New Staging System and Prognostic Model for Malignant Phyllodes Tumor Patients without Distant Metastasis: A Development and Validation Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
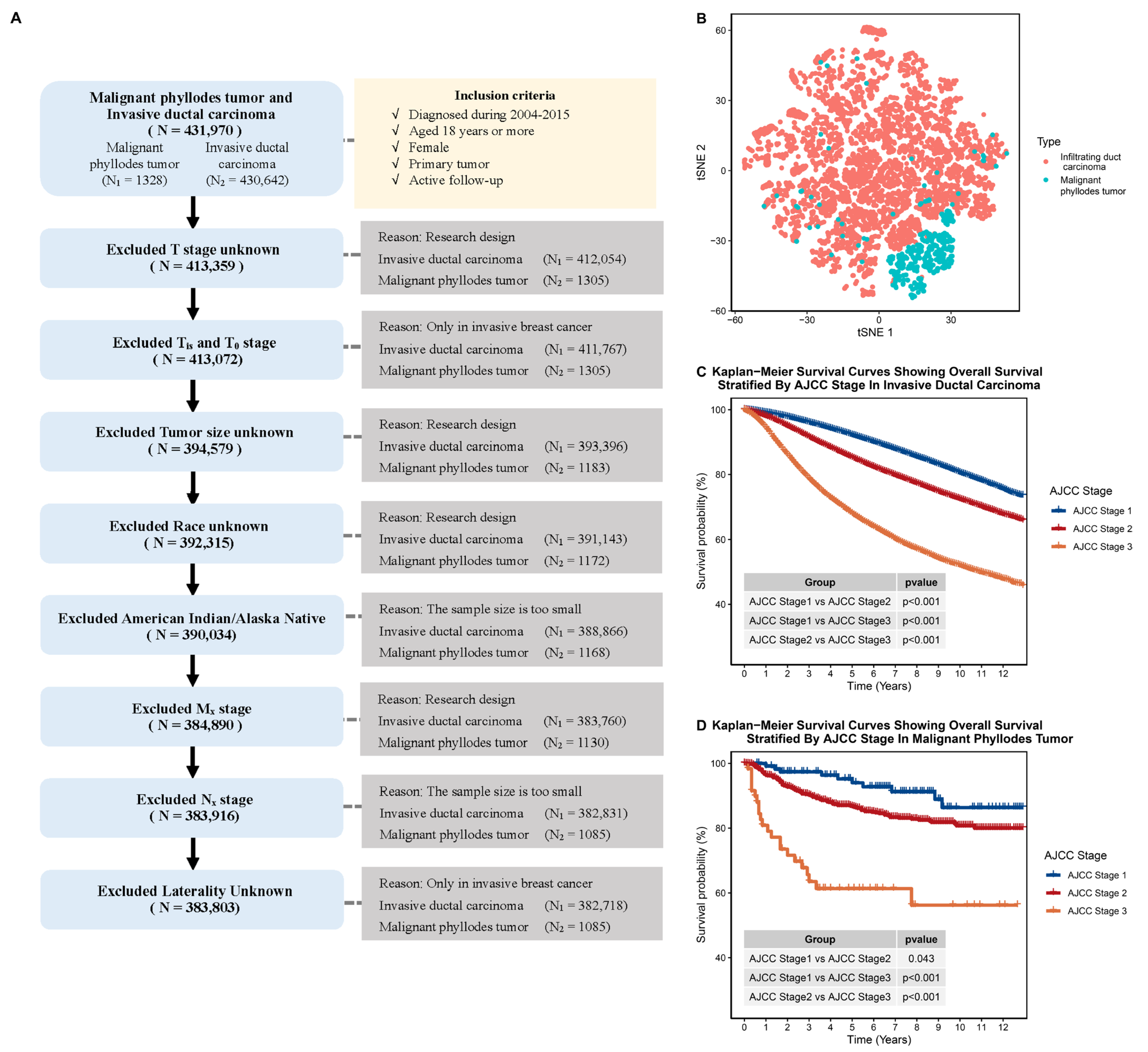
2.1. Data Collection
2.2. Comparing the Clinical Characteristics of IDC and MPTB
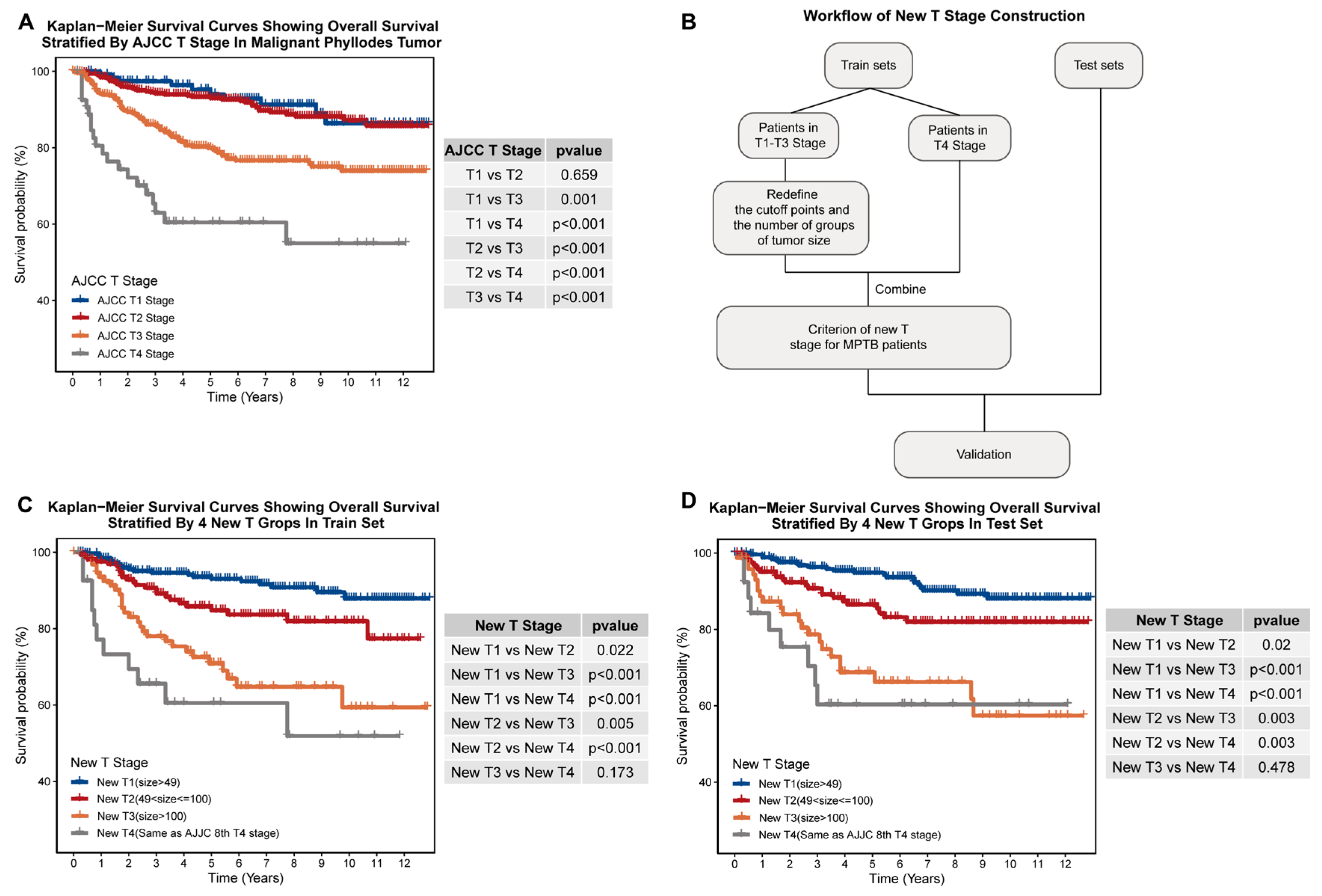
2.3. Construction of New T-Stage and Age Groupings
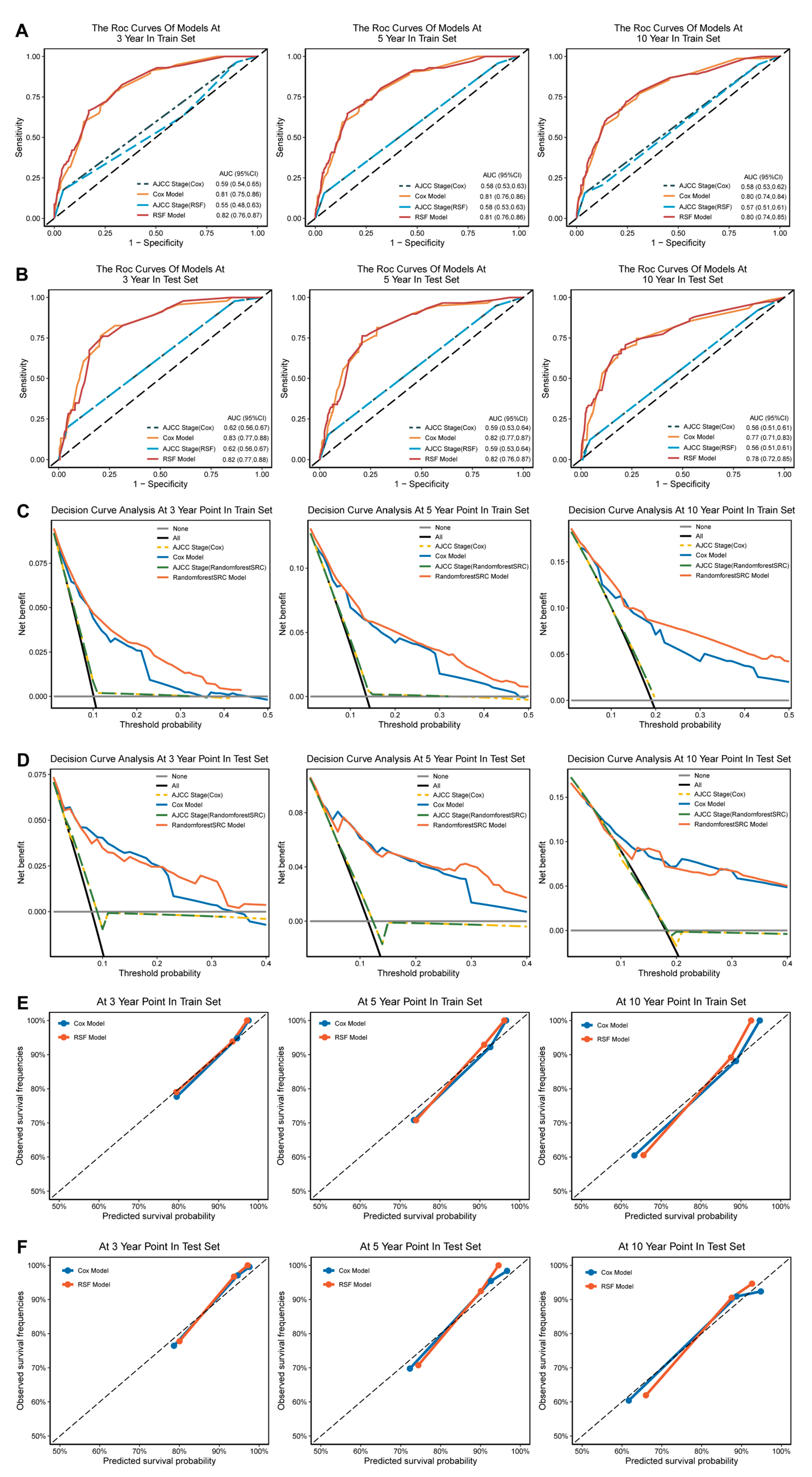
2.4. Construction of Prognostic Models
2.5. Validation of Prognostic Models
2.6. Statistical Analysis
3. Results
3.1. Malignant Phyllodes Tumor of the Breast Has Vastly Different Clinical Characteristics to Invasive Ductal Carcinoma
3.2. Our New T-Staging System Exhibits Better Prognosis-Distinguishing Ability Than the AJCC T-Staging System in Both Internal and External Cohorts
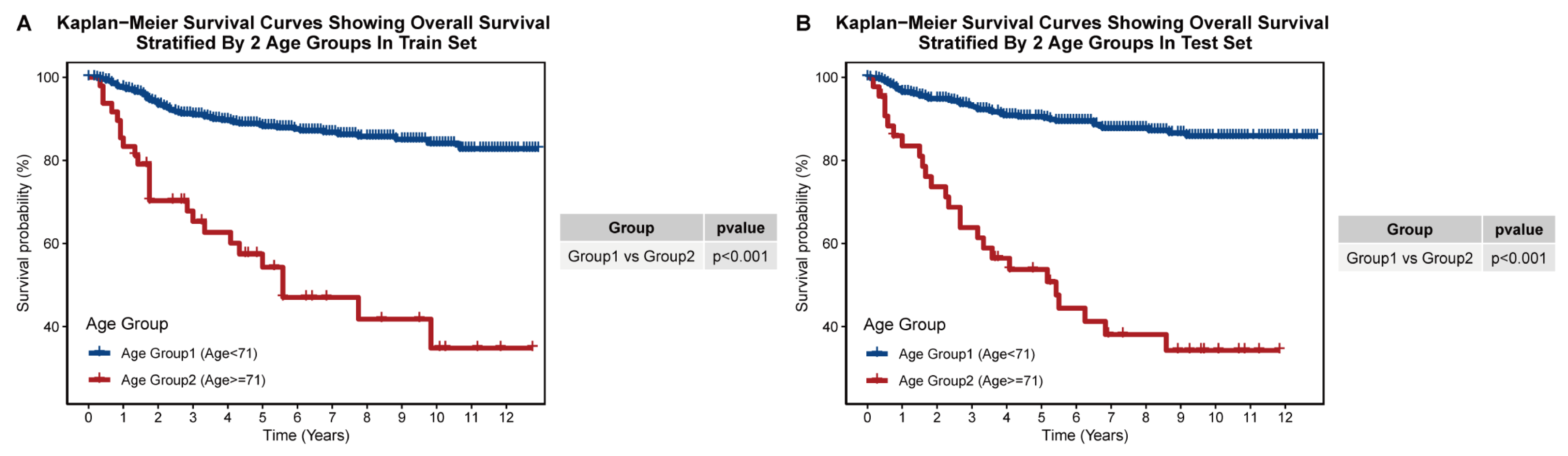
3.3. Construction of Prognostic Age-Stratification System
3.4. Construction of MPTB Survival Prognosis Models
3.5. Calibration and Validation
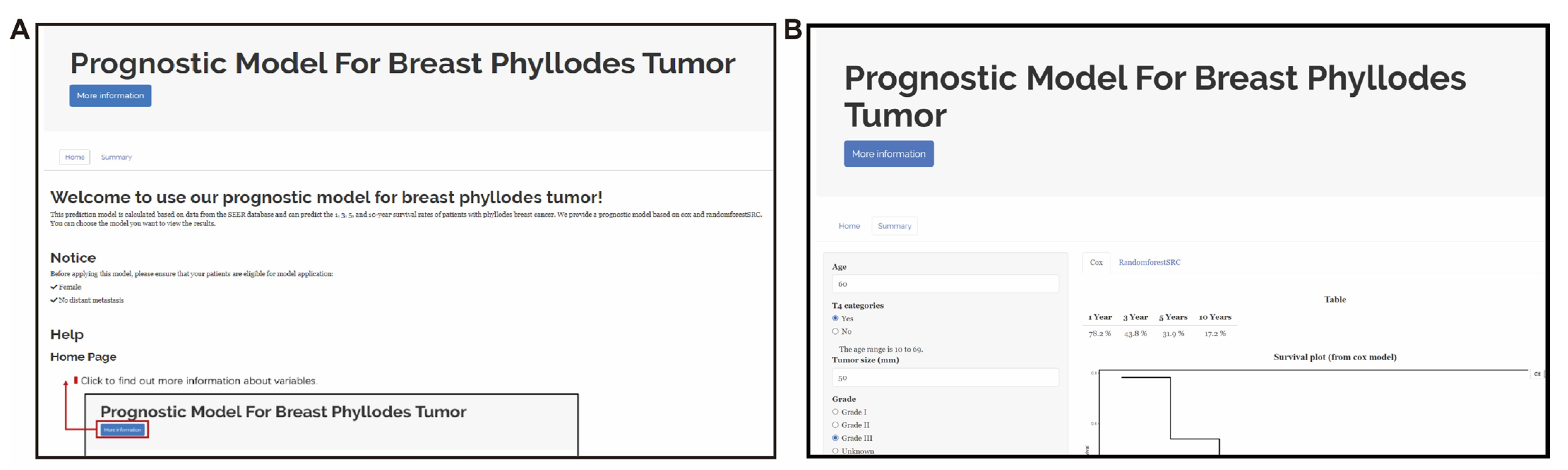
3.6. Web-Based App Enables Researchers and Clinicians to Easily Share and Use Our New MPTB Staging System and Prognostic Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macdonald, O.K.; Lee, C.M.; Tward, J.D.; Chappel, C.D.; Gaffney, D.K. Malignant phyllodes tumor of the female breast: Association of primary therapy with cause-specific survival from the Surveillance, Epidemiology, and End Results (SEER) program. Cancer 2006, 107, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kleer, C.G. Phyllodes Tumor of the Breast: Histopathologic Features, Differential Diagnosis, and Molecular/Genetic Updates. Arch. Pathol. Lab. Med. 2016, 140, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Ramdass, M.J.; Dindyal, S. Phyllodes breast tumour showing invasive squamous-cell carcinoma with invasive ductal, clear-cell, secretory, and squamous components. Lancet Oncol. 2006, 7, 880. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Eo, S.-H.; Hong, S.-M.; Cho, H. K-adaptive partitioning for survival data: The Kaps add-on package for R. arXiv 2013, arXiv:1306.4615v3. [Google Scholar]
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M.S. Random survival forests. Ann. Appl. Stat. 2008, 2, 841–860. [Google Scholar] [CrossRef]
- Taylor, J.M. Random Survival Forests. J. Thorac. Oncol. 2011, 6, 1974–1975. [Google Scholar] [CrossRef]
- Cetin, S.; Ulgen, A.; Dede, I.; Li, W. On Fair Performance Comparison between Random Survival Forest and Cox Regression: An Example of Colorectal Cancer Study. Sci. Med. J. 2021, 3, 66–76. [Google Scholar] [CrossRef]
- Kurt Omurlu, I.; Ture, M.; Tokatli, F. The comparisons of random survival forests and Cox regression analysis with simulation and an application related to breast cancer. Expert Syst. Appl. 2009, 36, 8582–8588. [Google Scholar] [CrossRef]
- Gower, J.C.J.B. A general coefficient of similarity and some of its properties. Biometrics 1971, 857–871. [Google Scholar] [CrossRef]
- Van der Maaten, L.; Hinton, G. Visualizing data using t-SNE. JMLR 2008, 9, 2579–2605. [Google Scholar]
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Saville, B.R.; Lewis, R.J. Decision curve analysis. JAMA 2015, 313, 409–410. [Google Scholar] [CrossRef]
- Austin, P.C.; Harrell, F.E., Jr.; van Klaveren, D. Graphical calibration curves and the integrated calibration index (ICI) for survival models. Stat. Med. 2020, 39, 2714–2742. [Google Scholar] [CrossRef] [PubMed]
- Kleinbaum, D.G.; Klein, M. Kaplan-Meier survival curves and the log-rank test. In Survival Analysis; Springer: Berlin/Heidelberg, Germany, 2012; pp. 55–96. [Google Scholar]
- Ferreira, J.A.; Zwinderman, A.H. On the Benjamini–Hochberg method. Ann. Statist. 2006, 34, 1823, 1827–1849. [Google Scholar] [CrossRef]
- Du, M.; Haag, D.G.; Lynch, J.W.; Mittinty, M.N. Comparison of the Tree-Based Machine Learning Algorithms to Cox Regression in Predicting the Survival of Oral and Pharyngeal Cancers: Analyses Based on SEER Database. Cancers 2020, 12, 2802. [Google Scholar] [CrossRef]
- Moncada-Torres, A.; van Maaren, M.C.; Hendriks, M.P.; Siesling, S.; Geleijnse, G. Explainable machine learning can outperform Cox regression predictions and provide insights in breast cancer survival. Sci. Rep. 2021, 11, 6968. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 484–493. [Google Scholar] [CrossRef]
- Koukourakis, I.M.; Zygogianni, A.; Kouloulias, V.; Koukourakis, M.I. Successful Treatment of a Locally Recurrent and Metastatic Malignant Phyllodes Tumor with Accelerated Radiotherapy and Nab-Paclitaxel, Cisplatin, and Liposomal Doxorubicin Chemotherapy. Chemotherapy 2021, 66, 82–86. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yamagishi, S.; Kohno, T.; Tajiri, R.; Gondo, T.; Yoshimoto, N.; Kusano, N. Effective Treatment of a Malignant Breast Phyllodes Tumor with Doxorubicin-Ifosfamide Therapy. Case Rep. Oncol. Med. 2019, 2019, 2759650. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Chen, C.J.; Kuo, S.J. Effect of Lipodox in combination with bevacizumab in a patient with a metastatic malignant phyllodes breast tumor: A case report. Oncol. Lett. 2017, 14, 6685–6689. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, L.; Hu, W.; Yan, J.; Qian, X.; Zhu, L. Apatinib treatment is effective for metastatic malignant phyllodes tumors of the breast: A case report. BMC Womens Health 2021, 21, 218. [Google Scholar] [CrossRef] [PubMed]
- Parkes, A.; Wang, W.L.; Patel, S.; Leung, C.H.; Lin, H.; Conley, A.P.; Somaiah, N.; Araujo, D.M.; Zarzour, M.; Livingston, J.A.; et al. Outcomes of systemic therapy in metastatic phyllodes tumor of the breast. Breast Cancer Res. Treat. 2021, 186, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Huang, H.; Guo, M.; Chen, J.; Wu, W.; Li, W.; Xu, X.; Lin, X.; Fu, W.; Yao, Y.; et al. Breast Phyllodes Tumors Recruit and Repolarize Tumor-Associated Macrophages via Secreting CCL5 to Promote Malignant Progression, Which Can Be Inhibited by CCR5 Inhibition Therapy. Clin. Cancer Res. 2019, 25, 3873–3886. [Google Scholar] [CrossRef]
| Characteristics | Overall | Invasive Ductal Carcinoma | Phyllodes Tumor | p Value |
|---|---|---|---|---|
| (n = 383,803) | (n = 382,718) | (n = 1085) | ||
| Age at diagnosis (years) (median [Q1, Q3]) | 59.00 [50.00, 69.00] | 59.00 [50.00, 69.00] | 51.00 [43.00, 60.00] | <0.001 |
| Race (%) | ||||
| White | 307,371 (80.1) | 306,565 (80.1) | 806 (74.3) | <0.001 |
| Black | 42,359 (11.0) | 42,246 (11.0) | 113 (10.4) | |
| Asian or Pacific Islander | 34,073 (8.9) | 33,907 (8.9) | 166 (15.3) | |
| Marital status (%) | ||||
| Never married/Unknown | 70,605 (18.4) | 70,273 (18.4) | 332 (30.6) | <0.001 |
| Ever married | 313,198 (81.6) | 312,445 (81.6) | 753 (69.4) | |
| Tumor size (median [Q1, Q3]) | 16.00 [10.00, 26.00] | 16.00 [10.00, 26.00] | 50.00 [30.00, 90.00] | <0.001 |
| T stage (%) | ||||
| T1 | 238,792 (62.2) | 238,673 (62.4) | 119 (11.0) | <0.001 |
| T2 | 116,232 (30.3) | 115,782 (30.3) | 450 (41.5) | |
| T3 | 17,907 (4.7) | 17,445 (4.6) | 462 (42.6) | |
| T4 | 10,872 (2.8) | 10,818 (2.8) | 54 (5.0) | |
| Lymph node metastasis (%) | ||||
| None/Unknown | 260,222 (67.8) | 259,149 (67.7) | 1073 (98.9) | <0.001 |
| Yes | 123,581 (32.2) | 123,569 (32.3) | 12 (1.1) | |
| 8th TNM stage (%) | ||||
| I | 192,360 (50.1) | 192,241 (50.2) | 119 (11.0) | <0.001 |
| II | 144,838 (37.7) | 143,932 (37.6) | 906 (83.5) | |
| III | 46,605 (12.1) | 46,545 (12.2) | 60 (5.5) | |
| Laterality (%) | ||||
| Left—origin of primary | 194,348 (50.6) | 193,835 (50.6) | 513 (47.3) | 0.029 |
| Right—origin of primary | 189,455 (49.4) | 188,883 (49.4) | 572 (52.7) | |
| Site of primary tumor (%) | ||||
| Central | 19,380 (5.0) | 19,304 (5.0) | 76 (7.0) | <0.001 |
| UIQ | 47,464 (12.4) | 47,373 (12.4) | 91 (8.4) | |
| LIQ | 22,463 (5.9) | 22,435 (5.9) | 28 (2.6) | |
| UOQ | 136,855 (35.7) | 136,542 (35.7) | 313 (28.8) | |
| LOQ | 28,510 (7.4) | 28,440 (7.4) | 70 (6.5) | |
| Overlap | 129,131 (33.6) | 128,624 (33.6) | 507 (46.7) | |
| Grade (%) | ||||
| Grade I | 75,476 (19.7) | 75,328 (19.7) | 148 (13.6) | <0.001 |
| Grade II | 154,420 (40.2) | 154,293 (40.3) | 127 (11.7) | |
| Grade III | 143,826 (37.5) | 143,557 (37.5) | 269 (24.8) | |
| Unknown | 10,081 (2.6) | 9540 (2.5) | 541 (49.9) | |
| ER (%) | ||||
| Negative | 78,786 (20.5) | 78,708 (20.6) | 78 (7.2) | <0.001 |
| Positive | 293,015 (76.3) | 292,985 (76.6) | 30 (2.8) | |
| Unknown | 12,002 (3.1) | 11,025 (2.9) | 977 (90.0) | |
| PR (%) | ||||
| Negative | 116,220 (30.3) | 116,140 (30.3) | 80 (7.4) | <0.001 |
| Positive | 252,929 (65.9) | 252,903 (66.1) | 26 (2.4) | |
| Unknown | 14,654 (3.8) | 13,675 (3.6) | 979 (90.2) | |
| Radiotherapy (%) | ||||
| None/Unknown | 180,484 (47.0) | 179,636 (46.9) | 848 (78.2) | <0.001 |
| Yes | 203,319 (53.0) | 203,082 (53.1) | 237 (21.8) | |
| Chemotherapy (%) | ||||
| None/Unknown | 213,584 (55.6) | 212,531 (55.5) | 1053 (97.1) | <0.001 |
| Yes | 170,219 (44.4) | 170,187 (44.5) | 32 (2.9) | |
| Surgery (%) | ||||
| None/Unknown | 12,346 (3.2) | 12,336 (3.2) | 10 (0.9) | <0.001 |
| Yes | 371,457 (96.8) | 370,382 (96.8) | 1075 (99.1) |
| Characteristics | Overall | Train | Test | p Value |
|---|---|---|---|---|
| (n = 1085) | (n = 553) | (n = 532) | ||
| Age at diagnosis (years) (median [Q1, Q3]) | 51.00 [43.00, 60.00] | 51.00 [43.00, 61.00] | 52.00 [43.00, 59.00] | 0.768 |
| Race (%) | ||||
| White | 806 (74.3) | 419 (75.8) | 387 (72.7) | 0.478 |
| Black | 113 (10.4) | 56 (10.1) | 57 (10.7) | |
| Asian or Pacific Islander | 166 (15.3) | 78 (14.1) | 88 (16.5) | |
| Marital status (%) | ||||
| Never married/Unknown | 332 (30.6) | 184 (33.3) | 148 (27.8) | 0.06 |
| Ever married | 753 (69.4) | 369 (66.7) | 384 (72.2) | |
| Tumor size (median [Q1, Q3]) | 50.00 [30.00, 90.00] | 50.00 [31.00, 94.00] | 50.00 [30.00, 85.00] | 0.225 |
| T stage (%) | ||||
| T1 | 119 (11.0) | 53 (9.6) | 66 (12.4) | 0.501 |
| T2 | 450 (41.5) | 230 (41.6) | 220 (41.4) | |
| T3 | 462 (42.6) | 242 (43.8) | 220 (41.4) | |
| T4 | 54 (5.0) | 28 (5.1) | 26 (4.9) | |
| Lymph node metastasis (%) | ||||
| None/Unknown | 1073 (98.9) | 546 (98.7) | 527 (99.1) | 0.824 |
| Yes | 12 (1.1) | 7 (1.3) | 5 (0.9) | |
| 8th TNM stage (%) | ||||
| I | 119 (11.0) | 53 (9.6) | 66 (12.4) | 0.331 |
| II | 906 (83.5) | 469 (84.8) | 437 (82.1) | |
| III | 60 (5.5) | 31 (5.6) | 29 (5.5) | |
| Laterality (%) | ||||
| Left—origin of primary | 513 (47.3) | 252 (45.6) | 261 (49.1) | 0.276 |
| Right—origin of primary | 572 (52.7) | 301 (54.4) | 271 (50.9) | |
| Site of primary tumor (%) | ||||
| Central | 76 (7.0) | 41 (7.4) | 35 (6.6) | 0.45 |
| UIQ | 91 (8.4) | 40 (7.2) | 51 (9.6) | |
| LIQ | 28 (2.6) | 15 (2.7) | 13 (2.4) | |
| UOQ | 313 (28.8) | 152 (27.5) | 161 (30.3) | |
| LOQ | 70 (6.5) | 33 (6.0) | 37 (7.0) | |
| Overlap | 507 (46.7) | 272 (49.2) | 235 (44.2) | |
| Grade (%) | ||||
| Grade I | 148 (13.6) | 72 (13.0) | 76 (14.3) | 0.245 |
| Grade II | 127 (11.7) | 70 (12.7) | 57 (10.7) | |
| Grade III | 269 (24.8) | 148 (26.8) | 121 (22.7) | |
| Unknown | 541 (49.9) | 263 (47.6) | 278 (52.3) | |
| ER (%) | ||||
| Negative | 78 (7.2) | 41 (7.4) | 37 (7.0) | 0.86 |
| Positive | 30 (2.8) | 14 (2.5) | 16 (3.0) | |
| Unknown | 977 (90.0) | 498 (90.1) | 479 (90.0) | |
| PR (%) | ||||
| Negative | 80 (7.4) | 42 (7.6) | 38 (7.1) | 0.854 |
| Positive | 26 (2.4) | 12 (2.2) | 14 (2.6) | |
| Unknown | 979 (90.2) | 499 (90.2) | 480 (90.2) | |
| Chemotherapy (%) | ||||
| None/Unknown | 1053 (97.1) | 533 (96.4) | 520 (97.7) | 0.252 |
| Yes | 32 (2.9) | 20 (3.6) | 12 (2.3) | |
| Surgery (%) | ||||
| None/Unknown | 10 (0.9) | 4 (0.7) | 6 (1.1) | 0.704 |
| Yes | 1075 (99.1) | 549 (99.3) | 526 (98.9) | |
| Radiotherapy (%) | ||||
| None/Unknown | 848 (78.2) | 433 (78.3) | 415 (78.0) | 0.137 |
| Radiation after surgery | 233 (21.5) | 116 (21.0) | 117 (22.0) | |
| Radiation prior to surgery | 4 (0.4) | 4 (0.7) | 0 (0.0) |
| Classification | Stage | Definition |
|---|---|---|
| New T stage | New T stage 1 | Tumor < 49 mm in greatest dimension. |
| New T stage 2 | Tumor ≥ 49 mm but < 100 mm in greatest dimension. | |
| New T stage 3 | Tumor size ≥ 100 mm in greatest dimension. | |
| New T stage 4 | Tumor of any size with direct extension to chest wall or skin. | |
| Age Group | Age Group 1 | Age < 71. |
| Age Group 2 | Age ≥ 71. |
| Characteristics | Univariate | Multivariable | |||
|---|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | ||
| Race | White | ||||
| Black | 1.500 (0.824, 2.730) | 0.185 | - | - | |
| Asian or Pacific Islander | 0.908 (0.465, 1.772) | 0.776 | - | - | |
| Marital status | Never married/Unknown | ||||
| Ever married | 1.163 (0.724, 1.867) | 0.533 | - | - | |
| Lymph node metastasis | None/Unknown | ||||
| Yes | 4.367 (1.375, 13.871) | 0.012 | 1.871 (0.564, 6.210) | 0.306 | |
| Laterality | Left—origin of primary | ||||
| Right—origin of primary | 1.054 (0.685, 1.621) | 0.813 | - | - | |
| Radiotherapy | None/Unknown | ||||
| Yes | 1.223 (0.746, 2.006) | 0.425 | - | - | |
| Chemotherapy | None/Unknown | ||||
| Yes | 2.840 (1.309, 6.159) | 0.008 | 2.407 (1.081, 5.363) | 0.032 | |
| Surgery | None/Unknown | ||||
| Yes | 0.109 (0.027, 0.446) | 0.002 | 0.077 (0.018, 0.336) | 0.001 | |
| Grade | Grade I | ||||
| Grade II | 0.704 (0.199, 2.495) | 0.586 | 0.913 (0.253, 3.303) | 0.890 | |
| Grade III | 3.130 (1.311, 7.472) | 0.010 | 2.599 (1.063, 6.354) | 0.036 | |
| Unknown | 2.277 (0.966, 5.367) | 0.060 | 1.795 (0.753, 4.282) | 0.187 | |
| Site of primary tumor | Central | ||||
| UIQ | 0.738 (0.241, 2.257) | 0.594 | - | - | |
| LIQ | 2.046 (0.669, 6.257) | 0.209 | - | - | |
| UOQ | 0.621 (0.266, 1.451) | 0.271 | - | - | |
| LOQ | 0.671 (0.202, 2.229) | 0.515 | - | - | |
| Overlap | 0.982 (0.464, 2.082) | 0.963 | - | - | |
| New T Stage | New T Stage 1 | ||||
| New T Stage 2 | 1.992 (1.109, 3.579) | 0.021 | 1.844 (1.016, 3.347) | 0.044 | |
| New T Stage 3 | 4.451 (2.524, 7.849) | 0.000 | 4.102 (2.276, 7.393) | 0.000 | |
| New T Stage 4 | 7.187 (3.460, 14.929) | 0.000 | 5.539 (2.595, 11.823) | 0.000 | |
| Age Group | Age Group 1 | ||||
| Age Group 2 | 5.188 (3.227, 8.341) | 0.000 | 5.173 (3.122, 8.571) | 0.000 | |
| ER | Negative | ||||
| Positive | 0.276 (0.036, 2.142) | 0.218 | - | - | |
| Unknown | 0.535 (0.283, 1.009) | 0.053 | - | - | |
| PR | Negative | ||||
| Positive | 0.305 (0.039, 2.367) | 0.256 | - | - | |
| Unknown | 0.540 (0.286, 1.018) | 0.057 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Z.; Quan, Q.; Wang, Q.; Jiang, J.; Peng, R. New Staging System and Prognostic Model for Malignant Phyllodes Tumor Patients without Distant Metastasis: A Development and Validation Study. J. Clin. Med. 2023, 12, 1889. https://doi.org/10.3390/jcm12051889
Ruan Z, Quan Q, Wang Q, Jiang J, Peng R. New Staging System and Prognostic Model for Malignant Phyllodes Tumor Patients without Distant Metastasis: A Development and Validation Study. Journal of Clinical Medicine. 2023; 12(5):1889. https://doi.org/10.3390/jcm12051889
Chicago/Turabian StyleRuan, Zhaohui, Qi Quan, Qianyu Wang, Jiaxin Jiang, and Roujun Peng. 2023. "New Staging System and Prognostic Model for Malignant Phyllodes Tumor Patients without Distant Metastasis: A Development and Validation Study" Journal of Clinical Medicine 12, no. 5: 1889. https://doi.org/10.3390/jcm12051889
APA StyleRuan, Z., Quan, Q., Wang, Q., Jiang, J., & Peng, R. (2023). New Staging System and Prognostic Model for Malignant Phyllodes Tumor Patients without Distant Metastasis: A Development and Validation Study. Journal of Clinical Medicine, 12(5), 1889. https://doi.org/10.3390/jcm12051889






