Cancer and Myotonic Dystrophy
Abstract
1. Introduction
Myopathies and Cancer
2. Mortality in DM
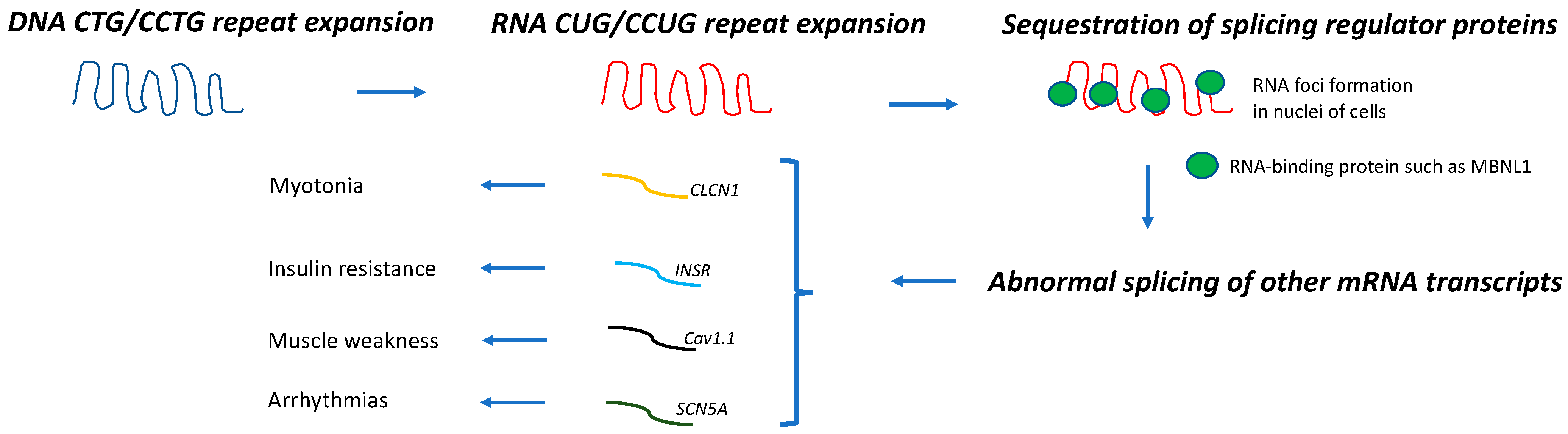
3. Carcinogenesis in DM
4. Pilomatricomas
5. Other Benign Tumors
6. Current Malignancy Screening Recommendations in DM
7. Susceptibility to General Anesthesia, Opiates, and Sedatives
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harper, P.S. Myotonic Dystrophy, 3rd ed.; W.B. Saunders: London, UK; New York, NY, USA, 2001. [Google Scholar]
- Johnson, N.E.; Butterfield, R.J.; Mayne, K.; Newcomb, T.; Imburgia, C.; Dunn, D.; Duval, B.; Feldkamp, M.L.; Weiss, R.B. Population Based Prevalence of Myotonic Dystrophy Type 1 Using Genetic Analysis of State-wide Blood Screening Program. Neurology 2021, 96, e1045–e1053. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Pizzuti, A.; Fenwick, R.G.; King, J.; Rajnarayan, S.; Dunne, P.W.; Dubel, J.; Nasser, G.A.; Ashizawa, T.; de Jong, P.; et al. An Unstable Triplet Repeat in a Gene Related to Myotonic Muscular Dystrophy. Science 1992, 255, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Suominen, T.; Bachinski, L.L.; Auvinen, S.; Hackman, P.; Baggerly, K.A.; Angelini, C.; Peltonen, L.; Krahe, R.; Udd, B. Population frequency of myotonic dystrophy: Higher than expected frequency of myotonic dystrophy type 2 (DM2) mutation in Finland. Eur. J. Hum. Genet. 2011, 19, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Liquori, C.L.; Ricker, K.; Moseley, M.L.; Jacobsen, J.F.; Kress, W.; Naylor, S.L.; Day, J.W.; Ranum, L.P.W. Myotonic Dystrophy Type 2 Caused by a CCTG Expansion in Intron 1 of ZNF9. Science 2001, 293, 864–867. [Google Scholar] [CrossRef]
- Brook, J.D.; McCurrach, M.E.; Harley, H.G.; Buckler, A.J.; Church, D.; Aburatani, H.; Hunter, K.; Stanton, V.P.; Thirion, J.-P.; Hudson, T.; et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 1992, 68, 799–808. [Google Scholar] [CrossRef]
- Udd, B.; Krahe, R. The myotonic dystrophies: Molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012, 11, 891–905. [Google Scholar] [CrossRef]
- Savkur, R.S.; Philips, A.V.; Cooper, T.A. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat. Genet. 2001, 29, 40–47. [Google Scholar] [CrossRef]
- Freyermuth, F.; Rau, F.; Kokunai, Y.; Linke, T.; Sellier, C.; Nakamori, M.; Kino, Y.; Arandel, L.; Jollet, A.; Thibault, C.; et al. Splicing misregulation of SCN5A contributes to cardiac-conduction delay and heart arrhythmia in myotonic dystrophy. Nat. Commun. 2016, 7, 11067. [Google Scholar] [CrossRef]
- Tang, Z.Z.; Yarotskyy, V.; Wei, L.; Sobczak, K.; Nakamori, M.; Eichinger, K.; Moxley, R.T.; Dirksen, R.T.; Thornton, C.A. Muscle weakness in myotonic dystrophy associated with misregulated splicing and altered gating of CaV1.1 calcium channel. Hum. Mol. Genet. 2012, 21, 1312–1324. [Google Scholar] [CrossRef]
- Koehorst, E.; Ballester-Lopez, A.; Arechavala-Gomeza, V.; Martínez-Piñeiro, A.; Nogales-Gadea, G. The Biomarker Potential of miRNAs in Myotonic Dystrophy Type I. J. Clin. Med. 2020, 9, 3939. [Google Scholar] [CrossRef]
- Gonzalez-Perez, P.; Amato, A. Myopathies and Cancer. In Effects of Cancer Treatment on the Nervous System, 1st ed.; Grisold, W., Ed.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2021; Volume 2, pp. 344–368. [Google Scholar]
- Tanboon, J.; Nishino, I. Update on dermatomyositis. Curr. Opin. Neurol. 2022, 35, 611–621. [Google Scholar] [CrossRef] [PubMed]
- De Die-Smulders, C.E.; Höweler, C.J.; Thijs, C.; Mirandolle, J.F.; Anten, H.B.; Smeets, H.J.; Chandler, K.E.; Geraedts, J.P. Age and causes of death in adult-onset myotonic dystrophy. Brain 1998, 121 Pt 8, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.; Allard, P.; Potvin, L.; Prévost, C.; Bégin, P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology 1999, 52, 1658. [Google Scholar] [CrossRef] [PubMed]
- MladenoviĆ, J.; Pekmezovic, T.; Todorovic, S.; Savic, D.; Romac, S.; Apostolski, S.; Rakocevic-Stojanovic, V. Survival and mortality of myotonic dystrophy type 1 (Steinert’s disease) in the population of Belgrade. Eur. J. Neurol. 2006, 13, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Groh, W.J.; Groh, M.R.; Shen, C.; Monckton, D.G.; Bodkin, C.L.; Pascuzzi, R.M. Survival and CTG repeat expansion in adults with myotonic dystrophy type 1. Muscle Nerve 2011, 43, 648–651. [Google Scholar] [CrossRef]
- Gadalla, S.M.; Pfeiffer, R.M.; Kristinsson, S.Y.; Björkholm, M.; Hilbert, J.E.; Moxley, R.T.; Landgren, O.; Greene, M.H. Quantifying Cancer Absolute Risk and Cancer Mortality in the Presence of Competing Events after a Myotonic Dystrophy Diagnosis. PLoS ONE 2013, 8, e79851. [Google Scholar] [CrossRef]
- Fernández-Torrón, R.; García-Puga, M.; Emparanza, J.I.; Maneiro, M.; Cobo, A.M.; Poza, J.J.; Espinal, J.B.; Zulaica, M.; Ruiz, I.; Martorell, L.; et al. Cancer risk in DM1 is sex-related and linked to miRNA-200/141 downregulation. Neurology 2016, 87, 1250–1257. [Google Scholar]
- Gadalla, S.M.; Lund, M.; Pfeiffer, R.M.; Gørtz, S.; Mueller, C.M.; Moxley, R.T.; Kristinsson, S.Y.; Björkholm, M.; Shebl, F.M.; Hilbert, J.E.; et al. Cancer Risk Among Patients With Myotonic Muscular Dystrophy. JAMA 2011, 306, 2480–2486. [Google Scholar] [CrossRef]
- Das, M.; Iii, R.T.M.; Hilbert, J.E.; Martens, W.B.; Letren, L.; Greene, M.H.; Gadalla, S.M. Correlates of tumor development in patients with myotonic dystrophy. J. Neurol. 2012, 259, 2161–2166. [Google Scholar] [CrossRef]
- Win, A.K.; Perattur, P.G.; Pulido, J.S.; Pulido, C.M.; Lindor, N.M. Increased Cancer Risks in Myotonic Dystrophy. Mayo Clin. Proc. 2012, 87, 130–135. [Google Scholar] [CrossRef]
- Abbott, D.; Johnson, N.E.; Cannon-Albright, L.A. A population-based survey of risk for cancer in individuals diagnosed with myotonic dystrophy. Muscle Nerve 2016, 54, 783–785. [Google Scholar] [CrossRef]
- Alsaggaf, R.; Wang, Y.; Marini-Bettolo, C.; Wood, L.; Nikolenko, N.; Lochmüller, H.; Greene, M.H.; Gadalla, S.M. Benign and malignant tumors in the UK myotonic dystrophy patient registry. Muscle Nerve 2017, 57, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Osanai, R.; Kinoshita, M.; Hirose, K.; Homma, T.; Kawabata, I. CTG triplet repeat expansion in a laryngeal carcinoma from a patient with myotonic dystrophy. Muscle Nerve 2000, 23, 804–806. [Google Scholar] [CrossRef]
- Bañuls, J.; Botella, R.; Palau, F.; Ramón, R.; Díaz, C.; Payá, A.; Carnero, L.; Vergara, G. Tissue and tumor mosaicism of the myotonin protein kinase gene trinucleotide repeat in a patient with multiple basal cell carcinomas associated with myotonic dystrophy. J. Am. Acad. Dermatol. 2004, 50 (Suppl. 2), S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Jinnai, K.; Sugio, T.; Mitani, M.; Hashimoto, K.; Takahashi, K. Elongation of (CTG)n repeats in myotonic dystrophy protein kinase gene in tumors associated with myotonic dystrophy patients. Muscle Nerve 1999, 22, 1271–1274. [Google Scholar] [CrossRef]
- Kinoshita, M.; Igarashi, A.; Komori, T.; Tamura, H.; Hayashi, M.; Kinoshita, K.; Deguchi, T.; Hirose, K. Differences in CTG triplet repeat expansions in an ovarian cancer and cyst from a patient with myotonic dystrophy. Muscle Nerve 1997, 20, 622–624. [Google Scholar] [CrossRef]
- Mohamed, S.; Pruna, L.; Kaminsky, P. Increasing risk of tumors in myotonic dystrophy type 1. Presse Med. 2013, 42 Pt 1, e281–e284. [Google Scholar] [CrossRef]
- Wang, Y.; Pfeiffer, R.M.; AlSaggaf, R.; Meeraus, W.; Gage, J.C.; Anderson, L.; Bremer, R.C.; Nikolenko, N.; Lochmuller, H.; Greene, M.H.; et al. Risk of skin cancer among patients with myotonic dystrophy type 1 based on primary care physician data from the U.K. Clinical Practice Research Datalink. Int. J. Cancer 2017, 142, 1174–1181. [Google Scholar] [CrossRef]
- Alsaggaf, R.; George, D.M.M.S.; Zhan, M.; Pfeiffer, R.M.; Wang, Y.; Wagner, K.R.; Greene, M.H.; Amr, S.; Gadalla, S.M. Cancer Risk in Myotonic Dystrophy Type I: Evidence of a Role for Disease Severity. JNCI Cancer Spectr. 2018, 2, pky052. [Google Scholar] [CrossRef]
- Emparanza, J.I.; de Munain, A.L.; Greene, M.H.; Matheu, A.; Fernández-Torrón, R.; Gadalla, S.M. Cancer phenotype in myotonic dystrophy patients: Results from a meta-analysis. Muscle Nerve 2018, 58, 517–522. [Google Scholar] [CrossRef]
- D’Ambrosio, E.S.; Chuang, K.; David, W.S.; Amato, A.A.; Gonzalez-Perez, P. Frequency and Type of Cancers in Myotonic Dystrophy. A Retrospective Cross-Sectional Study. Muscle Nerve, 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.L.; Leoncini, E.; Masciullo, M.; Modoni, A.; Gadalla, S.M.; Massa, R.; Rastelli, E.; Terracciano, C.; Antonini, G.; Bucci, E.; et al. Increased risk of tumor in DM1 is not related to exposure to common lifestyle risk factors. J. Neurol. 2016, 263, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.M.; Hilbert, J.E.; Martens, W.; Thornton, C.A.; Moxley, R.T.; Greene, M.H. Hypothesis: Neoplasms in myotonic dystrophy. Cancer Causes Control. 2009, 20, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Zemtsov, A. Association between basal, squamous cell carcinomas, dysplastic nevi and myotonic muscular dystrophy indicates an important role of RNA-binding proteins in development of human skin cancer. Arch. Dermatol. Res. 2009, 302, 169–170. [Google Scholar] [CrossRef]
- Kajino, Y.; Yamaguchi, A.; Hashimoto, N.; Matsuura, A.; Sato, N.; Kikuchi, K. beta-Catenin gene mutation in human hair follicle-related tumors. Pathol. Int. 2001, 51, 543–548. [Google Scholar] [CrossRef]
- Lazar, A.J.F.; Calonje, E.; Grayson, W.; Tos, A.D.; Mihm, M.C.; Redston, M.; McKee, P.H. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J. Cutan. Pathol. 2005, 32, 148–157. [Google Scholar] [CrossRef]
- Chan, E.F.; Gat, U.; McNiff, J.M.; Fuchs, E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat. Genet. 1999, 21, 410–413. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, W.; Yang, W.; Wang, Y.; Kong, H.; Wang, L.; Yan, L.; Xu, G.; Fei, J.; Fu, J.; et al. Wnt pathway is involved in pleomorphic adenomas induced by overexpression of PLAG1 in transgenic mice. Int. J. Cancer 2005, 118, 643–648. [Google Scholar] [CrossRef]
- Harris, S.; Moncrieff, C.; Johnson, K. Myotonic dystrophy: Will the real gene please step forward! Hum. Mol. Genet. 1996, 5, 1417–1423. [Google Scholar] [CrossRef]
- Wooster, R.; Cleton-Jansen, A.-M.; Collins, N.; Mangion, J.; Cornelis, R.; Cooper, C.; Gusterson, B.; Ponder, B.; von Deimling, A.; Wiestler, O.; et al. Instability of short tandem repeats (microsatellites) in human cancers. Nat. Genet. 1994, 6, 152–156. [Google Scholar] [CrossRef]
- Lee, E.; Lee, T.A.; Yoo, H.J.; Lee, S.; Park, B. CNBP controls tumor cell biology by regulating tumor-promoting gene expression. Mol. Carcinog. 2019, 58, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Zampetti, A.; Silvestri, G.; Manco, S.; Khamis, K.; Masciullo, M.; Bianchi, M.L.E.; Damiani, A.; Santoro, M.; Linder, D.; Bewley, A.; et al. Dysplastic nevi, cutaneous melanoma, and other skin neoplasms in patients with myotonic dystrophy type 1: A cross-sectional study. J. Am. Acad. Dermatol. 2015, 72, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.J.; Ashizawa, T.; Monckton, D.G.; Caskey, C.T.; Richards, C.S. Somatic heterogeneity of the CTG repeat in myotonic dystrophy is age and size dependent. Am. J. Hum. Genet. 1995, 56, 114–122. [Google Scholar] [PubMed]
- Yencha, M.W. Head and neck pilomatricoma in the pediatric age group: A retrospective study and literature review. Int. J. Pediatr. Otorhinolaryngol. 2001, 57, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Fama’, F.; Ieni, A.; Tchernev, G.; Chokoeva, A.A.; Maximov, G.K.; Wollina, U.; Lotti, T.; Patterson, J.W.; Fioranelli, M.; Roccia, M.G.; et al. Pilomatrixoma of the breast in a patient with type 1 myotonic dystrophy: Successful surgical approach. J. Biol. Regul. Homeost. Agents 2016, 30, 1–6. [Google Scholar] [PubMed]
- Ciriacks, K.; Knabel, D.; Waite, M.B. Syndromes associated with multiple pilomatricomas: When should clinicians be concerned? Pediatr. Dermatol. 2020, 37, 9–17. [Google Scholar] [CrossRef]
- Salerni, E.; Bonatti, M.L.; D’Aurizio, C.; Baldassarre, M.; D’Alessandro, E.; Prencipe, M. Multiple pilomatrixomas and myotonic dystrophy: A case report. Riv. di Neurol. 1988, 58, 124–126. [Google Scholar]
- Park, J.-S.; Park, D. Cancer frequency among the patients with myotonic dystrophy in the South Korean population using the national health insurance database. J. Neurol. Sci. 2020, 420, 117212. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.; Evans, A.; Green, C.; Affleck, A. A newly growing asymptomatic facial lesion. Clin. Exp. Dermatol. 2022, 47, 1609–1612. [Google Scholar] [CrossRef]
- Rübben, A.; Wahl, R.U.; Eggermann, T.; Dahl, E.; Ortiz-Brüchle, N.; Cacchi, C. Mutation analysis of multiple pilomatricomas in a patient with myotonic dystrophy type 1 suggests a DM1-associated hypermutation phenotype. PLoS ONE 2020, 15, e0230003. [Google Scholar] [CrossRef]
- Ashizawa, T.; Gagnon, C.; Groh, W.J.; Gutmann, L.; Johnson, N.E.; Meola, G.; Moxley, R.; Pandya, S.; Rogers, M.T.; Simpson, E.; et al. Consensus-based care recommendations for adults with myotonic dystrophy type 1. Neurol. Clin. Pract. 2018, 8, 507–520. [Google Scholar] [CrossRef]
- Cantwell, A.R.; Reed, W.B. Myotonia atrophica and multiple calcifying epithelioma of Malherbe. Acta Dermato-Venereol. 1965, 45, 387–390. [Google Scholar] [PubMed]
- Ben Hamou, A.; Espiard, S.; Do Cao, C.; Ladsous, M.; Loyer, C.; Moerman, A.; Boury, S.; Kyheng, M.; Dhaenens, C.M.; Tiffreau, V.; et al. Systematic thyroid screening in myotonic dystrophy: Link between thyroid volume and insulin resistance. Orphanet. J. Rare Dis. 2019, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Alsaggaf, R.; George, D.M.M.S.; Zhan, M.; Pfeiffer, R.M.; Wang, Y.; Anderson, L.A.; Liu, Z.; Koshiol, J.; Bauer, A.J.; Wagner, K.R.; et al. Benign tumors in myotonic dystrophy type I target disease-related cancer sites. Ann. Clin. Transl. Neurol. 2019, 6, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.J.L.; Tey, H.L. Multiple pilomatricomas: Case presentation and review of the literature. Dermatol. Online J. 2010, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Álvarez, L.; Batalla, A.; Iglesias-Puzas, Á.; Álvarez, C.; Flórez, Á. Multiple Pilomatricomas: A Retrospective Study and Literature Review. Am. J. Dermatopathol. 2019, 41, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Kentley, J.; Nasir, S.; Lloyd, K.; Markiewicz, D.; Harwood, C.A. Multiple pilomatrixomas as a presentation of myotonic dystrophy. Clin. Exp. Dermatol. 2019, 44, e149–e150. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lachance, A.; Sinclair, D.; Asgari, M.M. Multiple basal cell carcinomas in a patient with myotonic dystrophy type 1. BMJ Case Rep. 2019, 12, e227233. [Google Scholar] [CrossRef]
- Hirai, T.; Yamanaka, A.; Fujimoto, T.; Takahashi, A.; Takayama, Y.; Yamianaka, K. Multiple thymoma with myotonic dystrophy. Jpn. J. Thorac. Cardiovasc. Surg. 2001, 49, 457–460. [Google Scholar] [CrossRef]
- Reimund, J.M.; Duclos, B.; Chamouard, P.; Warter, J.M.; Weill, J.P.; Baumann, R. Intestinal carcinoid tumor and myotonic dystrophy. Dig. Dis. Sci. 1992, 37, 1922–1925. [Google Scholar] [CrossRef]
- Rosenberg, N.L.; DiLiberti, J.H.; Andrews, A.M.; Buist, N.R. Myotonic dystrophy and hyperparathyroidism: Association with neurofibromatosis and multiple endocrine adenomatosis type 2A. J. Neurol. Neurosurg. Psychiatry 1988, 51, 1578–1580. [Google Scholar] [CrossRef] [PubMed]
- Schoser, B.; Montagnese, F.; Bassez, G.; Fossati, B.; Gamez, J.; Heatwole, C.; Hilbert, J.; Kornblum, C.; Kostera-Pruszczyk, A.; Krahe, R.; et al. Consensus-based care recommendations for adults with myotonic dystrophy type 2. Neurol. Clin. Pract. 2019, 9, 343–353. [Google Scholar] [CrossRef]
- Johnson, W.; Marshall, G. Observations on Thomsen’s Disease. QJM Int. J. Med. 1915, 8, 114–128. [Google Scholar] [CrossRef]
- Dundee, J.W. Thiopentone in dystrophia myotonica. Anesth. Analg. 1952, 31, 257–326. [Google Scholar] [CrossRef]
- Bourke, T.D.; Zuck, D. Thipentone in dystrophia myotonica. Br. J. Anaesth. 1957, 29, 35–38. [Google Scholar] [CrossRef]
- Mathieu, J.; Allard, P.; Gobeil, G.; Girard, M.; De Braekeleer, M.; Begin, P. Anesthetic and surgical complications in 219 cases of myotonic dystrophy. Neurology 1997, 49, 1646–1650. [Google Scholar] [CrossRef]
- Kaufman, L. Anaesthesia in dystrophia myotonica: A review of the hazards of anaesthesia. Proc. R. Soc. Med. 1960, 53, 183–188. [Google Scholar] [CrossRef]
- Sinclair, J.L.; Reed, P.W. Risk factors for perioperative adverse events in children with myotonic dystrophy. Pediatr. Anesth. 2009, 19, 740–747. [Google Scholar] [CrossRef]
- Kim, C.-S.; Park, J.-M.; Park, D.; Kim, D.-H. Opioid use may be associated with postoperative complications in myotonic dystrophy type 1 with high-grade muscular impairment. Sci. Rep. 2021, 11, 8. [Google Scholar] [CrossRef]
- Azar, I. The response of Patients with Neuromuscular Disorders to Muscle Relaxants: A Review. Anesthesiology 1984, 61, 173–187. [Google Scholar] [CrossRef]
- Ferschi, M.; Moxley, R.; Day, J.W.; Gropper, M. Practical Suggestions for the Anesthetic Management of a Myotonic Dystrophy Patient. Myotonic Dystrophy Foundation. Care and a Cure. Available online: Myotonic.org/sites/default/files/MDF_LongForm_AnesGuidelines_01C.pdf (accessed on 6 February 2023).
- Thiel, R.E. The myotonic response to suxamethonium. Br. J. Anaesth. 1967, 39, 815–821. [Google Scholar] [CrossRef]
- Diefenbach, C.; Lynch, J.; Abel, M.; Buzello, W. Vecuronium for Muscle Relaxation in Patients with Dystrophia Myotonica. Obstet. Anesth. Dig. 1993, 76, 872–874. [Google Scholar] [CrossRef]
- Veyckemans, F.; Scholtes, J.-L. Myotonic Dystrophies type 1 and 2: Anesthetic care. Pediatr. Anesth. 2013, 23, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Kirzinger, L.M.; Schmidt, A.; Kornblum, C.; Schneider-Gold, C.; Kress, W.; Schoser, B. Side effects of anesthesia in DM2 as compared to DM1: A comparative retrospective study. Eur. J. Neurol. 2010, 17, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Van den Bersselaar, L.R.; Heytens, L.; Silva, H.C.A.; Reimann, J.; Tasca, G.; Díaz-Cambronero, Ó.; Løkken, N.; Hellblom, A.; Hopkins, P.M.; Rueffert, H.; et al. European Neuromuscular Centre consensus statement on anaesthesia in patients with neuromuscular disorders. Eur. J. Neurol. 2022, 29, 3486–3507. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, J.; Gomes, T.B.; Snape, M.; Nikolenko, N.; McMorn, A.; Evans, S.; Yaroshinsky, A.; Della Pasqua, O.; Oosterholt, S.; Lochmüller, H. A Phase 2 Study of AMO-02 (Tideglusib) in Congenital and Childhood-Onset Myotonic Dystrophy Type 1 (DM1). Pediatr. Neurol. 2020, 112, 84–93. [Google Scholar] [CrossRef] [PubMed]
| Study (Ref) | Country/Registry | Unspecified DM (n) | DM1 (n) | Age at Death (Years Old) | Cancer-Related Death (% of Patients) |
|---|---|---|---|---|---|
| de Die-Smulders CE et al., 1998 [14] | The Netherlands | 180 | --- | Mean: 54 95% IC: 52.0–56.7 | 10% |
| Mathieu J et al., 1999 [15] | Canada | 367 | --- | Mean: 53.2 (range: 24–81) | 11% |
| Mladenovic J et al., 2006 [16] | Belgrade | --- | 101 | Mean: 56.7 Average mortality rate: 0.5/106 | 1% |
| Groh WJ et al., 2011 [17] | United States | --- | 406 | Mean: 54 (range: 21–79) | ~6% |
| Gadalla SM et al., 2013 [18] | Swedish Patient Registry/ Swedish Cause of Death Registry | 1081 | --- | Median: 49.8 95% CI: 39.8–53.8 | 10% |
| Fernandez-Torron et al., 2016 [19] | Spain | --- | 424 | --- | 15.3% |
| Reference | N | Thyroid | Cutaneous Melanoma | Pancreas | Colon | Endometrium | Ovary | Prostate | Testes | Brain | Eye |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gadalla et al., 2011 [20] | 1658 | ↑SIR 7.1 (1.8–19.3) | ––– | ↑SIR 3.2 (1.0–7.6) | ↑SIR 2.9 (1.5–5.1) | ↑SIR 7.6 (4.0–13.2) | ↑SIR 5.2 (2.3–10.2) | SIR 0.7 (0.2–1.9) | SIR 1.4 (0.1–6.8) | ↑SIR 5.3 (2.3–10.4) | ↑SIR 12.0 (2.0–39.6) |
| Win et al., 2012 [22] | 307 | ↑SIR 5.54 (1.80–12.93) | SIR 2.05 (0.42–6.00) | ––– | SIR 1.09 (0.22–3.18) | SIR 1.07 (0.03–5.98) | SIR 1.66 (0.04–9.25) | SIR 2.21 (0.95–4.35) | SIR 5.09 (0.62–18.38) | SIR 1.54 (0.04–8.57) | ↑SIR 27.54 (3.34–99.49) |
| Mohamed et al., 2013 [29] | 109 | ––– | RR 7.1 (0.8–25.8) | ––– | RR 5.0 (0.6–18.2) | ↑RR 21.7 (2.4–78.5) | RR 9.3 (0.1–51.5) | ––– | ––– | ––– | ––– |
| Abbott et al., 2016 [23] | 281 | RR 3.78 (0.67–13.65) | RR 0.89 (0.0–4.20) | ––– | RR 2.15 (0.11–11.99) | ↑RR 6.98 (1.24–25.22) | RR 1.43 (0.25–5.16) | ↑RR 10.74 (1.91–38.79) | ––– | ––– | |
| Fernandez-Torron et al., 2016 [19] | 424 | ↑SIR 23.33 (9.38–48.08) | SIR 1.72 (0.04–9.61) | ––– | SIR 2.06 (0.94–3.92) | ↑SIR 6.86 (2.23–16.02) | ↑SIR 8.33 (1.72–24.31) | SIR 0.46 (0.06–1.67) | SIR 14.25 (0.35–79.6) | ↑SIR 9.80 (3.18–22.88) | |
| Wang et al., 2018 [30] | 1061 | ––– | HR: 2.40 (0.56–10.31) | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– |
| Alsaggaf et al., 2018 [31] | 927 | ↑HR 15.93 (2.45–103.64) | ↑HR 5.98 (1.24–28.79) | ↑HR 2.96 (0.30–29.38) | HR 1.82 (0.32–10.31) | ↑HR 14.88 (2.14–103.67) | ––– | ––– | HR 4.99 (0.46–53.78) | ––– | |
| HR 1.81 (0.21–15.23) | HR 1.12 (0.37–3.45) | HR 0.32 (0.04–2.62) | HR 2.03 (0.23–17.68) | ||||||||
| Emparanza et al., 2018 [32] | 2779 (meta-analysis) | ↑pSIR = 8.52 (3.62–20.1) | ↑pSIR = 2.45 (1.31–4.58) | ––– | ↑pSIR = 2.2 (1.39–3.49) | ↑pSIR = 7.48 (4.72–11.8) | ↑pSIR = 5.56 (2.99–10.3) | ––– | ↑pSIR = 5.95 (2.34–15.1) | ––– | ––– |
| Pathogenic Mechanism | References |
|---|---|
| 1. Upregulation of Wnt/B-catenin pathway | [35] |
| 2. Alterations of mRNAs transcripts (tumor suppressor genes or oncogenes) | [36] |
| 3. B-catenin mutations (CTNNB1) | [37,38,39] |
| 4. PLAG1 overexpression | [40] |
| 5. DMPK repeat expansion | [41,42] |
| 6. CNBP repeat expansion | [43] |
| Ref. | Type of Study/Country | Patients with DM (N) | N of Tumors (N) | Thyroid | Skin | GI | Breast | Sex (Female)-Related | Sex (Male)-Related | Hematologic | Salivary Glands | Brain | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ben Hamou et al., 2019 [55] | Retrospective Observational/ US | 115 (DM1) | N/A | Nodule = 61% (70/115), of which 50% with benign cytology * | |||||||||
| Alsaggaf et al., 2018 [24] | Cross-Sectional, Self-reported/ UK | 220 (DM1 and DM2) | 39 (N/A) | 6 (15.4) ^ | 4 (10.3%) | 0 (0) | 2 (5.1) | 11 (28.2) | 0 (0) | 0 (0) | 4 (10.3) | 10 (25.6) | |
| Alsaggaf et al., 2018 [31] | Retrospective Observational/ UK | 927 (DM1) | 138 (14%) vs. 844 (6%) DM1 vs. DM1-free | HR 10.4 (3.9–27.5) | HR 1.4 (1.1–1.9) | HR 4.3 (1.8–10.4) | Uterine fibroids: HR: 2.7 (1.2–5.9); Uterine polyps: HR 9.6 (1.2–77.5) | HR 8.4 (2.5–28.5) | |||||
| Mueller et al., 2009 [35] | Review of Case Reports/ US | N/A | N/A | ^^ | ^^ | ^^ | ^^ | ^^ | ^^ | ^^ | ^^ |
| Pre-operative assessment | History: age at diagnosis, cardiac, pulmonary problems, obstructive sleep apnea, central apnea, prior history of surgery-related complications, medications. |
| Exam: general and neurological examination, muscle function (MIRS), craniofacial abnormalities (indicating difficult airway). | |
| Evaluations: blood glucose, electrolytes, hemoglobin, LFTs, thyroid function, CK, ECG, chest X-ray, echocardiogram, Holter examination, PFTs, sleep oximetry or polysomnography. | |
| Specialists: primary care provider, neuromuscular specialist, cardiologist, pulmonologist, anesthesiologist. | |
| Operative management | Schedules: Patients with DM should avoid prolonged fasting and hypoglycemia. |
| Monitor: pulse oximetry, telemetry, invasive cardiac monitoring. | |
| Temperature control: both that of the patient and that of the operating room. | |
| Induction agents | Premedication: avoid. Benzodiazepines can cause central respiratory depression. |
| Halogenated agents: no increased risk of MH. | |
| Hypnotics: slow titration. Propofol-induced pain can provoke myotonia. Etomidate-induced pain can provoke myoclonic movements. | |
| Muscle relaxants: unpredictable response to succinylcholine. Non-repolarizing agents, such as rocuronium or cis-atracurium, are preferred. | |
| Opioids: fentanyl, sufentanil, or remifentanil have been used. | |
| Reversal of muscle relaxation: cholinesterase inhibitors are contraindicated. | |
| Recovery | Extubation: fully awake and with the support of NIV. |
| Prolonged PACU stay, with continuous monitoring of O2 and surveillance for signs of rhabdomyolysis. Risk of delayed respiratory problems, aspiration, and ileus. | |
| Analgesia: NSAIDs and paracetamol are safe. Caution with opioids. Tramadol has been used. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosio, E.S.; Gonzalez-Perez, P. Cancer and Myotonic Dystrophy. J. Clin. Med. 2023, 12, 1939. https://doi.org/10.3390/jcm12051939
D’Ambrosio ES, Gonzalez-Perez P. Cancer and Myotonic Dystrophy. Journal of Clinical Medicine. 2023; 12(5):1939. https://doi.org/10.3390/jcm12051939
Chicago/Turabian StyleD’Ambrosio, Eleonora S., and Paloma Gonzalez-Perez. 2023. "Cancer and Myotonic Dystrophy" Journal of Clinical Medicine 12, no. 5: 1939. https://doi.org/10.3390/jcm12051939
APA StyleD’Ambrosio, E. S., & Gonzalez-Perez, P. (2023). Cancer and Myotonic Dystrophy. Journal of Clinical Medicine, 12(5), 1939. https://doi.org/10.3390/jcm12051939






