Improved Early Detection Models of Pharyngocutaneous Fistula after Total Laryngectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. PCF Definition and Management
2.3. Clinical Factors Used for Early PCF Detection
2.4. Statistical Analysis to Compare the Clinical Factors between the Fistula and the No Fistula Groups
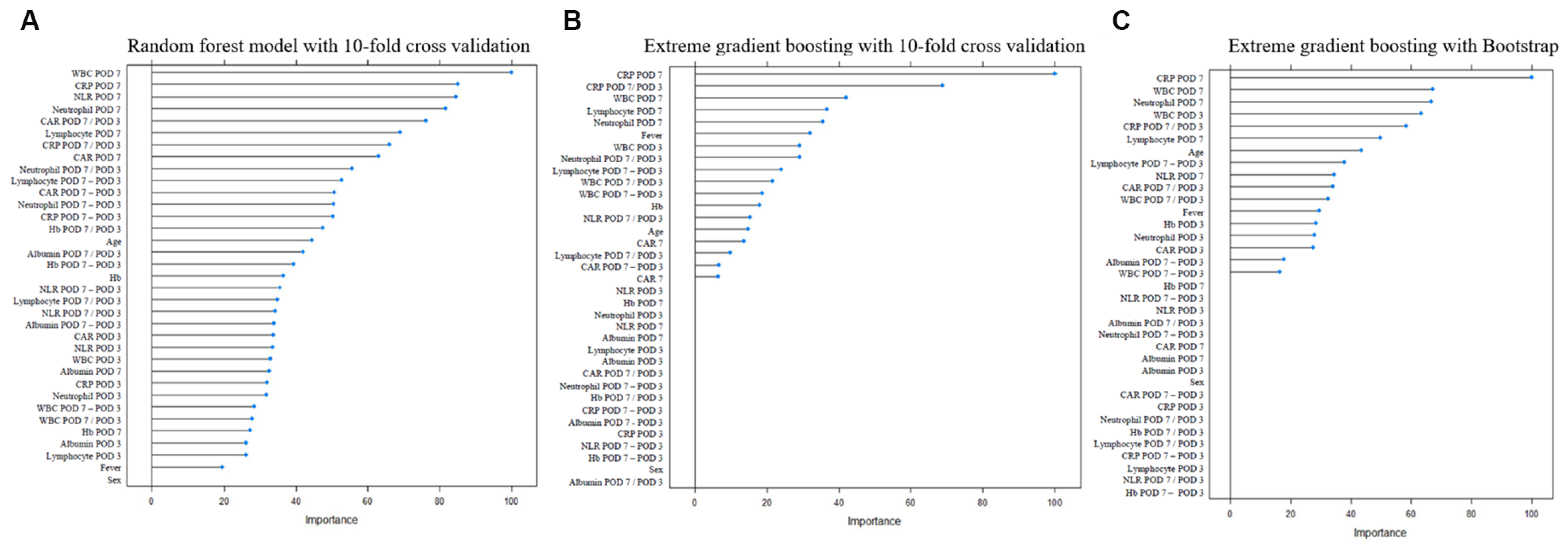
2.5. Development of PCF Prediction Models
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasan, Z.; Dwivedi, R.C.; Gunaratne, D.A.; Virk, S.A.; Palme, C.E.; Riffat, F. Systematic review and meta-analysis of the complications of salvage total laryngectomy. Eur. J. Surg. Oncol. 2017, 43, 42–51. [Google Scholar] [CrossRef]
- Makitie, A.A.; Niemensivu, R.; Hero, M.; Keski-Santti, H.; Back, L.; Kajanti, M.; Lehtonen, H.; Atula, T. Pharyngocutaneous fistula following total laryngectomy: A single institution’s 10-year experience. Eur. Arch. Otorhinolaryngol. 2006, 263, 1127–1130. [Google Scholar] [CrossRef]
- Wang, M.; Xun, Y.; Wang, K.; Lu, L.; Yu, A.; Guan, B.; Yu, C. Risk factors of pharyngocutaneous fistula after total laryngectomy: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2020, 277, 585–599. [Google Scholar] [CrossRef]
- Sifrer, R.; Anicin, A.; Pohar, M.P.; Zargi, M.; Pukl, P.; Soklic, T.; Strojan, P. Pharyngocutaneous fistula: The incidence and the risk factors. Eur. Arch. Otorhinolaryngol. 2016, 273, 3393–3399. [Google Scholar] [CrossRef] [PubMed]
- Sassler, A.M.; Esclamado, R.M.; Wolf, G.T. Surgery after organ preservation therapy. Analysis of wound complications. Arch. Otolaryngol. Head Neck Surg. 1995, 121, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.S.; Berkey, B.A.; Forastiere, A.; Cooper, J.; Maor, M.; Goepfert, H.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Outcome of salvage total laryngectomy following organ preservation therapy: The Radiation Therapy Oncology Group trial 91-11. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markou, K.D.; Vlachtsis, K.C.; Nikolaou, A.C.; Petridis, D.G.; Kouloulas, A.I.; Daniilidis, I.C. Incidence and predisposing factors of pharyngocutaneous fistula formation after total laryngectomy. Is there a relationship with tumor recurrence? Eur. Arch. Otorhinolaryngol. 2004, 261, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.V.; Aires, F.T.; Dedivitis, R.A.; Kulcsar, M.A.; Ramos, D.M.; Cernea, C.R.; Brandao, L.G. Efficacy of pectoralis major muscle flap for pharyngocutaneous fistula prevention in salvage total laryngectomy: A systematic review. Head Neck 2016, 38 (Suppl. 1), E2317–E2321. [Google Scholar] [CrossRef] [PubMed]
- Cavalot, A.L.; Gervasio, C.F.; Nazionale, G.; Albera, R.; Bussi, M.; Staffieri, A.; Ferrero, V.; Cortesina, G. Pharyngocutaneous fistula as a complication of total laryngectomy: Review of the literature and analysis of case records. Otolaryngol. Head Neck Surg. 2000, 123, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Moses, B.L.; Eisele, D.W.; Jones, B. Radiologic assessment of the early postoperative total-laryngectomy patient. Laryngoscope 1993, 103, 1157–1160. [Google Scholar] [CrossRef]
- White, H.N.; Golden, B.; Sweeny, L.; Carroll, W.R.; Magnuson, J.S.; Rosenthal, E.L. Assessment and incidence of salivary leak following laryngectomy. Laryngoscope 2012, 122, 1796–1799. [Google Scholar] [CrossRef]
- Amin, J.; Ortlip, T.E.; Cohen, D.; Vakharia, K.; Lubek, J.E. The utility of barium swallow studies for evaluation of pharyngocutaneous fistula after total laryngectomy. Arch. Otorhinoraryngol. Head Neck Surg. 2020, 4, 4. [Google Scholar] [CrossRef]
- Friedman, M.; Venkatesan, T.K.; Yakovlev, A.; Lim, J.W.; Tanyeri, H.M.; Caldarelli, D.D. Early detection and treatment of postoperative pharyngocutaneous fistula. Otolaryngol. Head Neck Surg. 1999, 121, 378–380. [Google Scholar] [CrossRef]
- Nassar, A.A.; Ibrahim, H.O. Surgical emphysema as an early sign for pharyngocutaneous fistula following total laryngectomy: A case report. Egypt J. Otolaryngol. 2021, 37, 1–4. [Google Scholar] [CrossRef]
- Goncalves, A.J.; de Souza, J.A., Jr.; Menezes, M.B.; Kavabata, N.K.; Suehara, A.B.; Lehn, C.N. Pharyngocutaneous fistulae following total laryngectomy comparison between manual and mechanical sutures. Eur. Arch. Otorhinolaryngol. 2009, 266, 1793–1798. [Google Scholar] [CrossRef]
- Narayan, M.; Limbachiya, S.; Balasubramanian, D.; Subramaniam, N.; Thankappan, K.; Iyer, S. Efficacy of small-volume gastrografin videofluoroscopic screening for detecting pharyngeal leaks following total laryngectomy. J. Laryngol. Otol. 2020, 134, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Krouse, J.H.; Metson, R. Barium swallow is a predictor of salivary fistula following laryngectomy. Otolaryngol. Head Neck Surg. 1992, 106, 254–257. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; De Cillis, G.; Marchiori, C.; Carpene, S.; Da Mosto, M.C. Multivariate analysis of risk factors for pharyngocutaneous fistula after total laryngectomy. Eur. Arch. Otorhinolaryngol. 2008, 265, 929–936. [Google Scholar] [CrossRef]
- Kiong, K.L.; Tan, N.C.; Skanthakumar, T.; Teo, C.E.H.; Soo, K.C.; Tan, H.K.; Roche, E.; Yee, K.; Iyer, N.G. Salivary fistula: Blue dye testing as part of an algorithm for early diagnosis. Laryngoscope Investig. Otolaryngol. 2017, 2, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Carsuzaa, F.; Capitaine, A.L.; Ferrie, J.C.; Apert, V.; Tonnerre, D.; Frasca, D.; Dufour, X. Pharyngocutaneous fistulas after total laryngectomy or pharyngolaryngectomy: Place of video-fluoroscopic swallowing study. Head Neck 2020, 42, 3638–3646. [Google Scholar] [CrossRef]
- van la Parra, R.F.; Kon, M.; Schellekens, P.P.; Braunius, W.W.; Pameijer, F.A. The prognostic value of abnormal findings on radiographic swallowing studies after total laryngectomy. Cancer Imaging 2007, 7, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Larsen, L.R.; Schuller, D.E. Wound amylase levels as an early indicator of orocutaneous fistulae. Laryngoscope 1984, 94, 1302–1306. [Google Scholar] [CrossRef]
- Fielder, C.P.; Morton, R.P. Wound amylase estimation and the prediction of pharyngocutaneous fistulae. Clin. Otolaryngol. Allied Sci. 1989, 14, 101–105. [Google Scholar] [CrossRef]
- Morton, R.P.; Mehanna, H.; Hall, F.T.; McIvor, N.P. Prediction of pharyngocutaneous fistulas after laryngectomy. Otolaryngol. Head Neck Surg. 2007, 136, S46–S49. [Google Scholar] [CrossRef]
- Aydogan, L.B.; Kiroglu, M.; Tuncer, U.; Soylu, L. The wound amylase concentration in the prediction of pharyngocutaneous fistula. Otolaryngol. Head Neck Surg. 2003, 129, 414–416. [Google Scholar] [CrossRef]
- Koob, I.; Pickhard, A.; Buchberger, M.; Boxberg, M.; Reiter, R.; Piontek, G.; Strassen, U. Bradykinin Receptor B1 and C-Reactive Protein as Prognostic Factors for Pharyngocutaneous Fistula Development After Laryngectomy. Head Neck Pathol. 2020, 14, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yucel, A.; Yucel, H.; Aydemir, F.; Mutaf, M.; Eryilmaz, M.A.; Arbag, H. Development of Pharyngocutaneous Fistula after Total Laryngectomy: The Predictive Value of C-reactive Protein/Albumin Ratio. Acta Medica (Hradec Kralove) 2020, 63, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Nakayama, M.; Gosho, M.; Nishimura, B.; Takahashi, K.; Yoshimura, T.; Senarita, M.; Ohara, H.; Akizuki, H.; Wada, T.; et al. Inflammation-Based Score (Combination of Platelet Count and Neutrophil-to-Lymphocyte Ratio) Predicts Pharyngocutaneous Fistula After Total Laryngectomy. Laryngoscope 2022, 132, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Livaditi, O.; Kotanidou, A.; Psarra, A.; Dimopoulou, I.; Sotiropoulou, C.; Augustatou, K.; Papasteriades, C.; Armaganidis, A.; Roussos, C.; Orfanos, S.E.; et al. Neutrophil CD64 expression and serum IL-8: Sensitive early markers of severity and outcome in sepsis. Cytokine 2006, 36, 283–290. [Google Scholar] [CrossRef]
- Park, B.H.; Kang, Y.A.; Park, M.S.; Jung, W.J.; Lee, S.H.; Lee, S.K.; Kim, S.Y.; Kim, S.K.; Chang, J.; Jung, J.Y.; et al. Delta neutrophil index as an early marker of disease severity in critically ill patients with sepsis. BMC Infect. Dis. 2011, 11, 299. [Google Scholar] [CrossRef] [Green Version]
- Lowsby, R.; Gomes, C.; Jarman, I.; Lisboa, P.; Nee, P.A.; Vardhan, M.; Eckersley, T.; Saleh, R.; Mills, H. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg. Med. J. 2015, 32, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Erdag, M.A.; Arslanoglu, S.; Onal, K.; Songu, M.; Tuylu, A.O. Pharyngocutaneous fistula following total laryngectomy: Multivariate analysis of risk factors. Eur. Arch. Otorhinolaryngol. 2013, 270, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, G.; Doundoulakis, G.; Terzakis, G.; Dokianakis, G. Pharyngocutaneous fistula after total laryngectomy: Incidence, cause, and treatment. Ann. Otol. Rhinol. Laryngol. 1994, 103, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Loaec, E.; Vaillant, P.Y.; Bonne, L.; Marianowski, R. Negative-pressure wound therapy for the treatment of pharyngocutaneous fistula. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2014, 131, 351–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, S.; Costa, J.; Bartosch, I.; Correia, B.; Silva, A. Management of Pharyngocutaneous Fistula with Negative-Pressure Wound Therapy. J. Craniofac. Surg. 2017, 28, e364–e367. [Google Scholar] [CrossRef] [PubMed]
- Andrews, B.T.; Smith, R.B.; Hoffman, H.T.; Funk, G.F. Orocutaneous and pharyngocutaneous fistula closure using a vacuum-assisted closure system. Ann. Otol. Rhinol. Laryngol. 2008, 117, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Paydarfar, J.A.; Birkmeyer, N.J. Complications in head and neck surgery: A meta-analysis of postlaryngectomy pharyngocutaneous fistula. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locatello, L.G.; Licci, G.; Maggiore, G.; Gallo, O. Non-Surgical Strategies for Assisting Closure of Pharyngocutaneous Fistula after Total Laryngectomy: A Systematic Review of the Literature. J. Clin. Med. 2021, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Bomeli, S.R.; Desai, S.C.; Johnson, J.T.; Walvekar, R.R. Management of salivary flow in head and neck cancer patients--a systematic review. Oral Oncol. 2008, 44, 1000–1008. [Google Scholar] [CrossRef]
- Abu Eta, R.; Eviatar, E.; Gavriel, H. Hyperbaric oxygen therapy as an alternative to surgery for non-healing pharyngocutaneous fistula. Eur. Arch. Otorhinolaryngol. 2016, 273, 3857–3861. [Google Scholar] [CrossRef]
| No Fistula | Fistula | p-Value | |
|---|---|---|---|
| Patient no. (%) | 177 (67.3) | 86 (32.7) | Total = 263 |
| Sex (M:F, n, %) | 156:21 (88.1:11.9) | 75:11 (87.2:12.8) | 0.842 |
| Hospital days (mean ± SD) | 22.4 ± 23.8 | 55.9 ± 45.1 | <0.001 |
| Age (mean ± SD) | 64.9 ± 9.5 | 64.6 ± 10.4 | 0.077 |
| Primary surgery extent (n, %) | 0.329 | ||
| Total laryngectomy | 144 (81.4) | 65 (75.6) | |
| Total laryngectomy with pharyngoesophagectomy | 33 (18.6) | 21 (24.4) | |
| Neck lymphatic surgery (n, %) | 0.284 | ||
| No neck dissection | 130 (73.4) | 69 (80.2) | |
| Neck dissection | 47 (26.6) | 17 (19.8) | |
| Flap (n, %) | 0.368 | ||
| No flap | 135 (76.3) | 61 (70.9) | |
| Flap reconstruction | 42 (23.7) | 25 (29.1) | |
| Previous radiation or chemoradiation therapy | 0.527 | ||
| None | 80 (45.2) | 37 (43.1) | |
| Radiation therapy | 49 (27.6) | 20 (23.2) | |
| Chemoradiation therapy | 48 (27.2) | 29 (33.7) |
| No Fistula | Fistula | p-Value | |
|---|---|---|---|
| Fever >38.0 °C by POD 3 (n, %) | 22 (12.4) | 30 (34.9) | <0.001 |
| Blood labs POD 3 | |||
| WBC (mean ± SD, ×103/µL) | 11.4 (4.2) | 13.0 (4.7) | 0.040 |
| CRP (mean ± SD, mg/dL) | 6.9 (5.3) | 7.3 (5.3) | 0.799 |
| Albumin (mean ± SD, g/dL) | 3.1 (0.5) | 3.0 (0.5) | 0.022 |
| CAR (mean ± SD) | 2.3 (2.0) | 2.5 (1.8) | 0.734 |
| Hb (mean ± SD, g/dL) | 10.9 (1.5) | 10.5 (1.5) | 0.028 |
| Neutrophils (%) | 85.3 (6.7) | 86.9 (6.6) | 0.023 |
| Lymphocytes (%) | 7.9 (4.9) | 7.0 (4.9) | 0.024 |
| NLR (mean ±S D) | 16.4 (12.5) | 21.6 (18.1) | 0.026 |
| Blood labs POD 7 | |||
| WBC (mean ± SD, ×103/µL) | 7.2 (2.7) | 9.7 (3.5) | <0.001 |
| CRP (mean ± SD, mg/dL) | 2.7 (2.8) | 4.8 (4.0) | <0.001 |
| Albumin (mean ± SD, g/dL) | 3.4 (0.4) | 3.3 (0.4) | 0.051 |
| CAR (mean ± SD) | 0.8 (0.9) | 1.5 (1.4) | <0.001 |
| Hb (mean ± SD, g/dL) | 11.0 (1.4) | 10.8 (1.4) | 0.082 |
| Neutrophils (%) | 71.7 (10.2) | 79.7 (8.3) | <0.001 |
| Lymphocytes (%) | 16.6 (7.6) | 11.0 (5.4) | <0.001 |
| NLR (mean ± SD) | 5.7 (3.6) | 10.2 (10.4) | <0.001 |
| Ratio of POD 7:POD 3 values | |||
| WBC (mean ± SD, ×103/µL) | 0.7 (0.3) | 0.9 (0.7) | 0.001 |
| CRP (mean ± SD, mg/dL) | 0.6 (0.8) | 1.0 (1.0) | <0.001 |
| Albumin (mean ± SD, g/dL) | 1.1 (0.2) | 1.1 (0.2) | 0.292 |
| CAR (mean ± SD) | 0.5 (0.7) | 0.9 (1.0) | <0.001 |
| Hb (mean ± SD, g/dL) | 1.0 (0.1) | 1.0 (0.2) | 0.374 |
| Neutrophils (%) | 0.8 (0.1) | 0.9 (0.1) | <0.001 |
| Lymphocytes (%) | 2.6 (1.6) | 2.2 (1.5) | 0.013 |
| NLR (mean ± SD) | 0.4 (0.3) | 0.7 (0.6) | 0.001 |
| Leakage on fistulography (n, %) * | 5 (3.0) | 29 (38.2) | <0.001 |
| Model 0 | Model 1 | Model 2 | |
|---|---|---|---|
| Analyzed clinical factors | Fistulography, | Fistulography, neutrophils (POD7) | Fistulography, WBC (POD7), neutrophil ratio (POD7:POD3) |
| Performance of prediction (AUROC) | |||
| Apparent performance on whole dataset | 0.68 | 0.82 | 0.83 |
| 10-fold CV | 0.69 | 0.81 | 0.84 |
| Bootstrap | 0.68 | 0.82 | 0.82 |
| Hold-out test set | - | 0.77 | 0.75 |
| Diagnostic power of prediction models | |||
| Sensitivity | 0.37 | 0.52 | 0.83 |
| Specificity | 0.97 | 0.91 | 0.73 |
| Positive predictive value | 0.85 | 0.73 | 0.60 |
| Negative predictive value | 0.75 | 0.80 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, Y.; Lee, H.S.; Jung, S.; Lee, C.; Kim, Y.; Chung, M.K.; Jeong, H.-S.; Baek, C.-H.; Ahn, J.H.; Son, Y.-I.; et al. Improved Early Detection Models of Pharyngocutaneous Fistula after Total Laryngectomy. J. Clin. Med. 2023, 12, 1851. https://doi.org/10.3390/jcm12051851
Heo Y, Lee HS, Jung S, Lee C, Kim Y, Chung MK, Jeong H-S, Baek C-H, Ahn JH, Son Y-I, et al. Improved Early Detection Models of Pharyngocutaneous Fistula after Total Laryngectomy. Journal of Clinical Medicine. 2023; 12(5):1851. https://doi.org/10.3390/jcm12051851
Chicago/Turabian StyleHeo, Yujin, Hyun Suk Lee, Sungha Jung, Changhee Lee, Younghac Kim, Man Ki Chung, Han-Sin Jeong, Chung-Hwan Baek, Joong Hyun Ahn, Young-Ik Son, and et al. 2023. "Improved Early Detection Models of Pharyngocutaneous Fistula after Total Laryngectomy" Journal of Clinical Medicine 12, no. 5: 1851. https://doi.org/10.3390/jcm12051851





