Severe Microcytic Anemia Caused by Complex Hereditary Spherocytosis and X-Linked Sideroblastic Anemia with Mutations in SPTB and ALAS2 Genes
Abstract
1. Background
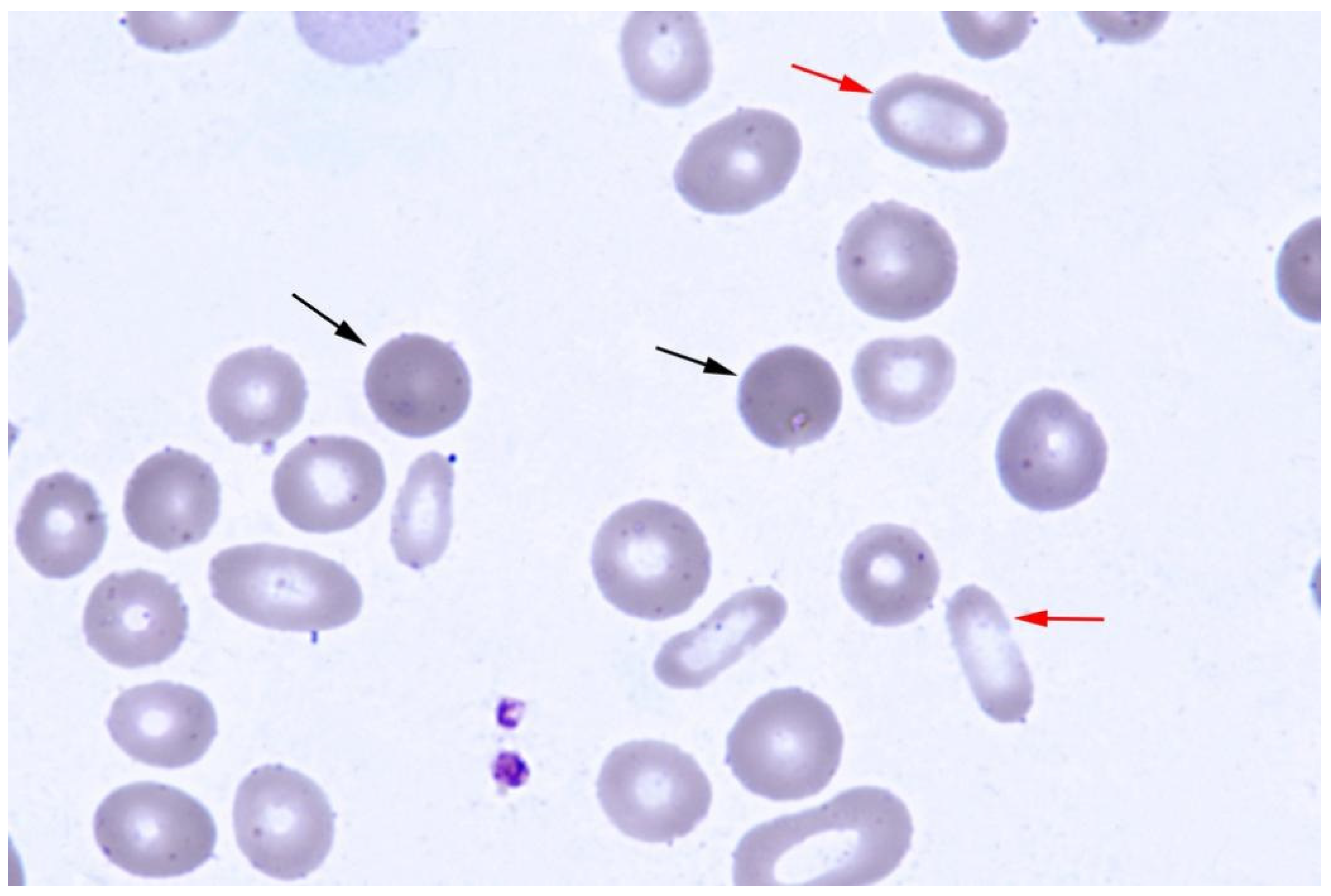
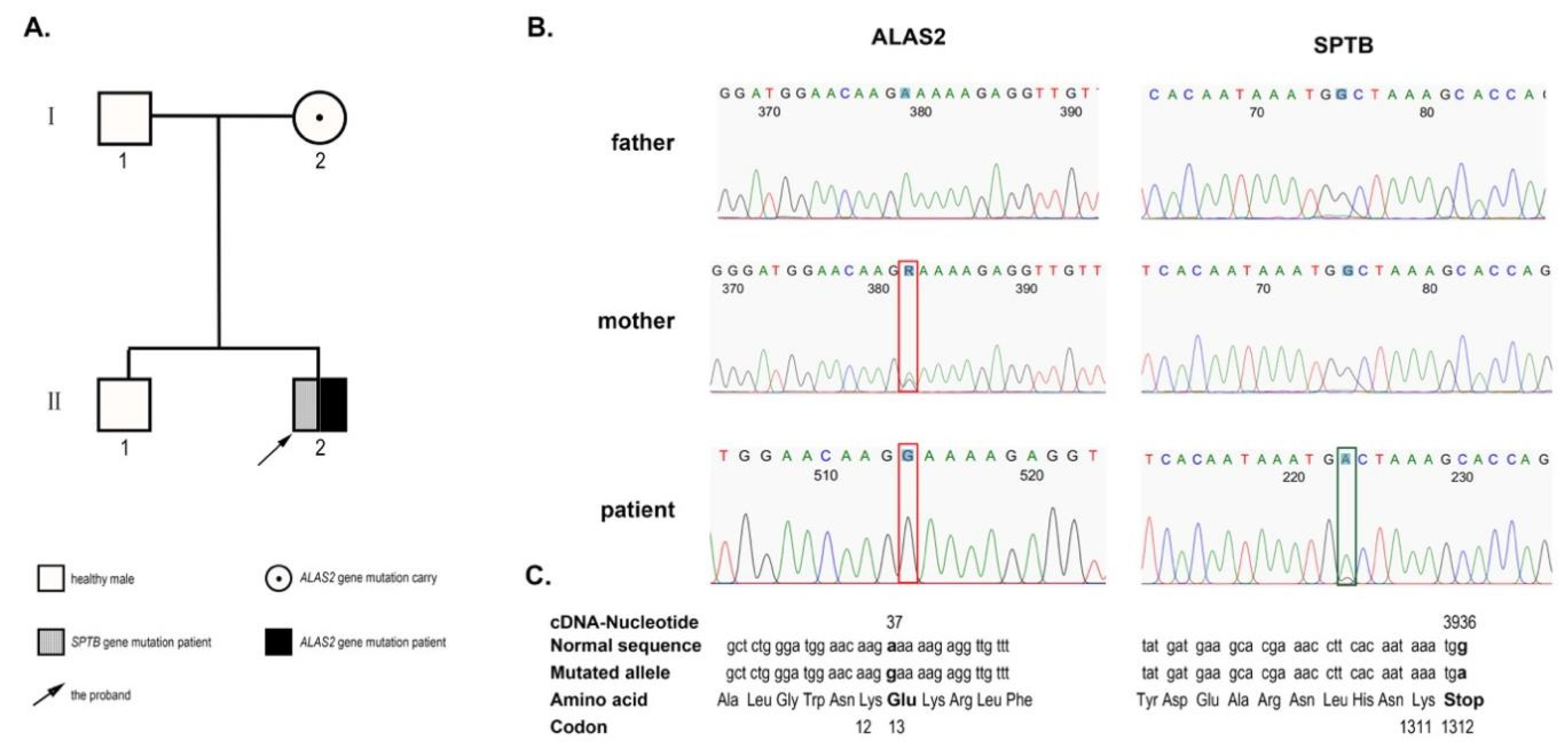
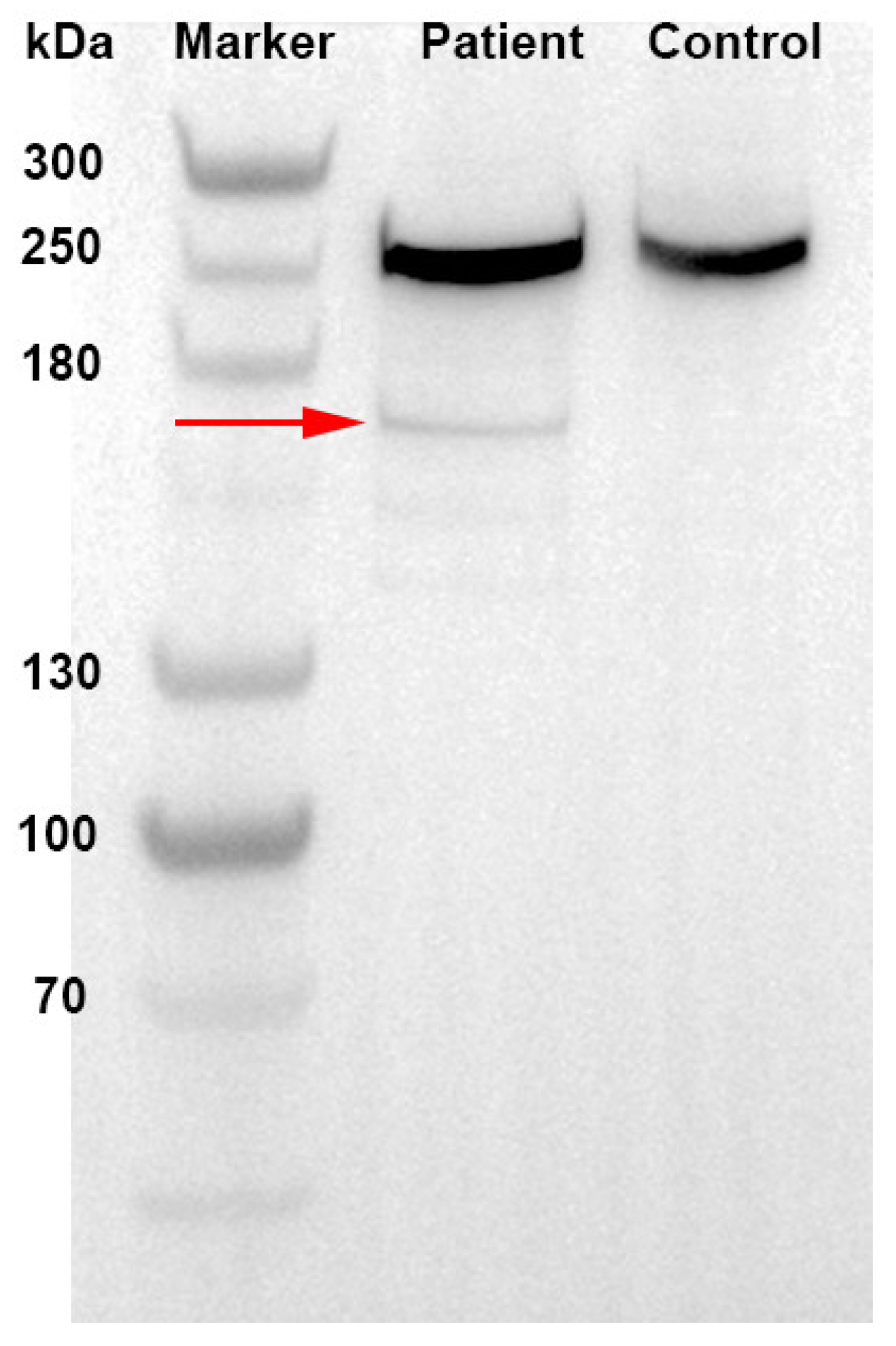
2. Case Presentation
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | autosomal dominant |
| ALAS | 5-aminolevulinic acid synthase |
| SPTA | spectrin, alpha |
| HS | hereditary spherocytosis |
| NGS | Next-generation sequencing |
| PLP | pyridoxal phosphate |
| RBCs | red blood cells |
| SPTB | spectrin, beta |
| sTfR | serum transferring receptor |
| XLSA | X-linked sideroblastic anemia |
References
- Costa, L.D.; Galimand, J.; Fenneteau, O.; Mohandas, N. Hereditary spherocytosis, elliptocytosis, and other red cell membrane disorders. Blood Rev. 2013, 27, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Ducamp, S.; Fleming, M.D. The molecular genetics of sideroblastic anemia. Blood 2019, 133, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; May, A.; Bergamaschi, G.; Cerani, P.; Rosti, V.; Bishop, D.F. Familial-skewed X-chromosome inactivation as a predisposing factor for late-onset X-linked sideroblastic anemia in carrier females. Blood 2000, 96, 4363–4365. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Bishop, D.F. The molecular biology and pyridoxine responsiveness of X-linked sideroblastic anaemia. Haematologica 1998, 83, 56–70. [Google Scholar] [PubMed]
- Shohet, S.B. Spectrin and spherocytosis. N. Engl. J. Med. 1982, 306, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, S.; Gallagher, P.G.; Mohandas, N. Hereditary spherocytosis. Lancet 2008, 372, 1411–1426. [Google Scholar] [CrossRef] [PubMed]
- Bennett, V.; Healy, J. Membrane domains based on ankyrin and spectrin associated with cell-cell interactions. Cold Spring Harb. Perspect. Biol. 2009, 1, a003012. [Google Scholar] [CrossRef] [PubMed]
- Fermo, E.; Vercellati, C.; Marcello, A.P.; Keskin, E.Y.; Perrotta, S.; Zaninoni, A.; Brancaleoni, V.; Zanella, A.; Giannotta, J.A.; Barcellini, W.; et al. Targeted Next Generation Sequencing and Diagnosis of Congenital Hemolytic Anemias: A Three Years Experience Monocentric Study. Front. Physiol. 2021, 12, 684569. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, H.; Vassiliadis, J.N.; Murray, J.; Njolstad, P.R.; Rogus, J.J.; Ballas, S.K.; Schaffer, F.; Jarolim, P.; Brabec, V.; Palek, J. Characterization of the underlying molecular defect in hereditary spherocytosis associated with spectrin deficiency. Blood 1997, 90, 398–406. [Google Scholar] [PubMed]
- Miraglia del Giudice, E.; Lombardi, C.; Francese, M.; Nobili, B.; Conte, M.L.; Amendola, G. Frequent de novo monoallelic expression of beta-spectrin gene (SPTB) in children with hereditary spherocytosis and isolated spectrin deficiency. Br. J. Haematol. 1998, 101, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Shao, S.M.; Liu, J.; Zeng, C.M.; Han, Y.; Zhang, X.R. Neonatal hereditary spherocytosis caused by a de novo frameshift mutation of the SPTB gene characterized by hydrops fetalis. Medicine 2021, 100, e24804. [Google Scholar] [CrossRef] [PubMed]
- Tole, S.; Dhir, P.; Pugi, J.; Drury, L.J.; Butchart, S.; Fantauzzi, M.; Langer, J.C.; Baker, J.M.; Blanchette, V.S.; Kirby-Allen, M.; et al. Genotype–phenotype correlation in children with hereditary spherocytosis. Br. J. Haematol. 2020, 191, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.X.; Yang, W.R.; Zhao, X.; Jin, L.P.; Zhang, L.; Zhou, K.; Li, Y.; Ye, L.; Li, J.P.; Fan, H.H.; et al. The characteristic of hereditary spherocytosis related gene mutation in 37 Chinese hereditary spherocytisis patients. Zhonghua Xue Ye Xue Za Zhi 2018, 39, 898–903. [Google Scholar] [PubMed]
- Park, J.; Jeong, D.C.; Yoo, J.; Jang, W.; Chae, H.; Kim, J.; Kwon, A.; Choi, H.; Lee, J.; Chung, N.-G.; et al. Mutational characteristics of ANK1 and SPTB genes in hereditary spherocytosis. Clin. Genet. 2016, 90, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Shoolingin-Jordan, P.M.; Al-Daihan, S.; Alexeev, D.; Baxter, R.L.; Bottomley, S.S.; Kahari, I.D.; Roy, I.; Sarwar, M.; Sawyer, L.; Wang, S.-F. 5-Aminolevulinic acid synthase: Mechanism, mutations and medicine. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2003, 1647, 361–366. [Google Scholar] [CrossRef]
- Ducamp, S.; Kannengiesser, C.; Touati, M.; Garcon, L.; Guerci-Bresler, A.; Guichard, J.F.-o.; Vermylen, C.; Dochir, J.; Poirel, H.A.; Fouyssac, F.; et al. Sideroblastic anemia: Molecular analysis of the ALAS2 gene in a series of 29 probands and functional studies of 10 missense mutations. Hum. Mutat. 2011, 32, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Aivado, M.; Gattermann, N.; Rong, A.; Giagounidis, A.A.; Prall, W.C.; Czibere, A.; Hildebrandt, B.; Haas, R.; Bottomley, S.S. X-linked sideroblastic anemia associated with a novel ALAS2 mutation and unfortunate skewed X-chromosome inactivation patterns. Blood Cells Mol. Dis. 2006, 37, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.C.; Sadlon, T.J.; Schwarz, Q.P.; Matthews, C.S.; Wise, P.D.; Cox, L.L.; Bottomley, S.S.; May, B.K. The major splice variant of human 5-aminolevulinate synthase-2 contributes significantly to erythroid heme biosynthesis. Int. J. Biochem. Cell. Biol. 2004, 36, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Harigae, H.; Furuyama, K. Hereditary sideroblastic anemia: Pathophysiology and gene mutations. Int. J. Hematol. 2010, 92, 425–431. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhang, H.; Qin, Y.; Liu, T. Severe Microcytic Anemia Caused by Complex Hereditary Spherocytosis and X-Linked Sideroblastic Anemia with Mutations in SPTB and ALAS2 Genes. J. Clin. Med. 2023, 12, 1990. https://doi.org/10.3390/jcm12051990
Zhou J, Zhang H, Qin Y, Liu T. Severe Microcytic Anemia Caused by Complex Hereditary Spherocytosis and X-Linked Sideroblastic Anemia with Mutations in SPTB and ALAS2 Genes. Journal of Clinical Medicine. 2023; 12(5):1990. https://doi.org/10.3390/jcm12051990
Chicago/Turabian StyleZhou, Jianying, Hang Zhang, Yao Qin, and Ting Liu. 2023. "Severe Microcytic Anemia Caused by Complex Hereditary Spherocytosis and X-Linked Sideroblastic Anemia with Mutations in SPTB and ALAS2 Genes" Journal of Clinical Medicine 12, no. 5: 1990. https://doi.org/10.3390/jcm12051990
APA StyleZhou, J., Zhang, H., Qin, Y., & Liu, T. (2023). Severe Microcytic Anemia Caused by Complex Hereditary Spherocytosis and X-Linked Sideroblastic Anemia with Mutations in SPTB and ALAS2 Genes. Journal of Clinical Medicine, 12(5), 1990. https://doi.org/10.3390/jcm12051990






