Consequences of Obesity on Short-Term Outcomes in Patients Who Underwent Off-Pump Coronary Artery Bypass Grafting Surgery
Abstract
:1. Introduction
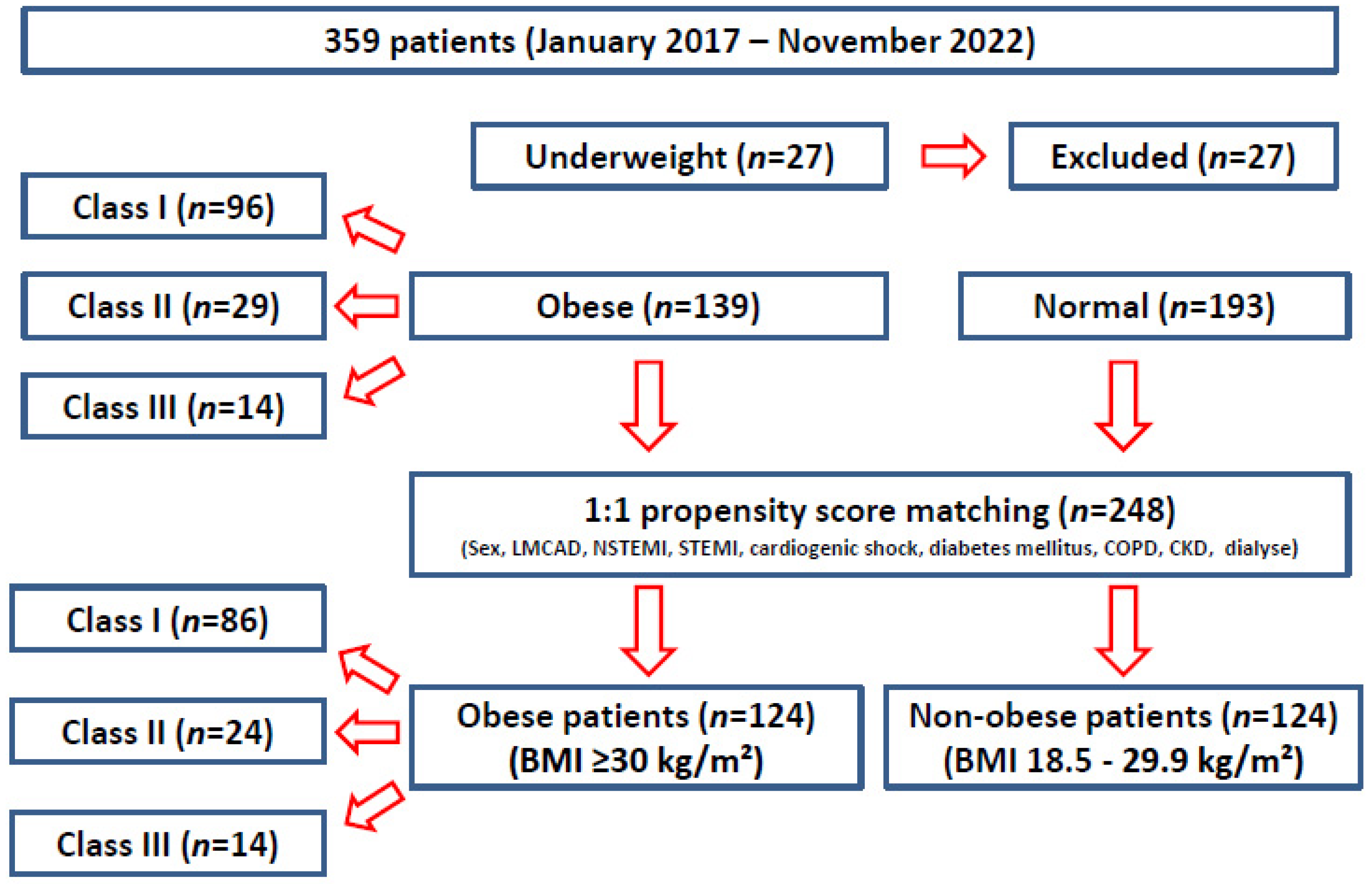
2. Materials and Methods
2.1. Definition of Obesity
- Underweight: BMI <18.5 kg/m2
- Normal weight: BMI 18.5–24.9 kg/m2
- Overweight: BMI 25.0–29.9 kg/m2
- Obese class I: BMI 30.0–34.9 kg/m2
- Obese class II: BMI 35.0–39.9 kg/m2
- Obese class III: BMI >40.0 kg/m2
2.2. Surgical Procedure
2.3. Data Collection
- patients’ baseline characteristics (age, gender, Euroscore II, ejection fraction, left main coronary artery disease, history of Non-ST-elevation myocardial infarction (NSTEMI), history of ST-elevation myocardial infarction (STEMI), cardiogenic shock, previous stenting, previous stroke, reoperation rate, diabetes mellitus, hyperlipidaemia, peripheral vascular disease, arterial hypertension, pulmonary hypertension, chronic obstructive pulmonary disease, chronic kidney disease and dialysis);
- intraoperative characteristics (use of both internal thoracic arteries, total arterial revascularization, T-graft technique, endoscopic saphenous vein harvesting, heartstring use, catecholamine use, temporary pacer use, duration of bypass surgery, extracorporeal membrane oxygenation (ECMO) use, and intra-aortic balloon pump (IABP) use);
- postoperative data (transient ischemic attack (TIA), stroke, delirium, low cardiac output syndrome (LCOS), CK, CK-MB, lactate, creatinine, acute kidney injury, dialysis, wound infection, plastic covering, permanent pacemaker implantation (PPI), bleeding with reoperation, intensive care unit (ICU) stay, hospital stay, and in-hospital mortality);
- primary and secondary endpoints due to obesity classes I, II, and III;
- combined risk factors of in-hospital mortality (age, body mass index, diabetes mellitus, STEMI, NSTEMI, and reoperation rate).
2.4. Outcome Analysis
2.5. Ethics
2.6. Statistical Methods
3. Results
3.1. Preoperative Data
3.2. Intraoperative Characteristics
3.3. Postoperative Data
3.4. Postoperative Data in Obese Patients
3.5. Combined Risk Factors of In-Hospital Mortality
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caliskan, E.; Güsewell, S.; Seifert, B.; Theusinger, O.M.; Starck, C.T.; Pavicevic, J.; Reser, D.; Holubec, T.; Plass, A.; Falk, V.; et al. Does body mass index impact the early outcome of surgical revascularization? A comparison between off-pump and on-pump coronary artery bypass grafting. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 749–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghöfer, A.; Pischon, T.; Reinhold, T.; Apovian, C.M.; Sharma, A.M.; Willich, S.N. Obesity prevalence from a European perspective: A systematic review. BMC Public Health 2008, 8, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flegal, K.M.; Carroll, D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012, 307, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, P.; Eckel, R.H. Obesity and cardiovascular disease. Curr. Atheroscler. Rep. 2002, 4, 448–453. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Van Itallie, T.B. Body weight, health, and longevity. Ann. Intern. Med. 1984, 100, 285–295. [Google Scholar] [CrossRef]
- Sabzi, F.; Faraji, R. Effect of Body Mass Index on Postoperative Complications in Beating Coronary Artery Surgery. Ethiop. J. Health Sci. 2016, 26, 509–516. [Google Scholar] [CrossRef]
- Engelman, D.T.; Adams, D.H.; Byrne, J.G.; Aranki, S.F.; Collins, J.J., Jr.; Couper, G.S.; Allred, E.N.; Cohn, L.H.; Rizzo, R.J. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J. Thorac. Cardiovasc. Surg. 1999, 118, 866–873. [Google Scholar] [CrossRef] [Green Version]
- Reeves, B.C.; Ascione, R.; Chamberlain, M.H.; Angelini, G.D. Effect of body mass index on early outcomes in patients undergoing coronary artery bypass surgery. J. Am. Coll. Cardiol. 2003, 42, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Bhamidipati, C.M.; Seymour, K.A.; Cohen, N.; Rolland, R.; Dilip, K.A.; Lutz, C.J. Is body mass index a risk factor for isolated off-pump coronary revascularization? J. Card. Surg. 2011, 26, 565–571. [Google Scholar] [CrossRef]
- Keeling, W.B.; Kilgo, P.D.; Puskas, J.D.; Halkos, M.E.; Lattouf, O.M.; Guyton, R.A.; Thourani, V.H. Off-pump coronary artery bypass grafting attenuates morbidity and mortality for patients with low and high body mass index. J. Thorac. Cardiovasc. Surg. 2013, 146, 1442–1448. [Google Scholar] [CrossRef] [Green Version]
- Barandon, L.; Richebé, P.; Munos, E.; Calderon, J.; Lafitte, M.; Lafitte, S.; Couffinhal, T.; Roques, X. Off-pump coronary artery bypass surgery in very high-risk patients: Adjustment and preliminary results. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.M.; Poston, R.S.; Gammie, J.S.; Cardarelli, M.G.; Schwartz, K.; Sikora, J.A.H.; Yi, S.; Pierson, R.N.; Griffith, B.P. Off-pump versus on-pump coronary artery bypass grafting in consecutive patients: Decision-making algorithm and outcomes. Ann. Thorac. Surg. 2006, 81, 555–561. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Cartier, R.; Demers, P.; Bouchard, D.; Pellerin, M. Long-term results after systematic off-pump coronary artery bypass graft surgery in 1000 consecutive patients. Circulation 2006, 114, I-486–I-491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathoe, H.M.; van Dijk, D.; Jansen, E.W.; Suyker, W.J.; Diephuis, J.C.; van Boven, W.-J.; de la Rivière, A.B.; Borst, C.; Kalkman, C.J.; Grobbee, D.E.; et al. A comparison of on-pump and off-pump coronary bypass surgery in low-risk patients. N. Engl. J. Med. 2003, 348, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Krasivskyi, I.; Eghbalzadeh, K.; Ivanov, B.; Gerfer, S.; Großmann, C.; Sabashnikov, A.; Kuhn, E.; Mader, N.; Djordjevic, I.; Wahlers, T. Impact of Obesity on Early In-Hospital Outcomes after Coronary Artery Bypass Grafting Surgery in Acute Coronary Syndrome: A Propensity Score Matching Analysis. J. Clin. Med. 2022, 11, 6805. [Google Scholar] [CrossRef] [PubMed]
- Stamou, S.C.; Hill, P.C.; Haile, E.; Prince, S.; MacK, M.J.; Corso, P.J. Clinical outcomes of nonelective coronary revascularization with and without cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2006, 131, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shroyer, A.L.; Grover, F.L.; Hattler, B.; Collins, J.F.; McDonald, G.O.; Kozora, E.; Lucke, J.C.; Baltz, J.H.; Novitzky, D. On-pump versus off-pump coronary-artery bypass surgery. N. Engl. J. Med. 2009, 361, 1827–1837. [Google Scholar] [CrossRef]
- van Straten, A.H.; Bramer, S.; Hamad MA, S.; van Zundert, A.A.; Martens, E.J.; Schönberger, J.P.; de Wolf, A.M. Effect of body mass index on early and late mortality after coronary artery bypass grafting. Ann. Thorac. Surg. 2010, 89, 30–37. [Google Scholar] [CrossRef]
- Sun, X.; Hill, P.C.; Bafi, A.S.; Garcia, J.M.; Haile, E.; Corso, P.J.; Boyce, S.W. Is cardiac surgery safe in extremely obese patients (body mass index 50 or greater)? Ann. Thorac. Surg. 2009, 87, 540–546. [Google Scholar] [CrossRef]
- Filardo, G.; Adams, J.P. Effect of body mass index on mortality in patients undergoing isolated coronary artery bypass grafting. Ann. Thorac. Surg. 2010, 90, 1060. [Google Scholar] [CrossRef]
- Potapov, E.V.; Loebe, M.; Anker, S.; Stein, J.; Bondy, S.; Nasseri, B.A.; Sodian, R.; Hausmann, H.; Hetzer, R. Impact of body mass index on outcome in patients after coronary artery bypass grafting with and without valve surgery. Eur. Heart J. 2003, 24, 1933–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, B.; Grunwald, G.K.; Rumsfeld, J.S.; Hill, J.O.; Ho, P.M.; Wyatt, H.R.; Shroyer, A.L.W. Relationship of body mass index with outcomes after coronary artery bypass graft surgery. Ann. Thorac. Surg. 2007, 84, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Vassiliades, T.A.; Nielsen, J.L.; Lonquist, J.L. Effects of obesity on outcomes in endoscopically assisted coronary artery bypass operations. Heart Surg. Forum 2003, 6, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Prapas, S.N.; Panagiotopoulos, I.A.; Ayyad, M.A.S.; Protogeros, D.A.; Linardakis, I.N.; Kotsis, V.N.; Katinioti, A.A.; Michalopoulos, A.S. Impact of obesity on outcome of patients undergoing off-pump coronary artery bypass grafting using aorta no-touch technique. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 234–237. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, G.; Haan, C.K.; Peterson, E.D.; Coombs, L.P.; Cruzzavala, J.L.; Murray, G.F. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: A study from the Society of Thoracic Surgeons’ database. Ann. Thorac. Surg. 2002, 74, 1125–1131. [Google Scholar] [CrossRef]
- Mahajan, R.; Stokes, M.; Elliott, A.; A Munawar, D.; Khokhar, K.B.; Thiyagarajah, A.; Hendriks, J.; Linz, D.; Gallagher, C.; Kaye, D.; et al. Complex interaction of obesity, intentional weight loss and heart failure: A systematic review and meta-analysis. Heart 2020, 106, 58–68. [Google Scholar] [CrossRef]
- Oreopoulos, A.; Padwal, R.; Norris, C.M.; Mullen, J.C.; Pretorius, V.; Kalantar-Zadeh, K. Effect of obesity on short- and long-term mortality postcoronary revascularization: A meta-analysis. Obesity 2008, 16, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Bor, D.H.; Rose, R.M.; Modlin, J.F.; Weintraub, R.; Friedland, G.H. Mediastinitis after cardiovascular surgery. Rev. Infect. Dis. 1983, 5, 885–897. [Google Scholar] [CrossRef]
- Abboud, C.S.; Wey, S.B.; Baltar, V.T. Risk factors for mediastinitis after cardiac surgery. Ann. Thorac. Surg. 2004, 77, 676–683. [Google Scholar] [CrossRef]
- Milano, C.A.; Kesler, K.; Archibald, N.; Sexton, D.J.; Jones, R.H. Mediastinitis after coronary artery bypass graft surgery. Risk factors and long-term survival. Circulation 1995, 92, 2245–2251. [Google Scholar] [CrossRef]
- Seyfer, A.E.; Shriver, G.D.; Miller, T.R.; Graeber, G.M. Sternal blood flow after median sternotomy and mobilization of the internal mammary arteries. Surgery 1988, 104, 899–904. [Google Scholar]
- Kouchoukos, N.T.; Wareing, T.H.; Murphy, S.F.; Pelate, C.; Marshall, W.G. Risks of bilateral internal mammary artery bypass grafting. Ann. Thorac. Surg. 1990, 49, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, O.J.; Slottosch, I.; Wendt, D.; Welp, H.; Schiller, W.; Martens, S.; Choi, Y.-H.; Welz, A.; Pisarenko, J.; Neuhäuser, M.; et al. Surgical revascularization for acute coronary syndromes: A report from the North Rhine-Westphalia surgical myocardial infarction registry. Eur. J. Cardiothorac. Surg. 2020, 58, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, O.J.; Schlachtenberger, G.; Wendt, D.; Choi, Y.; Slottosch, I.; Welp, H.; Schiller, W.; Martens, S.; Welz, A.; Neuhäuser, M.; et al. Early Clinical Outcomes of Surgical Myocardial Revascularization for Acute Coronary Syndromes Complicated by Cardiogenic Shock: A Report From the North-Rhine-Westphalia Surgical Myocardial Infarction Registry. J. Am. Heart Assoc. 2019, 8, e012049. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Christopher, E.B.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, E.L.; McCullough, J.N.; Ross, C.S.; Kramer, R.S.; Westbrook, B.M.; Klemperer, J.D.; Leavitt, B.J.; Brown, J.R.; Olmstead, E.; Hernandez, F.; et al. Optimal Timing From Myocardial Infarction to Coronary Artery Bypass Grafting on Hospital Mortality. Ann. Thorac. Surg. 2017, 103, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Axelsson, T.A.; Mennander, A.; Malmberg, M.; Gunn, J.; Jeppsson, A.; Gudbjartsson, T. Is emergency and salvage coronary artery bypass grafting justified? The Nordic Emergency/Salvage coronary artery bypass grafting study. Eur. J. Cardiothorac. Surg. 2016, 49, 1451–1456. [Google Scholar] [CrossRef] [Green Version]
- Davierwala, P.M.; Verevkin, A.; Leontyev, S.; Misfeld, M.; Borger, M.A.; Mohr, F.W. Does Timing of Coronary Artery Bypass Surgery Affect Early and Long-Term Outcomes in Patients With Non-ST-Segment-Elevation Myocardial Infarction? Circulation 2015, 132, 731–740. [Google Scholar] [CrossRef] [Green Version]
- Parikh, S.V.; de Lemos, J.A.; Jessen, M.E.; Brilakis, E.S.; Ohman, E.M.; Chen, A.Y.; Wang, T.Y.; Peterson, E.D.; Roe, M.T.; Holper, E.M. Timing of in-hospital coronary artery bypass graft surgery for non-ST-segment elevation myocardial infarction patients results from the National Cardiovascular Data Registry ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With The Guidelines). JACC Cardiovasc. Interv. 2010, 3, 419–427. [Google Scholar]
- Abbott, J.D.; Ahmed, H.N.; Vlachos, H.A.; Selzer, F.; Williams, D.O. Comparison of outcome in patients with ST-elevation versus non-ST-elevation acute myocardial infarction treated with percutaneous coronary intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am. J. Cardiol. 2007, 100, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, T.M.; Galal, W.; Tjeertes, E.K.M.; Hoeks, S.E.; Verhagen, H.J.; Stolker, R.J. The obesity paradox in the surgical population. Surgeon 2013, 11, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Forlivesi, S.; Cappellari, M.; Bonetti, B. Obesity paradox and stroke: A narrative review. Eat. Weight Disord. 2021, 26, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Oesch, L.; Tatlisumak, T.; Arnold, M.; Sarikaya, H. Obesity paradox in stroke—Myth or reality? A systematic review. PLoS ONE 2017, 12, e0171334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masoudkabir, F.; Yavari, N.; Jameie, M.; Pashang, M.; Sadeghian, S.; Salarifar, M.; Jalali, A.; Tafti, S.H.A.; Abbasi, K.; Omran, A.S.; et al. The association between different body mass index levels and midterm surgical revascularization outcomes. PLoS ONE 2022, 17, e0274129. [Google Scholar] [CrossRef]
- Virani, S.S.; Nambi, V.; Lee, V.V.; Elayda, M.A.; Pan, W.; Petersen, L.A.; Wilson, J.M.; Willerson, J.T.; Ballantyne, C.M. Obesity: An independent predictor of in-hospital postoperative renal insufficiency among patients undergoing cardiac surgery? Texas Heart Inst. J. 2009, 36, 540–545. [Google Scholar]
- Wakasugi, M.; Narita, I. Prefecture-specific prevalence of overweight/obesity is associated with regional variation in the incidence of treated ESKD in Japan. Clin. Exp. Nephrol. 2023, 27, 132–140. [Google Scholar] [CrossRef]
- Stevens, J.; Truesdale, K.P.; Katz, E.G.; Cai, J. Impact of body mass index on incident hypertension and diabetes in Chinese Asians, American Whites, and American Blacks: The People’s Republic of China Study and the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2008, 167, 1365–1374. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L.F.; Shintani, A.; Ikizler, T.A.; Himmelfarb, J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J. Am. Soc. Nephrol. 2008, 19, 593–599. [Google Scholar] [CrossRef]
- Ejerblad, E.; Fored, C.M.; Lindblad, P.; Fryzek, J.; McLaughlin, J.K.; Nyrén, O. Obesity and risk for chronic renal failure. J. Am. Soc. Nephrol. 2006, 17, 1695–1702. [Google Scholar] [CrossRef] [Green Version]
- Moulton, M.J.; Creswell, L.L.; Mackey, M.E.; Cox, J.L.; Rosenbloom, M. Obesity is not a risk factor for significant adverse outcomes after cardiac surgery. Circulation 1996, 94 (Suppl. 9), II87–II92. [Google Scholar] [PubMed]
| Before PSM n = 332 | After PSM n = 248 | |||||
|---|---|---|---|---|---|---|
| Non-Obese (n = 193) | Obese (n = 139) | p-Value | Non-Obese (n = 124) | Obese (n = 124) | p-Value | |
| Age (years), (min/max) | 67(39/88) | 67(44/86) | 0.451 | 68(39/88) | 66(44/86) | 0.106 |
| Female gender, n (%) | 37 (19.2%) | 48 (34.5%) | 0.002 | 31 (25.0%) | 39 (31.5%) | 0.259 |
| Euroscore II (%), mean ± SD | 4.0 ± 2.6 | 4.2 ± 2.1 | 0.232 | 4.3 ± 2.9 | 4.0 ± 1.9 | 0.178 |
| LV-EF (%), mean ± SD | 48 ± 13 | 47 ± 14 | 0.354 | 47 ± 13 | 48 ± 14 | 0.281 |
| LM CAD, n (%) | 91 (48.1%) | 42 (30.9%) | 0.002 | 50 (40.3%) | 40 (32.3%) | 0.187 |
| History of NSTEMI, n (%) | 59 (30.7%) | 49 (35.5%) | 0.362 | 39 (31.5%) | 39 (31.5%) | 1.000 |
| History of STEMI, n (%) | 24 (12.6%) | 22 (15.9%) | 0.383 | 16 (12.9%) | 21 (16.9%) | 0.373 |
| Cardiogenic shock, n (%) | 15 (7.8%) | 21 (15.2%) | 0.033 | 13 (10.5%) | 19 (15.3%) | 0.256 |
| Previous PTCA, n (%) | 56 (29.2%) | 44 (32.1%) | 0.566 | 32 (25.8%) | 39 (31.5%) | 0.325 |
| Previous stroke, n (%) | 9 (4.7%) | 10 (7.3%) | 0.317 | 7 (5.6%) | 8 (6.5%) | 0.790 |
| Urgent procedure, n (%) | 15 (7.8%) | 21 (15.2%) | 0.033 | 13 (10.5%) | 19 (15.3%) | 0.256 |
| Reoperation, n (%) | 17 (8.9%) | 11 (8.2%) | 0.838 | 9 (7.3%) | 10 (8.3%) | 0.768 |
| DM, n (%) | 59 (30.7%) | 71 (51.1%) | <0.001 | 50 (40.3%) | 60 (48.4%) | 0.201 |
| Insulin-dependent DM (%) | 20 (10.4%) | 13 (9.4%) | 0.766 | 17 (13.7%) | 10 (8.1%) | 0.160 |
| HbA1C (%), (min/max) | 6.4(5.1/12.6) | 6.5(5.0/9.6) | 0.356 | 6.6(5.1/12.6) | 6.5(5.0/9.6) | 0.272 |
| Hyperlipidaemia, n (%) | 178 (92.7%) | 123 (88.5%) | 0.187 | 114 (91.9%) | 113 (91.1%) | 0.820 |
| PVD, n (%) | 39 (20.3%) | 33 (23.4%) | 0.394 | 31 (25.0%) | 29 (23.4%) | 0.767 |
| Arterial hypertension, n (%) | 184 (95.8%) | 133 (95.7%) | 0.947 | 120 (96.8%) | 120 (96.8%) | 1.000 |
| PH, n (%) | 5 (2.6%) | 7 (5.0%) | 0.247 | 1 (0.8%) | 4 (3.2%) | 0.370 |
| COPD, n (%) | 20 (10.4%) | 32 (23.4%) | 0.002 | 18 (14.5%) | 27 (21.8%) | 0.138 |
| Dialysis, n (%) | 2 (1.0%) | 9 (6.6%) | 0.006 | 2 (1.6%) | 5 (4.0%) | 0.446 |
| CKD, n (%) | 16 (8.3%) | 25 (18.2%) | 0.007 | 16 (12.9%) | 19 (15.3%) | 0.584 |
| Before PSM n = 332 | After PSM n = 248 | |||||
|---|---|---|---|---|---|---|
| Non-Obese (n = 193) | Obese (n = 139) | p-Value | Non-Obese (n = 124) | Obese (n = 124) | p-Value | |
| Use of two ITA grafts, n (%) | 110 (57.9%) | 68 (50.0%) | 0.158 | 62 (50.4%) | 61 (49.2%) | 0.849 |
| TAR, n (%) | 105 (55.3%) | 53 (39.5%) | 0.005 | 63 (51.2%) | 49 (40.2%) | 0.082 |
| T-graft technique, n (%) | 106 (55.8%) | 48 (35.8%) | <0.001 | 62 (50.4%) | 46 (37.7%) | 0.045 |
| Endoscopic SVG, n (%) | 52 (27.5%) | 49 (36.0%) | 0.102 | 42 (34.1%) | 43 (34.7%) | 0.930 |
| Heartstring, n (%) | 64 (34.0%) | 57 (41.9%) | 0.148 | 49 (40.5%) | 52 (41.9%) | 0.819 |
| Catecholamine use, n (%) | 185 (99.1%) | 133 (96.4%) | 0.204 | 117 (98.3%) | 120 (96.8%) | 0.442 |
| Temporary pacer, n (%) | 108 (56.8%) | 81 (60.0%) | 0.570 | 70 (56.9%) | 73 (59.8%) | 0.642 |
| ECMO intraoperative, n (%) | 0 (0.0%) | 2 (1.4%) | 0.174 | 0 (0.0%) | 2 (1.6%) | 0.498 |
| IABP intraoperative, n (%) | 5 (2.6%) | 2 (1.5%) | 0.704 | 3 (2.4%) | 2 (1.6%) | 0.658 |
| OP time, (min/max) | 169 (108/370) | 179 (80/330) | 0.974 | 169 (108/370) | 179 (80/330) | 0.865 |
| Before PSM n = 332 | After PSM n = 248 | |||||
|---|---|---|---|---|---|---|
| Non-Obese (n = 193) | Obese (n = 139) | p-Value | Non-Obese (n = 124) | Obese (n = 124) | p-Value | |
| TIA, n (%) | 3 (1.6%) | 10 (7.2%) | 0.009 | 2 (1.6%) | 8 (6.5%) | 0.102 |
| Stroke, n (%) | 1 (0.5%) | 0 (0.0%) | 0.395 | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Delirium, n (%) | 17 (8.9%) | 20 (14.5%) | 0.113 | 13 (10.6%) | 17 (13.7%) | 0.450 |
| LCOS after surgery, n (%) | 5 (2.6%) | 7 (5.1%) | 0.249 | 2 (1.7%) | 6 (4.8%) | 0.281 |
| CK, 48 h, U/L, mean ± SD | 635 ± 752 | 860 ± 1316 | 0.086 | 681 ± 761 | 909 ± 1361 | 0.162 |
| CK-MB, 48 h, U/L, mean ± SD | 39 ± 137 | 24 ± 20 | 0.256 | 46 ± 171 | 24 ± 21 | 0.215 |
| Lactate 48 h, mmol/L, mean ± SD | 1.5 ± 1.2 | 1.5 ± 1.1 | 0.785 | 1.5 ± 0.8 | 1.4 ± 0.7 | 0.516 |
| Creatinine 48 h, mg/dL, mean ± SD | 1.0 ± 0.4 | 1.4 ± 2.8 | 0.122 | 1.0 ± 0.3 | 1.4 ± 2.9 | 0.211 |
| Acute kidney failure, n (%) | 11 (5.8%) | 11 (8.0%) | 0.436 | 8 (6.6%) | 7 (5.6%) | 0.765 |
| Dialysis, n (%) | 1 (0.5%) | 14 (10.1%) | <0.001 | 1 (0.8%) | 9 (7.3%) | 0.019 |
| Wound infection, n (%) | 23 (12.0%) | 8 (5.8%) | 0.058 | 14 (11.3%) | 4 (3.2%) | 0.014 |
| Plastic covering, n (%) | 4 (2.1%) | 1 (0.7%) | 0.405 | 3 (2.4%) | 1 (0.8%) | 0.622 |
| PPI, n (%) | 4 (2.1%) | 2 (1.4%) | 0.662 | 4 (3.3%) | 1 (0.8%) | 0.211 |
| Bleeding with reoperation, n (%) | 10 (5.2%) | 3 (2.2%) | 0.162 | 8 (6.5%) | 3 (2.4%) | 0.216 |
| ICU stay, days, median(min/max) | 2 (1/3) | 2(1/6) | 0.147 | 2 (1/3) | 2 (1/5) | 0.314 |
| Hospital stay, days, median(min/max) | 9 (5/18) | 9 (5/20) | 0.195 | 9 (5/17) | 9 (5/19) | 0.058 |
| In-hospital mortality, n (%) | 3 (1.6%) | 5 (3.6%) | 0.286 | 2 (1.6%) | 3 (2.4%) | 0.651 |
| Before PSM Obesity Class (n = 139) | After PSM Obesity Class (124) | |||||||
|---|---|---|---|---|---|---|---|---|
| I (n = 96) | II (n = 29) | III (n =1 4) | p-Value | I (n = 86) | II (n = 24) | III (n = 14) | p-Value | |
| TIA, n (%) | 7 (7.3%) | 0 (0.0%) | 3 (21.4%) | 0.002 | 5 (5.8%) | 0 (0.0%) | 3 (21.4%) | 0.008 |
| Stroke, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Delirium, n (%) | 12 (12.5%) | 5 (17.9%) | 3 (21.4%) | 0.414 | 9 (10.5%) | 5 (20.8%) | 3 (21.4%) | 0.475 |
| LCOS after surgery, n (%) | 4 (4.2%) | 3 (10.7%) | 0 (0.0%) | 0.234 | 4 (4.7%) | 2 (8.3%) | 0 (0.0%) | 0.444 |
| Acute kidney failure, n (%) | 6 (6.3%) | 5 (17.9%) | 0 (0.0%) | 0.133 | 3 (3.5%) | 4 (16.7%) | 0 (0.0%) | 0.063 |
| Dialysis, n (%) | 5 (5.2%) | 8 (28.6%) | 1 (7.1%) | <0.001 | 3 (3.5%) | 5 (20.8%) | 1 (7.1%) | <0.001 |
| Wound infection, n (%) | 7 (7.3%) | 1 (3.6%) | 0 (0.0%) | 0.165 | 3 (3.5%) | 1 (4.2%) | 0 (0.0%) | 0.369 |
| Plastic covering, n (%) | 1 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0.790 | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) | 0.847 |
| PPI, n (%) | 1 (1.0%) | 1 (3.6%) | 0 (0.0%) | 0.399 | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) | 0.195 |
| Bleeding with reoperation, n (%) | 1 (1.0%) | 1 (3.6%) | 1 (7.1%) | 0.172 | 1 (1.2%) | 1 (4.2%) | 1 (7.1%) | 0.113 |
| In-hospital mortality, n (%) | 2 (2.1%) | 2 (7.1%) | 1 (7.1%) | 0.105 | 1 (1.2%) | 1 (4.2%) | 1 (7.1%) | 0.230 |
| Combined Risk Factors | Univariate Logistic Regression Model | Multivariate Logistic Regression Model | ||
|---|---|---|---|---|
| OR (CI 95%) | p-Value | OR (CI 95%) | p-Value | |
| Age | 1.037 (0.949–1.133) | 0.426 | 1.037 (0.957–1.123) | 0.372 |
| BMI | 1.005 (0.925–1.092) | 0.907 | 1.011 (0.961–1.062) | 0.681 |
| DM | 4.308 (0.779–23.815) | 0.094 | 4.629 (0.924–23.381) | 0.062 |
| STEMI | 7.909 (1.540–40.611) | 0.013 | 6.239 (1.507–25.823) | 0.012 |
| NSTEMI | 3.745 (0.718–19.518) | 0.117 | 2.064 (0.507–8.410) | 0.312 |
| Reoperation | 6.718 (1.324–34.084) | 0.022 | 7.320 (1.653–32.424) | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasivskyi, I.; Djordjevic, I.; Ivanov, B.; Eghbalzadeh, K.; Großmann, C.; Reichert, S.; Radwan, M.; Sandoval Boburg, R.; Sabashnikov, A.; Schlensak, C.; et al. Consequences of Obesity on Short-Term Outcomes in Patients Who Underwent Off-Pump Coronary Artery Bypass Grafting Surgery. J. Clin. Med. 2023, 12, 1929. https://doi.org/10.3390/jcm12051929
Krasivskyi I, Djordjevic I, Ivanov B, Eghbalzadeh K, Großmann C, Reichert S, Radwan M, Sandoval Boburg R, Sabashnikov A, Schlensak C, et al. Consequences of Obesity on Short-Term Outcomes in Patients Who Underwent Off-Pump Coronary Artery Bypass Grafting Surgery. Journal of Clinical Medicine. 2023; 12(5):1929. https://doi.org/10.3390/jcm12051929
Chicago/Turabian StyleKrasivskyi, Ihor, Ilija Djordjevic, Borko Ivanov, Kaveh Eghbalzadeh, Clara Großmann, Stefan Reichert, Medhat Radwan, Rodrigo Sandoval Boburg, Anton Sabashnikov, Christian Schlensak, and et al. 2023. "Consequences of Obesity on Short-Term Outcomes in Patients Who Underwent Off-Pump Coronary Artery Bypass Grafting Surgery" Journal of Clinical Medicine 12, no. 5: 1929. https://doi.org/10.3390/jcm12051929






