Strategic Approach to Aberrant Hepatic Arterial Anatomy during Laparoscopic Pancreaticoduodenectomy: Technique with Video
Abstract
1. Introduction
2. Materials and Methods
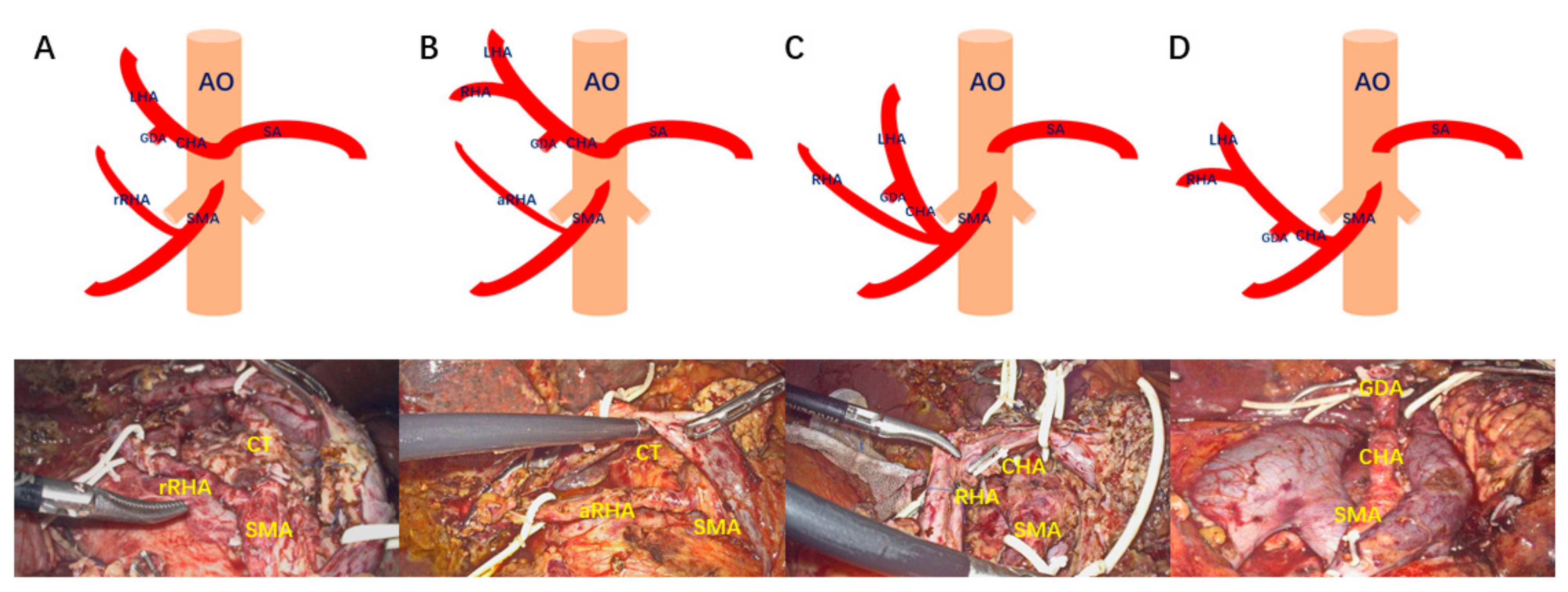
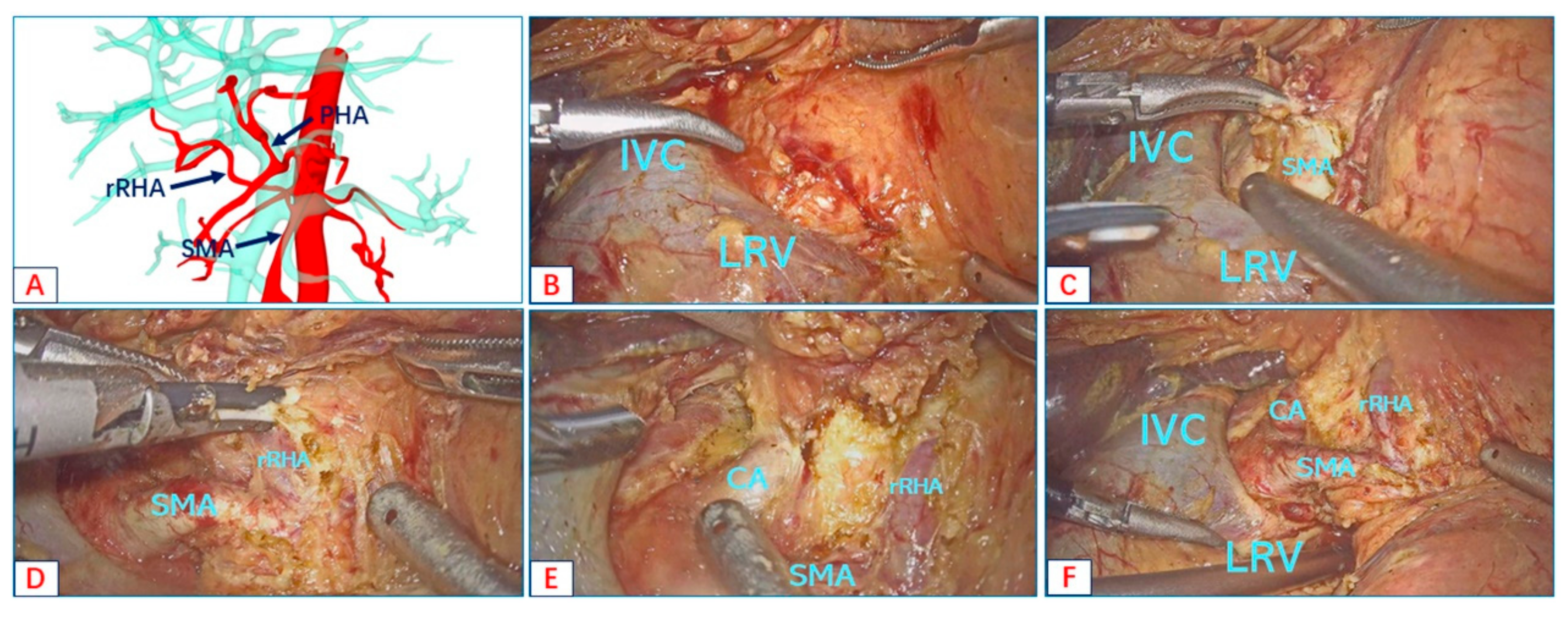
- Type A: Replaced right hepatic artery originates from the SMA;
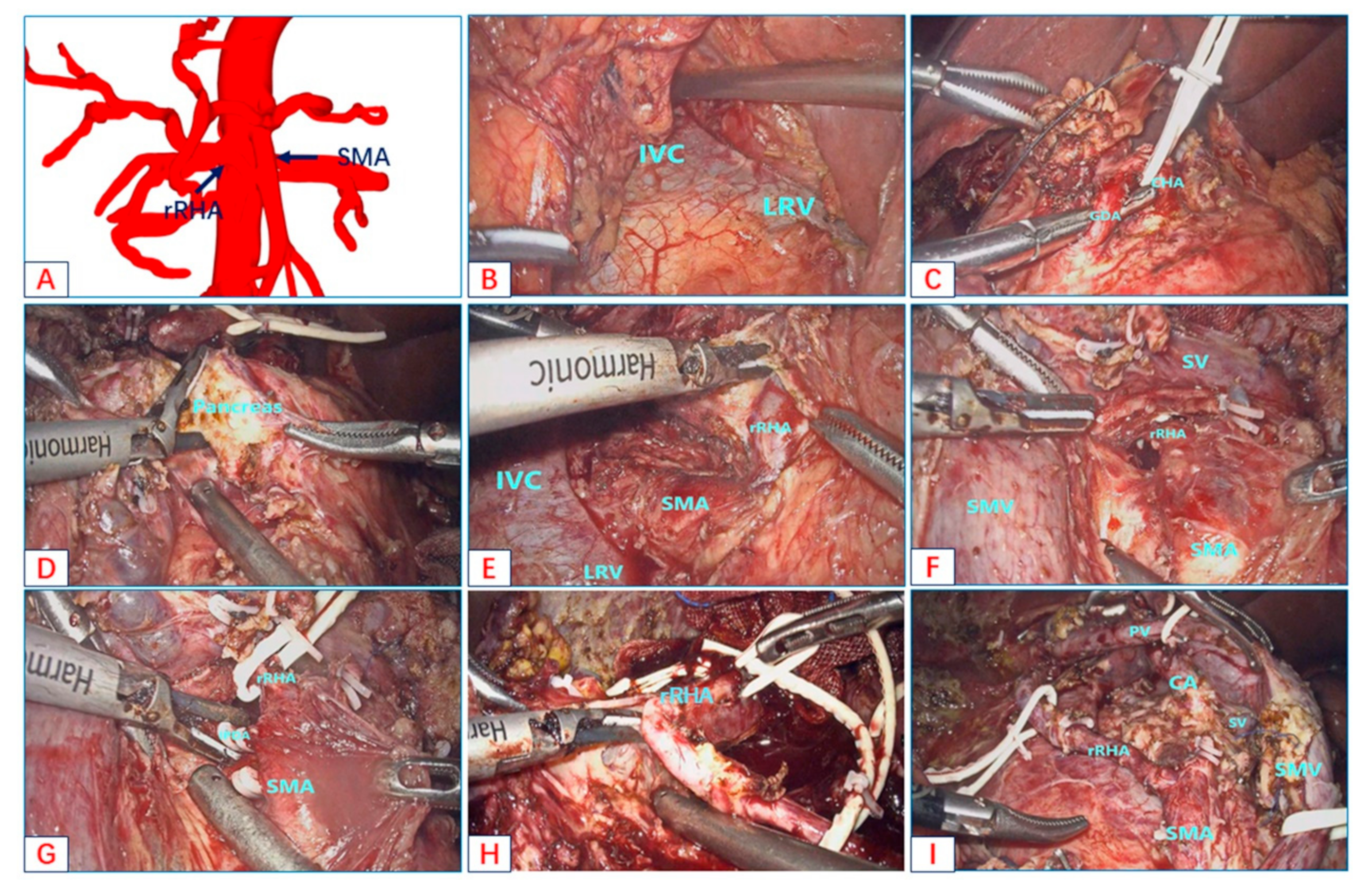
- Type B: Accessory right hepatic artery originates from the SMA;
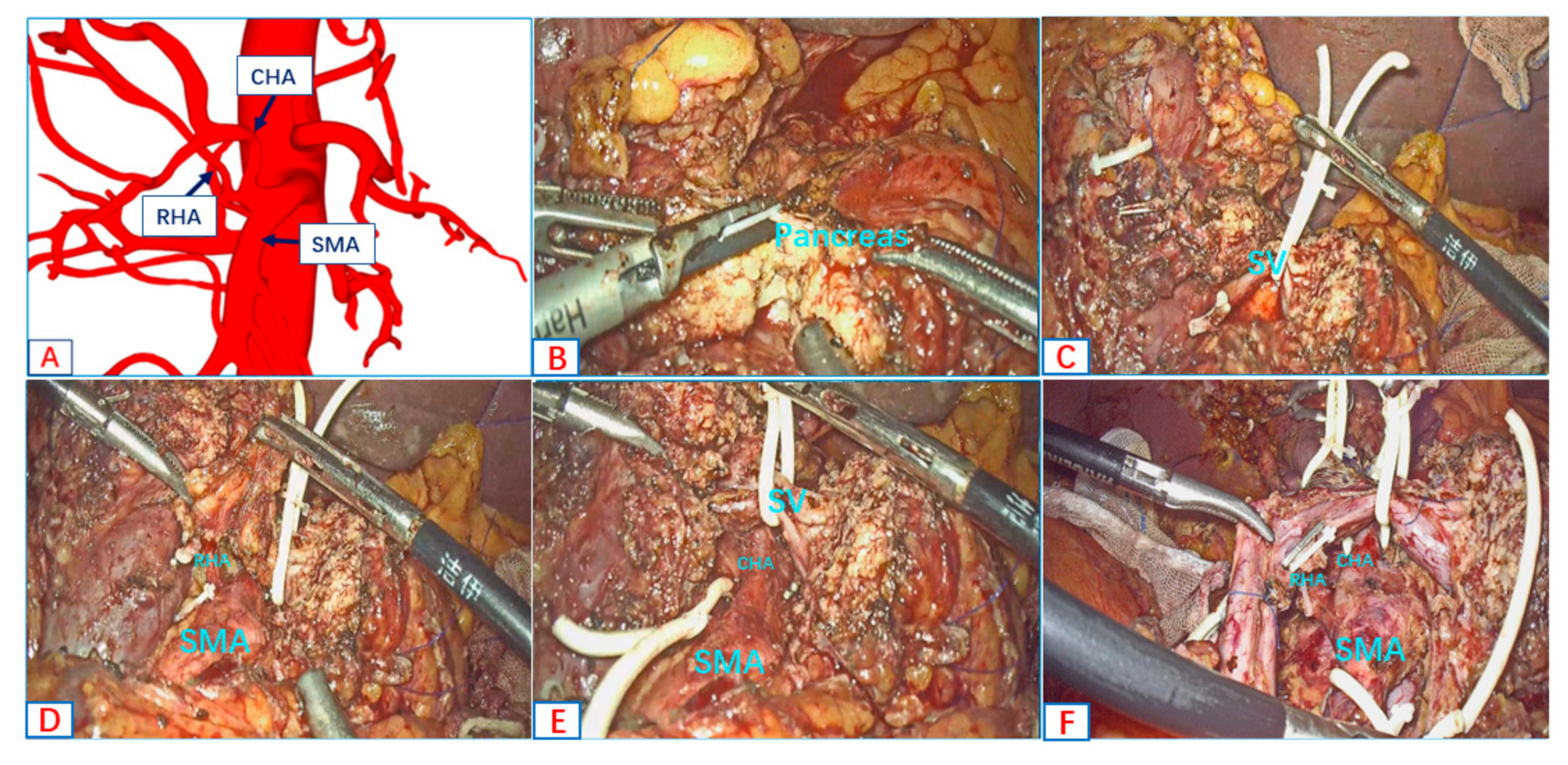
- Type C: Common hepatic artery and right hepatic artery originates from the SMA;
- Type D: Common hepatic artery originates from the SMA.
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, S.; Otsubo, T.; Koizumi, S.; Ariizumi, S.; Katagiri, S.; Watanabe, T.; Nakano, H.; Yamamoto, M. Anatomic variations of hepatic artery and new clinical classification based on abdominal angiographic images of 1200 cases. Hepatogastroenterology 2014, 61, 2345–2348. [Google Scholar] [PubMed]
- Miura, F.; Asano, T.; Amano, H.; Yoshida, M.; Toyota, N.; Wada, K.; Kato, K.; Hayano, K.; Kadowaki, S.; Shibuya, M.; et al. Eleven cases of postoperative hepatic infarction following pancreato-biliary surgery. J. Gastrointest. Surg. 2010, 14, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.S.; Birnbaum, B.; Jacobs, J.E. Hepatic infarction secondary to arterial insufficiency in native livers: CT findings in 10 patients. Radiology 1998, 208, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Kanazawa, S.; Hiraki, T.; Mimura, H.; Yasui, K.; Akaki, S.; Yagi, T.; Naomoto, Y.; Tanaka, N.; Hiraki, Y. Hepatic infarction following abdominal interventional procedures. Acta Med. Okayama 2004, 58, 97–106. [Google Scholar]
- Traverso, L.W.; Freeny, P. Pancreaticoduodenectomy. The importance of preserving hepatic blood flow to prevent biliary fistula. Am. Surg. 1989, 55, 421–426. [Google Scholar]
- Woods, M.S.; Traverso, L.W. Sparing a replaced common hepatic artery during pancreaticoduodenectomy. Am. Surg. 1993, 59, 719–721. [Google Scholar]
- Giani, A.; Mazzola, M.; Morini, L.; Zironda, A.; Bertoglio, C.L.; De Martini, P.; Magistro, C.; Ferrari, G. Hepatic vascular anomalies during totally laparoscopic pancreaticoduodenectomy: Challenging the challenge. Updates Surg. 2022, 74, 583–590. [Google Scholar] [CrossRef]
- Kim, J.H.; Gonzalez-Heredia, R.; Daskalaki, D.; Rashdan, M.; Masrur, M.; Giulianotti, P.C. Totally replaced right hepatic artery in pancreaticoduodenectomy: Is this anatomical condition a contraindication to minimally invasive surgery? HPB 2016, 18, 580–585. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Zenati, M.S.; Boone, B.A.; Steve, J.; Hogg, M.E.; Bartlett, D.L.; Zeh, H.J.; Zureikat, A.H. Robotic pancreaticoduodenectomy in the presence of aberrant or anomalous hepatic arterial anatomy: Safety and oncologic outcomes. HPB 2015, 17, 594–599. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Q.; Liu, S.; Zhang, W.; Ji, B.; Liu, Y. The Impact of Aberrant Hepatic Artery on Resection Margin and Outcomes of Laparoscopic Pancreatoduodenectomy: A Single-Center Report. World J. Surg. 2021, 45, 3183–3190. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Liu, S.; Wang, Y.; Liu, K.; Meng, L.; Chen, Q.; Jia, B.; Liu, Y. A single-center clinical study of hepatic artery variations in laparoscopic pancreaticoduodenectomy: A retrospective analysis of data from 218 cases. Medicine 2020, 99, e20403. [Google Scholar] [CrossRef]
- Pessaux, P.; Varma, D.; Arnaud, J. Pancreaticoduodenectomy: Superior mesenteric artery first approach. J. Gastrointest. Surg. 2006, 10, 607–611. [Google Scholar] [CrossRef]
- Butler, J.R.; Ahmad, S.A.; Katz, M.H.; Cioffi, J.L.; Zyromski, N.J. A systematic review of the role of periadventitial dissection of the superior mesenteric artery in affecting margin status after pancreatoduodenectomy for pancreatic adenocarcinoma. HPB 2016, 18, 305–311. [Google Scholar] [CrossRef]
- Peparini, N. Mesopancreas: A boundless structure, namely the rationale for dissection of the paraaortic area in pancreaticoduodenectomy for pancreatic head carcinoma. World J. Gastroenterol. 2015, 21, 2865–2870. [Google Scholar] [CrossRef]
- Bouassida, M.; Mighri, M.M.; Chtourou, M.F.; Sassi, S.; Touinsi, H.; Hajji, H.; Sassi, S. Retroportal lamina or mesopancreas? Lessons learned by anatomical and histological study of thirty three cadaveric dissections. Int. J. Surg. 2013, 11, 834–836. [Google Scholar] [CrossRef]
- Nappo, G.; Perinel, J.; El Bechwaty, M.; Adham, M. The Standardization of Pancreatoduodenectomy: Where Are We? Pancreas 2016, 45, 493–502. [Google Scholar] [CrossRef]
- Mangieri, C.W.; Valenzuela, C.D.; Erali, R.A.; Shen, P.; Howerton, R.; Clark, C.J. Prognostic Effect of Aberrant Right Hepatic Artery with Pancreaticoduodenectomy: Focus on Hepatic Recurrence. Ann. Surg. Oncol. 2022, 29, 3219–3228. [Google Scholar] [CrossRef]
- Mansour, S.; Damouny, M.; Obeid, M.; Farah, A.; Halloun, K.; Marjiyeh, R.; Ghalia, J.; Kluger, Y.; Khuri, S. Impact of Vascular Anomalies on Pancreatoduodenectomy Procedure. J. Clin. Med. Res. 2021, 13, 158–163. [Google Scholar] [CrossRef]
- Stauffer, J.A.; Bridges, M.D.; Turan, N.; Nguyen, J.H.; Martin, J.K. Aberrant right hepatic arterial anatomy and pancreaticoduodenectomy: Recognition, prevalence and management. HPB 2009, 11, 161–165. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, Y.-J.; Kim, C.-W.; Moon, K.-M.; Kim, M.-W. Clinical implications of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy. World J. Surg. 2009, 33, 1727–1732. [Google Scholar] [CrossRef]
- Rubio-Manzanares-Dorado, M.; Marín-Gómez, L.M.; Sanchez, D.A.; Suárez-Artacho, G.; Bernal-Bellido, C.; Serrano-Díaz-Canedo, J.; Álamo-Martínez, J.M.; Bravo, M.A.G.; Padillo-Ruiz, F.J. Implication of the presence of a variant hepatic artery during the Whipple procedure. Rev. Esp. Enferm. Dig. 2015, 107, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Velazquez, P.; Muller, X.; Malleo, G.; Park, J.; Hwang, H.; Napoli, N.; Javed, A.; Inoue, Y.; Beghdadi, N.; Kalisvaart, M.; et al. Benchmarks in Pancreatic Surgery: A Novel Tool for Unbiased Outcome Comparisons. Ann. Surg. 2019, 270, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ohigashi, H.; Ishikawa, O.; Eguchi, H.; Yamada, T.; Sasaki, Y.; Noura, S.; Takachi, K.; Miyashiro, I.; Murata, K.; Doki, Y.; et al. Early ligation of the inferior pancreaticoduodenal artery to reduce blood loss during pancreaticoduodenectomy. Hepatogastroenterology 2004, 51, 4–5. [Google Scholar] [PubMed]
- Okabayashi, T.; Shima, Y.; Sumiyoshi, T.; Kozuki, A.; Tokumaru, T.; Saisaka, Y. Right posterior approach for pancreaticoduodenectomy: A new technical approach. JOP 2015, 16, 41–44. [Google Scholar] [PubMed]
- Pedziwiatr, M.; Pisarska, M.; Małczak, P.; Major, P.; Wierdak, M.; Radkowiak, D.; Kulawik, J.; Dembiński, M.; Budzyński, A. Laparoscopic uncinate process first pancreatoduodenectomy-feasibility study of a modified ‘artery first’ approach to pancreatic head cancer. Langenbecks Arch. Surg. 2017, 402, 917–923. [Google Scholar] [CrossRef]
- Inoue, Y.; Saiura, A.; Yoshioka, R.; Ono, Y.; Takahashi, M.; Arita, J.; Takahashi, Y.; Koga, R. Pancreatoduodenectomy with Systematic Mesopancreas Dissection Using a Supracolic Anterior Artery-first Approach. Ann. Surg. 2015, 262, 1092–1101. [Google Scholar] [CrossRef]
- Sanjay, P.; Takaori, K.; Govil, S.; Shrikhande, S.V.; Windsor, J.A. ‘Artery-first’ approaches to pancreatoduodenectomy. Br. J. Surg. 2012, 99, 1027–1035. [Google Scholar] [CrossRef]
- Lupascu, C.; Moldovanu, R.; Andronic, D.; Ursulescu, C.; Vasiluţă, C.; Răileanu, G.; Fotea, V.; Târcoveanu, E. Posterior approach pancreaticoduodenectomy: Best option for hepatic artery anatomical variants. Hepatogastroenterology 2011, 58, 2112–2114. [Google Scholar]
- Noie, T.; Harihara, Y.; Inoue, K.; Kubota, K.; Takayama, T.; Makuuchi, M. Ventral dissection of replaced right hepatic artery during pancreatoduodenectomy. Hepatogastroenterology 2001, 48, 999–1000. [Google Scholar]
- Varty, P.P.; Yamamoto, H.; Farges, O.; Belghiti, J.; Sauvanet, A. Early retropancreatic dissection during pancreaticoduodenectomy. Am. J. Surg. 2005, 189, 488–491. [Google Scholar] [CrossRef]
- Okada, K.; Kawai, M.; Hirono, S.; Miyazawa, M.; Shimizu, A.; Kitahata, Y.; Tani, M.; Yamaue, H. A replaced right hepatic artery adjacent to pancreatic carcinoma should be divided to obtain R0 resection in pancreaticoduodenectomy. Langenbecks Arch. Surg. 2015, 400, 57–65. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kubota, K.; Rokkaku, K.; Nemoto, T.; Sakuma, A. Disposal of replaced common hepatic artery coursing within the pancreas during pancreatoduodenectomy: Report of a case. Surg. Today 2005, 35, 984–987. [Google Scholar] [CrossRef]
- Kim, P.T.; Temple, S.; Atenafu, E.G.; Cleary, S.P.; Moulton, C.-A.; McGilvray, I.D.; Gallinger, S.; Greig, P.D.; Wei, A.C. Aberrant right hepatic artery in pancreaticoduodenectomy for adenocarcinoma: Impact on resectability and postoperative outcomes. HPB 2014, 16, 204–211. [Google Scholar] [CrossRef]
- Loos, M.; Hackert, T.; Büchler, M.W. Arterial Resection in Pancreatic Cancer Surgery: Effective After a Learning Curve. Ann. Surg. 2022, 275, 759–768. [Google Scholar] [CrossRef]
| Clinical Data | Frequencies | Range/Percentage |
|---|---|---|
| Sex | ||
| Male | 16 | 66.67% |
| Female | 8 | 33.33% |
| Age (year) | 58.1 ± 12.1 | 51–75 |
| Aberrant hepatic arterial anatomy | ||
| rRHA | 10 | 41.67% |
| aRHA | 12 | 50.00% |
| CHA and aRHA | 1 | 4.17% |
| CHA | 1 | 4.17% |
| Disease | ||
| Pancreatic ductal adenocarcinoma | 14 | 58.33% |
| Cholangiocarcinoma | 4 | 16.67% |
| Adenocarcinoma of the duodenum | 2 | 8.33% |
| Ampullary carcinoma | 4 | 16.67% |
| Operation time (min) | 362 ± 60.43 | 325–510 |
| Blood loss (mL) | 256 ± 55.72 | 210–350 |
| Conversion to laparotomy | 0 | 0 |
| Postoperation ALT and AST | ||
| ALT (U/L) | 235 ± 25.65 | 184–276 |
| AST (U/L) | 180 ± 34.43 | 133–245 |
| Clavien III–IV complication | 0 | 0 |
| Postoperative pancreatic fistula | ||
| A | 6 | 25.00% |
| B | 4 | 16.67% |
| C | 0 | 0 |
| Tumor-free margins (mm) | 3.43 ± 0.78 | 2.7–4.3 |
| Standard LPD (n = 82) | AHAA-LPD (n = 24) | p Value | |
|---|---|---|---|
| BRPC | 8(9.8%) | 0(0%) | 0.112 |
| Neoadjuvant chemotherapy | 6(7.3%) | 0(0%) | 0.172 |
| OT | 338(280–396) | 344(330–388) | 0.173 |
| Blood loss | 500(375–800) | 250(222–281) | <0.001 |
| Overall complications | 44(53.6%) | 11(45.8%) | 0.500 |
| Clavien I–II complication | 38(46.3%) | 11(45.8%) | 0.965 |
| Clavien III–IV complication | 4(4.9%) | 0(0%) | 0.270 |
| 30-day mortality | 2(2.4%) | 0(0%) | 0.440 |
| POPF (A, B) | 27(32.9%) | 10(41.7%) | 0.430 |
| POPF (C) | 7(8.5%) | 0(0%) | 0.139 |
| DGE | 8(9.8%) | 3(12.5%) | 0.698 |
| BL | 4(4.9%) | 2(8.3%) | 0.519 |
| PH | 6(7.3%) | 1(4.2%) | 0.585 |
| PHS | 16(13–20) | 17(14–19) | 0.357 |
| Lymph node | 15(12–18) | 18(16–20) | <0.001 |
| R0 | 76(92.7%) | 24(100%) | 0.172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xu, J.; Lei, K.; You, K.; Liu, Z. Strategic Approach to Aberrant Hepatic Arterial Anatomy during Laparoscopic Pancreaticoduodenectomy: Technique with Video. J. Clin. Med. 2023, 12, 1965. https://doi.org/10.3390/jcm12051965
Wang J, Xu J, Lei K, You K, Liu Z. Strategic Approach to Aberrant Hepatic Arterial Anatomy during Laparoscopic Pancreaticoduodenectomy: Technique with Video. Journal of Clinical Medicine. 2023; 12(5):1965. https://doi.org/10.3390/jcm12051965
Chicago/Turabian StyleWang, Jiaguo, Jie Xu, Kai Lei, Ke You, and Zuojin Liu. 2023. "Strategic Approach to Aberrant Hepatic Arterial Anatomy during Laparoscopic Pancreaticoduodenectomy: Technique with Video" Journal of Clinical Medicine 12, no. 5: 1965. https://doi.org/10.3390/jcm12051965
APA StyleWang, J., Xu, J., Lei, K., You, K., & Liu, Z. (2023). Strategic Approach to Aberrant Hepatic Arterial Anatomy during Laparoscopic Pancreaticoduodenectomy: Technique with Video. Journal of Clinical Medicine, 12(5), 1965. https://doi.org/10.3390/jcm12051965





