The Influence of Conventional and Innovative Rehabilitation Methods on Brain Plasticity Induction in Patients with Multiple Sclerosis
Abstract
:1. Multiple Sclerosis and Physical Rehabilitation
2. Neuroplasticity–Molecular Basis
3. Markers of Neuroplasticity
3.1. BDNF and Neuroplasticity
3.2. The Role of Myokines
3.3. Adipokines and Multiple Sclerosis
3.4. Oxidative Stress
3.5. Epigenetic Inheritance
3.6. The Mirror Neuron System
4. Conventional Rehabilitation and Brain Plasticity
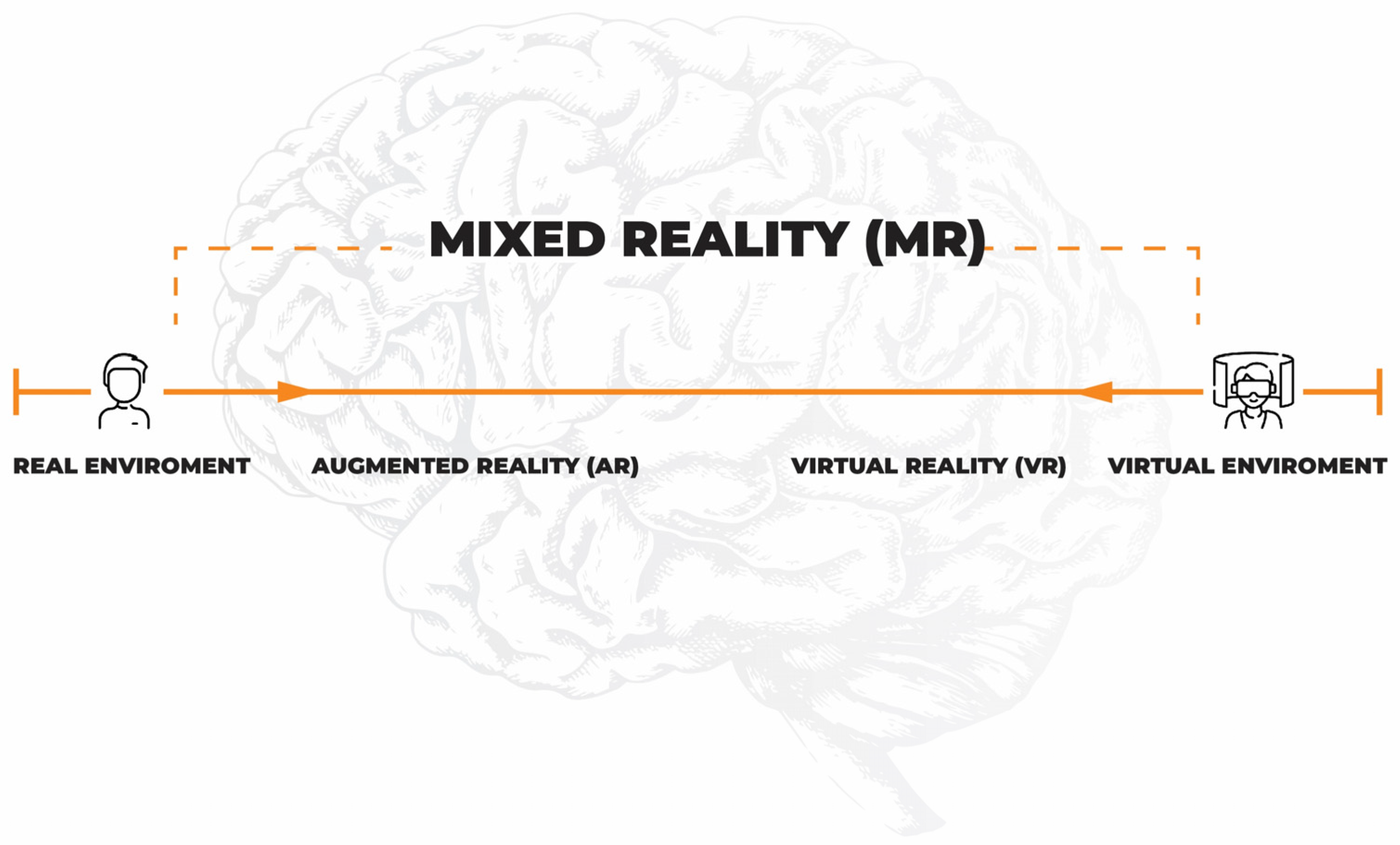
5. Virtual Reality and Neurorehabilitation
6. Other Symptoms and Rehabilitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Gil Moreno, M.J.; Cerezo García, M.; Marasescu, R.; Pinel González, A.; López Álvarez, L.; Aladro Benito, Y. Neuropsychological syndromes in multiple sclerosis. Psicothema 2013, 25, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Pérez, I.; Sánchez-Alcalá, M.; Nieto-Escámez, F.A.; Castellote-Caballero, Y.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual reality-based therapy improves fatigue, impact, and quality of life in patients with multiple sclerosis. A systematic review with a meta-analysis. Sensors 2021, 21, 7389. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Ascherio, A. Risk factors in the development of multiple sclerosis. Expert Rev. Clin. Immunol. 2007, 3, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Marks, S. The interplay between the immune and central nervous systems in neuronal injury. Neurology 2010, 74, S9–S16. [Google Scholar] [CrossRef]
- Dobson, R.; Ramagopalan, S.; Davis, A.; Giovannoni, G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: A meta-analysis of prevalence, prognosis and effect of latitude. J. Neurol. Neurosurg. Psychiatry 2013, 84, 909–914. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Cree, B.A.C.; Hauser, S.L. Ocrelizumab and Other CD20+ B-Cell-Depleting Therapies in Multiple Sclerosis. Neurotherapeutics 2017, 14, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Dennison, L.; Moss-morris, R.; Chalder, T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin. Psychol. Rev. 2009, 29, 141–153. [Google Scholar] [CrossRef]
- De Giglio, L.; Tommasin, S.; Petsas, N.; Pantano, P. The role of fMRI in the assessment of neuroplasticity in MS: A systematic review. Neural Plast. 2019, 2019, 5181649. [Google Scholar] [CrossRef]
- Milgram, P.; Takemura, H.; Utsumi, A.; Kishino, F. Augmented reality: A class of displays on the reality-virtuality continuum. Telemanipulator Telepresence Technol. 1995, 2351, 282–292. [Google Scholar] [CrossRef]
- Li, J.; Theng, Y.L.; Foo, S. Game-based digital interventions for depression therapy: A systematic review and meta-analysis. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 519–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabia, A.; Anger, W.H. Virtual reality for treatment compliance for people with serious mental illness. Issues Ment. Health Nurs. 2016, 37, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Triberti, S.; Repetto, C.; Riva, G. Psychological factors influencing the effectiveness of virtual reality-based analgesia: A systematic review. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.P.; Lomo, T. Long-Lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef] [PubMed]
- Huganir, R.L.; Nicoll, R.A. AMPARs and Synaptic Plasticity: The Last 25 Years. Neuron 2013, 80, 704–717. [Google Scholar] [CrossRef] [Green Version]
- Ksiazek-winiarek, D.J.; Szpakowski, P.; Glabinski, A. Neural Plasticity in Multiple Sclerosis: The Functional and Molecular Background. Neural Plast. 2015, 2015, 307175. [Google Scholar] [CrossRef] [Green Version]
- Lømo, T. Discovering long-term potentiation (LTP)—Recollections and reflections on what came after. Acta Physiol. 2018, 222, e12921. [Google Scholar] [CrossRef]
- Mori, F.; Rossi, S.; Piccinin, S.; Motta, C.; Mango, D.; Kusayanagi, H.; Bergami, A.; Studer, V.; Nicoletti, C.G.; Buttari, F.; et al. Synaptic Plasticity and PDGF Signaling Defects Underlie Clinical Progression in Multiple Sclerosis. J. Neurosci. 2013, 33, 19112–19119. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.; Mori, F.; Rossi, S.; Centonze, D. Disability in multiple sclerosis: When synaptic long-term potentiation fails. Neurosci. Biobehav. Rev. 2014, 43, 88–99. [Google Scholar] [CrossRef]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of Cell Survival by Secreted Proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef] [Green Version]
- Linker, R.; Gold, R.; Luhder, F. Function of neurotrophic factors beyond the nervous system: Inflammation and autoimmune demyelination. Crit. Rev. Immunol. 2009, 29, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Korte, M.; Carrolltt, P.; Wolf, E.; Brem, G.; Thoenent, H.; Bonhoeffer, T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 1995, 92, 8856–8860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokhtarzade, M.; Motl, R.; Negaresh, R.; Zimmer, P.; Khodadoost, M.; Baker, J.S.; Patel, D.; Majdinasab, N.; Ranjbar, R. Exercise-induced changes in neurotrophic factors and markers of blood-brain barrier permeability are moderated by weight status in multiple sclerosis. Neuropeptides 2018, 70, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wens, I.; Keytsman, C.; Deckx, N.; Cools, N.; Dalgas, U.; Eijnde, B. Brain derived neurotrophic factor in multiple sclerosis: Effect of 24 weeks endurance and resistance training. Eur. J. Neurol. 2016, 23, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, M.; Jason, L.; Peterson, J.; Herrington, J.; Mathews, H. Brain Derived Neurotrophic Factor is Decreased in Chronic Fatigue Syndrome and Multiple Sclerosis. J. Neurol. Neurophysiol. 2014, 12, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Castellano, V.; White, L.J. Serum brain-derived neurotrophic factor response to aerobic exercise in multiple sclerosis. J. Neurol. Sci. 2008, 269, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, D.; Vachapova, V.; Shihman, B.; Miler, A.; Karni, A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: Reversal by glatiramer acetate. J. Neuroimmunol. 2005, 167, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Greco, L.; Stipa, A.; Floridi, A.; Gallai, V. Brain-derived neurotrophic factor in patients with multiple sclerosis. J. Neuroimmunol. 2002, 132, 180–188. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood—Brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 7–9. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Contraction-Induced Myokine Production and Release: Is Skeletal Muscle an Endocrine Organ ? Exerc. Sport Sci. Rev. 2005, 33, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; Praag, H. Van Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Timper, K.; Denson, J.L.; Steculorum, S.M.; Rose-john, S.; Wunderlich, F.T.; Timper, K.; Denson, J.L.; Steculorum, S.M.; Heilinger, C.; Engstro, L. IL-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central IL-6 trans -Signaling Article IL-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central IL-6 trans -Signaling. Cell Rep. 2017, 19, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Tsigos, C.; Papanicolaou, D.; Defensor, R.; Mitsiadis, C.; Kyrou, I.; Chrousos, G. Dose Effects of Recombinant Human lnterleukin-6 on Pituitary Hormone Secretion and Energy Expenditure. Neuroendocrinology 1997, 66, 54–62. [Google Scholar] [CrossRef]
- Moon, H.Y.; Becke, A.; Berron, D.; Mattison, J.A.; Duzel, E.; Van Praag, H.; Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function Short Article Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padamsey, Z.; Mcguinness, L.; Bardo, S.J.; Hedegaard, A.; Hart, M.L.; Emptage, N.J.; Padamsey, Z.; Mcguinness, L.; Bardo, S.J.; Reinhart, M.; et al. Activity-Dependent Exocytosis of Lysosomes Regulates the Structural Plasticity of Dendritic Article Activity-Dependent Exocytosis of Lysosomes Regulates the Structural Plasticity of Dendritic Spines. Neuron 2016, 93, 132–146. [Google Scholar] [CrossRef] [Green Version]
- Olesen, J.; Kiilerich, K.; Pilegaard, H. PGC-1 α-mediated adaptations in skeletal muscle. Eur. J. Physiol. 2010, 460, 153–162. [Google Scholar] [CrossRef]
- Bostrom, P. A PGC1a dependent myokine that drives brownefatelike development of white fat and thermogenesis. Yearb. Endocrinol. 2012, 2012, 114–116. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Article Exercise Induces Hippocampal BDNF through a PGC-1 a/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasiñska, A.; Eusebio, M.; Kuna, P.; Gbiñski, A.; Pietruczuk, M. Evaluation of the relationship between leptin, resistin, adiponectin and natural regulatory T cells in relapsing-remitting multiple sclerosis. Neurol. Neurochir. Polska 2012, 46, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Correale, J.; Marrodan, M. Multiple sclerosis and obesity: The role of adipokines. Front. Immunol. 2022, 13, 1038393. [Google Scholar] [CrossRef] [PubMed]
- Polito, R.; Monda, V.; Nigro, E.; Messina, A.; Di Maio, G.; Giuliano, M.T.; Orrù, S.; Imperlini, E.; Calcagno, G.; Mosca, L.; et al. The Important Role of Adiponectin and Orexin-A, Two Key Proteins Improving Healthy Status: Focus on Physical Activity. Front. Physiol. 2020, 11, 356. [Google Scholar] [CrossRef] [Green Version]
- Signoriello, E.; Lus, G.; Polito, R.; Casertano, S.; Scudiero, O.; Coletta, M.; Rossi, F.; Nigro, E.; Daniele, A. Adiponectin profile at baseline is correlated to progression and severity of Multiple Sclerosis. Eur. J. Neurol. 2018, 26, 348–355. [Google Scholar] [CrossRef]
- Signoriello, E.; Mallardo, M.; Nigro, E.; Polito, R.; Casertano, S.; Di Pietro, A.; Coletta, M.; Rossi, F.; Lus, G.; Daniele, A.; et al. Adiponectin in Cerebrospinal Fluid from Patients Affected by Multiple Sclerosis Is Correlated with the Progression and Severity of Disease. Mol. Neurobiol. 2021, 58, 2663–2670. [Google Scholar] [CrossRef]
- Haider, L. Stress in the Pathogenesis of Multiple Sclerosis. Oxidative Med. Cell. Longev. 2015, 2015, 725370. [Google Scholar] [CrossRef]
- Adamczyk, B.; Adamczyk-Sowa, M. New Insights into the Role of Oxidative Stress Mechanisms in the Pathophysiology and Treatment of Multiple Sclerosis. Oxidative Med. Cell. Longev. 2016, 2016, 1973834. [Google Scholar] [CrossRef] [Green Version]
- Głąbiński, A.; Tawsek, N.; Bartosz, G. Increased generation of superoxide radicals in the bloGd of MS patienis. Acta Neurol. Scand 1993, 88, 174–177. [Google Scholar] [CrossRef]
- Tobore, T.O. Oxidative/Nitroxidative Stress and Multiple Sclerosis. J. Mol. Neurosci. 2020, 71, 506–514. [Google Scholar] [CrossRef]
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. BioMed Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chiang, W.C.; Yeh, Y.C.; Fan, S.C.; Yang, W.H.; Kuo, H.C.; Li, P.C. Effects of virtual reality-based motor control training on inflammation, oxidative stress, neuroplasticity and upper limb motor function in patients with chronic stroke: A randomized controlled trial. BMC Neurol. 2022, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Burggren, W. Epigenetic inheritance and its role in evolutionary biology: Re-evaluation and new perspectives. Biology 2016, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Conrad, R.J.; Verdin, E.; Ott, M. Lysine Acetylation Goes Global: From Epigenetics to Metabolism and Therapeutics. Chem. Rev. 2018, 118, 1216–1252. [Google Scholar] [CrossRef] [Green Version]
- Elsner, V.R.; Lovatel, G.A.; Bertoldi, K.; Vanzella, C.; Santos, F.M.; Spindler, C.; de Almeida, E.F.; Nardin, P.; Siqueira, I.R. Effect of different exercise protocols on histone acetyltransferases and histone deacetylases activities in rat hippocampus. Neuroscience 2011, 192, 580–587. [Google Scholar] [CrossRef]
- Collins, A.; Hill, L.E.; Chandramohan, Y.; Whitcomb, D.; Droste, S.K.; Reul, J.M.H.M. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS ONE 2009, 4, e4330. [Google Scholar] [CrossRef] [Green Version]
- Maejima, H.; Kanemura, N.; Kokubun, T.; Murata, K.; Takayanagi, K. Exercise enhances cognitive function and neurotrophin expression in the hippocampus accompanied by changes in epigenetic programming in senescence-accelerated mice. Neurosci. Lett. 2017, 665, 67–73. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Zhuang, Y.; Feng, J.; Ying, Z.; Fan, G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 2011, 33, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Winter, C.; Kern, F.; Gall, D.; Latoschik, M.E.; Pauli, P.; Käthner, I. Immersive virtual reality during gait rehabilitation increases walking speed and motivation: A usability evaluation with healthy participants and patients with multiple sclerosis and stroke. J. Neuroeng. Rehabil. 2021, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.M.; Otr, L.; Nilsen, D.M.; Otr, L.; Gillen, G.; Yoon, J.; Stein, J. Immersive Virtual Reality Mirror Therapy for Upper Limb Recovery After Stroke A Pilot Study. Am. J. Phys. Med. Rehabil. 2019, 98, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.; Poyade, M.; Rooney, S.; Paul, P.L. Upper limb rehabilitation interventions using virtual reality for people with multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2020, 47, 102610. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Fadiga, L.; Gallese, V.; Fogassi, L. Premotor cortex and the recognition of motor actions. Cogn. Brain Res. 1996, 3, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Naro, A.; Russo, M.; Leo, A.; De Luca, R.; Balletta, T.; Buda, A.; La Rosa, G.; Bramanti, A.; Bramanti, P. The role of virtual reality in improving motor performance as revealed by EEG: A randomized clinical trial. J. Neuroeng. Rehabil. 2017, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.G.; Maresca, G.; De Luca, R.; Stagnitti, M.C.; Porcari, B.; Ferrera, M.C.; Galletti, F.; Casella, C.; Manuli, A.; Calabrò, R.S. The Growing Use of Virtual Reality in Cognitive Rehabilitation: Fact, Fake or Vision? A Scoping Review. J. Natl. Med. Assoc. 2019, 111, 457–463. [Google Scholar] [CrossRef]
- Hickok, G. Eight Problems for the Mirror Neuron Theory of Action Understanding in Monkeys and Humans. J. Cogn. Neurosci. 2009, 21, 1229–1243. [Google Scholar] [CrossRef] [Green Version]
- Shobeiri, P.; Karimi, A.; Momtazmanesh, S.; Teixeira, A.L.; Teunissen, C.E.; van Wegen, E.E.H.; Hirsch, M.A.; Yekaninejad, M.S.; Rezaei, N. Exercise-induced increase in blood-based brain-derived neurotrophic factor (BDNF) in people with multiple sclerosis: A systematic review and meta-analysis of exercise intervention trials. PLoS ONE 2022, 17, e0264557. [Google Scholar] [CrossRef]
- Diechmann, M.D.; Campbell, E.; Coulter, E.; Paul, L.; Dalgas, U.; Hvid, L.G. Effects of Exercise Training on Neurotrophic Factors and Subsequent Neuroprotection in Persons with Multiple Sclerosis—A Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 1499. [Google Scholar] [CrossRef]
- Zivadinov, R.; Weinstock-Guttman, B.; Benedict, R.; Tamaño-Blanco, M.; Hussein, S.; Abdelrahman, N.; Durfee, J.; Ramanathan, M. Preservation of gray matter volume in multiple sclerosis patients with the Met allele of the rs6265 (Val66Met) SNP of brain-derived neurotrophic factor. Hum. Mol. Genet. 2007, 16, 2659–2668. [Google Scholar] [CrossRef] [Green Version]
- Dinacci, D.; Tessitore, A.; Russo, A.; De Bonis, M.L.; Lavorgna, L.; Picconi, O.; Sacco, R.; Bonavita, S.; Gallo, A.; Servillo, G.; et al. BDNF Val66Met polymorphism and brain volumes in multiple sclerosis. Neurol. Sci. 2011, 32, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Clarelli, F.; Cannizzaro, M.; Mascia, E.; Santoro, S.; Sorosina, M.; Ferrè, L.; Leocani, L.; Esposito, F. BDNF Val66Met Polymorphism Is Associated with Motor Recovery after Rehabilitation in Progressive Multiple Sclerosis Patients. Front. Neurol. 2022, 13, 790360. [Google Scholar] [CrossRef] [PubMed]
- Briken, S.; Rosenkranz, S.C.; Keminer, O.; Patra, S.; Ketels, G.; Heesen, C.; Hellweg, R.; Pless, O.; Schulz, K.H.; Gold, S.M. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J. Neuroimmunol. 2016, 299, 53–58. [Google Scholar] [CrossRef]
- Miller, L.; Paul, L.; Mattison, P.; McFadyen, A. Evaluation of a home-based physiotherapy programme for those with moderate to severe multiple sclerosis: A randomized controlled pilot study. Clin. Rehabil. 2011, 25, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Briken, S.; Gold, S.M.; Patra, S.; Vettorazzi, E.; Harbs, D.; Tallner, A.; Ketels, G.; Schulz, K.H.; Heesen, C. Effects of exercise on fitness and cognition in progressive MS: A randomized, controlled pilot trial. Mult. Scler. J. 2014, 20, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, E.; Coulter, E.H.; Mattison, P.G.; Miller, L.; McFadyen, A.; Paul, L. Physiotherapy rehabilitation for people with progressive multiple sclerosis: A systematic review. Arch. Phys. Med. Rehabil. 2016, 97, 141–151.e3. [Google Scholar] [CrossRef] [Green Version]
- Motl, R.W.; Mcauley, E.; Snook, E.M. Physical activity and multiple sclerosis: A meta-analysis. Mult. Scler. J. 2005, 11, 459–463. [Google Scholar] [CrossRef]
- Leonardi, S.; Maggio, M.G.; Russo, M.; Bramanti, A.; Arcadi, F.A.; Naro, A.; Calabrò, R.S.; De Luca, R. Cognitive recovery in people with relapsing/remitting multiple sclerosis: A randomized clinical trial on virtual reality-based neurorehabilitation. Clin. Neurol. Neurosurg. 2021, 208, 106828. [Google Scholar] [CrossRef]
- Cuesta-Gómez, A.; Sánchez-Herrera-Baeza, P.; Oña-Simbaña, E.D.; Martínez-Medina, A.; Ortiz-Comino, C.; Balaguer-Bernaldo-De-Quirós, C.; Jardón-Huete, A.; Cano-De-La-Cuerda, R. Effects of virtual reality associated with serious games for upper limb rehabilitation inpatients with multiple sclerosis: Randomized controlled trial. J. Neuroeng. Rehabil. 2020, 17, 90. [Google Scholar] [CrossRef]
- Waliño-Paniagua, C.N.; Gómez-Calero, C.; Jiménez-Trujillo, M.I.; Aguirre-Tejedor, L.; Bermejo-Franco, A.; Ortiz-Gutiérrez, R.M.; Cano-De-La-Cuerda, R. Effects of a Game-Based Virtual Reality Video Capture Training Program Plus Occupational Therapy on Manual Dexterity in Patients with Multiple Sclerosis: A Randomized Controlled Trial. J. Healthc. Eng. 2019, 2019, 9780587. [Google Scholar] [CrossRef] [Green Version]
- Ozdogar, A.T.; Ertekin, O.; Kahraman, T.; Yigit, P.; Ozakbas, S. Effect of video-based exergaming on arm and cognitive function in persons with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2020, 40, 101966. [Google Scholar] [CrossRef]
- Jonsdottir, J.; Perini, G.; Ascolese, A.; Bowman, T.; Montesano, A.; Lawo, M.; Bertoni, R. Unilateral arm rehabilitation for persons with multiple sclerosis using serious games in a virtual reality approach: Bilateral treatment effect? Mult. Scler. Relat. Disord. 2019, 35, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.S.; Fagundes, C.V.; dos Santos Mendes, F.A.; Leal, J.C. Effectiveness of Virtual Reality Rehabilitation in Persons with Multiple Sclerosis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Mult. Scler. Relat. Disord. 2021, 54, 103128. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhang, X.; Chen, P. Effects of Different Exercise Therapies on Balance Function and Functional Walking Ability in Multiple Sclerosis Disease Patients—A Network Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 7175. [Google Scholar] [CrossRef] [PubMed]
- Pruszyńska, M.; Milewska-Jędrzejczak, M.; Bednarski, I.; Szpakowski, P.; Głąbiński, A.; Tadeja, S.K. Towards Effective Telerehabilitation: Assessing Effects of Applying Augmented Reality in Remote Rehabilitation of Patients Suffering from Multiple Sclerosis. ACM Trans. Access. Comput. 2022, 15, 1–14. [Google Scholar] [CrossRef]
- Danzl, M.M.; Etter, N.M.; Andreatta, R.O.; Kitzman, P.H. Facilitating neurorehabilitation through principles of engagement. J. Allied Health 2012, 41, 35–41. [Google Scholar] [PubMed]
- Matamala-Gomez, M.; Maisto, M.; Montana, J.I.; Mavrodiev, P.A.; Baglio, F.; Rossetto, F.; Mantovani, F.; Riva, G.; Realdon, O. The role of engagement in teleneurorehabilitation: A systematic review. Front. Neurol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Backus, D.; Manella, C.; Bender, A.; Sweatman, M. Impact of massage therapy on fatigue, pain, and spasticity in people with multiple sclerosis: A pilot study. Int. J. Ther. Massage Bodyw. Res. Educ. Pract. 2016, 9, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Razazian, N.; Kazeminia, M.; Moayedi, H.; Daneshkhah, A.; Shohaimi, S. The impact of physical exercise on the fatigue symptoms in patients with multiple sclerosis: A systematic review and meta- analysis. BMC Neurol. 2020, 20, 93. [Google Scholar] [CrossRef]
- Armutlu, K.; Karabudak, R.; Nurlu, G. Physiotherapy Approaches in the Treatment of Ataxic Multiple Sclerosis: A Pilot Study. Neurorehabil. Neural Repair 2001, 15, 203–211. [Google Scholar] [CrossRef]
- Wiles, C.M.; Newcombe, R.G.; Fuller, K.J.; Shaw, S.; Furnival-Doran, J.; Pickersgill, T.P.; Morgan, A. Controlled randomised crossover trial of the effects of physiotherapy on mobility in chronic multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2001, 70, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Lord, S.E.; Wade, D.T.; Halligan, P.W. A comparison of two physiotherapy treatment approaches to improve walking in multiple sclerosis: A pilot randomized controlled study. Clin. Rehabil. 1998, 12, 477–486. [Google Scholar] [CrossRef]
- Salcı, Y.; Fil, A.; Armutlu, K.; Yildiz, F.G.; Kurne, A.; Aksoy, S.; Nurlu, G.; Karabudak, R. Effects of different exercise modalities on ataxia in multiple sclerosis patients: A randomized controlled study. Disabil. Rehabil. 2017, 39, 2626–2632. [Google Scholar] [CrossRef]
- Gibson-Horn, C. Balance-based torso-weighting in a patient with ataxia and multiple sclerosis: A case report. J. Neurol. Phys. Ther. 2008, 32, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Scheidler, A.M.; Kinnett-Hopkins, D.; Learmonth, Y.C.; Motl, R.; López-Ortiz, C. Targeted ballet program mitigates ataxia and improves balance in females with mild-Tomoderate multiple sclerosis. PLoS ONE 2018, 13, e0205382. [Google Scholar] [CrossRef]
- Landmeyer, N.C.; Bürkner, P.C.; Wiendl, H.; Ruck, T.; Hartung, H.P.; Holling, H.; Meuth, S.G.; Johnen, A. Disease-modifying treatments and cognition in relapsing-remitting multiple sclerosis: A meta-analysis. Neurology 2020, 94, E2373–E2383. [Google Scholar] [CrossRef]
- Gharakhanlou, R.; Wesselmann, L.; Rademacher, A.; Lampit, A.; Negaresh, R.; Kaviani, M.; Oberste, M.; Motl, R.W.; Sandroff, B.M.; Bansi, J.; et al. Exercise training and cognitive performance in persons with multiple sclerosis: A systematic review and multilevel meta-analysis of clinical trials. Mult. Scler. J. 2021, 27, 1977–1993. [Google Scholar] [CrossRef]
- Maggio, M.G.; Russo, M.; Foti, M.; Destro, M.; La, G.; Molonia, F.; Bramanti, P.; Lombardo, G.; De Luca, R.; Salvatore, R. Virtual reality in multiple sclerosis rehabilitation: A review on cognitive and motor outcomes. J. Clin. Neurosci. 2019, 65, 106–111. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milewska-Jędrzejczak, M.; Głąbiński, A. The Influence of Conventional and Innovative Rehabilitation Methods on Brain Plasticity Induction in Patients with Multiple Sclerosis. J. Clin. Med. 2023, 12, 1880. https://doi.org/10.3390/jcm12051880
Milewska-Jędrzejczak M, Głąbiński A. The Influence of Conventional and Innovative Rehabilitation Methods on Brain Plasticity Induction in Patients with Multiple Sclerosis. Journal of Clinical Medicine. 2023; 12(5):1880. https://doi.org/10.3390/jcm12051880
Chicago/Turabian StyleMilewska-Jędrzejczak, Marta, and Andrzej Głąbiński. 2023. "The Influence of Conventional and Innovative Rehabilitation Methods on Brain Plasticity Induction in Patients with Multiple Sclerosis" Journal of Clinical Medicine 12, no. 5: 1880. https://doi.org/10.3390/jcm12051880





