Followed up in an Outpatient Memory Care Facility. J. Clin. Med. 2023, 12, 1845. https://doi.org/10.3390/jcm12051845
Merla L, Montesi MC, Ticali J, Bais B, Cavarape A, Colussi G. COVID-19 Accelerated Cognitive Decline in Elderly Patients with Pre-Existing Dementia Followed up in an Outpatient Memory Care Facility. Journal of Clinical Medicine. 2023; 12(5):1845. https://doi.org/10.3390/jcm12051845
Chicago/Turabian StyleMerla, Lucia, Maria Cristina Montesi, Jessica Ticali, Bruno Bais, Alessandro Cavarape, and GianLuca Colussi. 2023. "COVID-19 Accelerated Cognitive Decline in Elderly Patients with Pre-Existing Dementia Followed up in an Outpatient Memory Care Facility" Journal of Clinical Medicine 12, no. 5: 1845. https://doi.org/10.3390/jcm12051845
APA StyleMerla, L., Montesi, M. C., Ticali, J., Bais, B., Cavarape, A., & Colussi, G. (2023). COVID-19 Accelerated Cognitive Decline in Elderly Patients with Pre-Existing Dementia Followed up in an Outpatient Memory Care Facility. Journal of Clinical Medicine, 12(5), 1845. https://doi.org/10.3390/jcm12051845






