Cycle Characteristics and Pregnancy Outcomes of Early Rescue Intracytoplasmic Sperm Injection Cycles in Normal and Hyper-Ovarian Response Women: A Six-Year Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Controlled Ovarian Stimulation and Oocyte Retrieval
2.3. Sperm Preparation
2.4. ICSI Procedure
2.5. Short-Term IVF Procedure, Early Second PB Assessment, and Early r-ICSI
2.6. Fertilization Assessment, Embryo Quality Categorization, and Embryo Transfer
2.7. Endometrial Preparation Protocol for Frozen-Thawed Embryo Transfers
2.8. Assessment of Pregnancy Outcomes
2.9. Ethical Approval
2.10. Statistical Analysis
3. Results
3.1. The Baseline and Cycle Characteristics in the Different Insemination Groups
3.2. Embryonic Laboratory Outcomes in the Different Insemination Groups
3.3. The Pregnancy, Delivery, and Neonatal Outcomes in the Different Insemination Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neri, Q.V.; Lee, B.; Rosenwaks, Z.; Machaca, K.; Palermo, G.D. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium 2014, 55, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Yeh, J. Calcium Oscillatory Patterns and Oocyte Activation During Fertilization: A Possible Mechanism for Total Fertilization Failure (TFF) in Human In Vitro Fertilization? Reprod. Sci. 2021, 28, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Combelles, C.M.; Morozumi, K.; Yanagimachi, R.; Zhu, L.; Fox, J.H.; Racowsky, C. Diagnosing cellular defects in an unexplained case of total fertilization failure. Hum. Reprod. 2010, 25, 1666–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.N.; Sasson, I.E.; Sammel, M.D.; Dokras, A. Does intracytoplasmic sperm injection improve the fertilization rate and decrease the total fertilization failure rate in couples with well-defined unexplained infertility? A systematic review and meta-analysis. Fertil. Steril. 2013, 100, 704–711. [Google Scholar] [CrossRef]
- Zagadailov, P.; Seifer, D.B.; Shan, H.; Zarek, S.M.; Hsu, A.L. Do state insurance mandates alter ICSI utilization? Reprod. Biol. Endocrinol.: RBE 2020, 18, 33. [Google Scholar] [CrossRef] [Green Version]
- Boulet, S.L.; Mehta, A.; Kissin, D.M.; Warner, L.; Kawwass, J.F.; Jamieson, D.J. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. Jama 2015, 313, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Dang, V.Q.; Vuong, L.N.; Luu, T.M.; Pham, T.D.; Ho, T.M.; Ha, A.N.; Truong, B.T.; Phan, A.K.; Nguyen, D.P.; Pham, T.N.; et al. Intracytoplasmic sperm injection versus conventional in-vitro fertilisation in couples with infertility in whom the male partner has normal total sperm count and motility: An open-label, randomised controlled trial. Lancet 2021, 397, 1554–1563. [Google Scholar] [CrossRef]
- Abbas, A.M.; Hussein, R.S.; Elsenity, M.A.; Samaha, I.I.; El Etriby, K.A.; Abd El-Ghany, M.F.; Khalifa, M.A.; Abdelrheem, S.S.; Ahmed, A.A.; Khodry, M.M. Higher clinical pregnancy rate with in-vitro fertilization versus intracytoplasmic sperm injection in treatment of non-male factor infertility: Systematic review and meta-analysis. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101706. [Google Scholar] [CrossRef]
- Beck-Fruchter, R.; Lavee, M.; Weiss, A.; Geslevich, Y.; Shalev, E. Rescue intracytoplasmic sperm injection: A systematic review. Fertil. Steril. 2014, 101, 690–698. [Google Scholar] [CrossRef]
- Ming, L.; Liu, P.; Qiao, J.; Lian, Y.; Zheng, X.; Ren, X.; Huang, J.; Wu, Y. Synchronization between embryo development and endometrium is a contributing factor for rescue ICSI outcome. Reprod. Biomed. Online 2012, 24, 527–531. [Google Scholar] [CrossRef] [Green Version]
- Ethics Committee of the American Society for Reproductive Medicine. Fertility treatment when the prognosis is very poor or futile. Fertil. Steril. 2009, 92, 1194–1197. [Google Scholar] [CrossRef]
- Chen, C.; Kattera, S. Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF. Hum. Reprod. 2003, 18, 2118–2121. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef]
- An, B.G.L.; Chapman, M.; Tilia, L.; Venetis, C. Is there an optimal window of time for transferring single frozen-thawed euploid blastocysts? A cohort study of 1170 embryo transfers. Hum. Reprod. 2022, 37, 2797–2807. [Google Scholar] [CrossRef]
- Qi, Q.; Luo, J.; Wang, Y.; Xie, Q. Effects of artificial cycles with and without gonadotropin-releasing hormone agonist pretreatment on frozen embryo transfer outcomes. J. Int. Med. Res. 2020, 48, 300060520918474. [Google Scholar] [CrossRef]
- Lewin, J.; Lukaszewski, T.; Sangster, P.; Williamson, E.; McEleny, K.; Al Wattar, B.H.; Yasmin, E. Reproductive outcomes following surgical sperm retrieval in couples with male factor subfertility: A 10-year retrospective national cohort. Fertil. Steril. 2022. [Google Scholar] [CrossRef]
- Practice Committees of the American Society for Reproductive Medicine. Society for Assisted Reproductive, T. Intracytoplasmic sperm injection (ICSI) for non-male factor infertility: A committee opinion. Fertil. Steril. 2012, 98, 1395–1399. [Google Scholar] [CrossRef]
- Paffoni, A.; Reschini, M.; Pisaturo, V.; Guarneri, C.; Palini, S.; Vigano, P. Should rescue ICSI be re-evaluated considering the deferred transfer of cryopreserved embryos in in-vitro fertilization cycles? A systematic review and meta-analysis. Reprod. Biol. Endocrinol.: RBE 2021, 19, 121. [Google Scholar] [CrossRef]
- Xiong, F.; Sun, Q.; Li, G.; Yao, Z.; Chen, P.; Wan, C.; Zhong, H.; Zeng, Y. Perinatal and neonatal outcomes of pregnancies after early rescue intracytoplasmic sperm injection in women with primary infertility compared with conventional intracytoplasmic sperm injection: A retrospective 6-year study. BMC Pregnancy Childbirth 2020, 20, 460. [Google Scholar] [CrossRef]
- Huang, B.; Qian, K.; Li, Z.; Yue, J.; Yang, W.; Zhu, G.; Zhang, H. Neonatal outcomes after early rescue intracytoplasmic sperm injection: An analysis of a 5-year period. Fertil. Steril. 2015, 103, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Ma, S.; Li, C.; Liu, H.; Zhao, H.; Li, L.J.; Li, Q.; Li, M. Intracytoplasmic Sperm Injection May Not Improve Clinical Outcomes Despite Its Positive Effect on Embryo Results: A Retrospective Analysis of 1130 Half-ICSI Treatments. Front. Endocrinol. 2022, 13, 877471. [Google Scholar] [CrossRef] [PubMed]
- Foong, S.C.; Fleetham, J.A.; O’Keane, J.A.; Scott, S.G.; Tough, S.C.; Greene, C.A. A prospective randomized trial of conventional in vitro fertilization versus intracytoplasmic sperm injection in unexplained infertility. J. Assist. Reprod. Genet. 2006, 23, 137–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pehlivan, T.; Rubio, C.; Ruiz, A.; Navarro, J.; Remohi, J.; Pellicer, A.; Simon, C. Embryonic chromosomal abnormalities obtained after rescue intracytoplasmic sperm injection of 1-day-old unfertilized oocytes. J. Assist. Reprod. Genet. 2004, 21, 55–57. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.H.; Son, W.Y.; Henderson, S.; Mahfoudh, A.; Dahan, M.; Holzer, H. Spindle examination in unfertilized eggs using the polarization microscope can assist rescue ICSI. Reprod. Biomed. Online 2013, 26, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Steward, R.G.; Lan, L.; Shah, A.A.; Yeh, J.S.; Price, T.M.; Goldfarb, J.M.; Muasher, S.J. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: An analysis of 256,381 in vitro fertilization cycles. Fertil. Steril. 2014, 101, 967–973. [Google Scholar] [CrossRef]
- Ma, C.; Xu, H.; Wang, H.; Feng, G.; Han, Y.; Alpadi, K.; Li, R.; Qiao, J. An online tool for predicting ovarian responses in unselected patients using dynamic inhibin B and basal antimullerian hormone levels. Front. Endocrinol. 2023, 14, 1074347. [Google Scholar] [CrossRef]
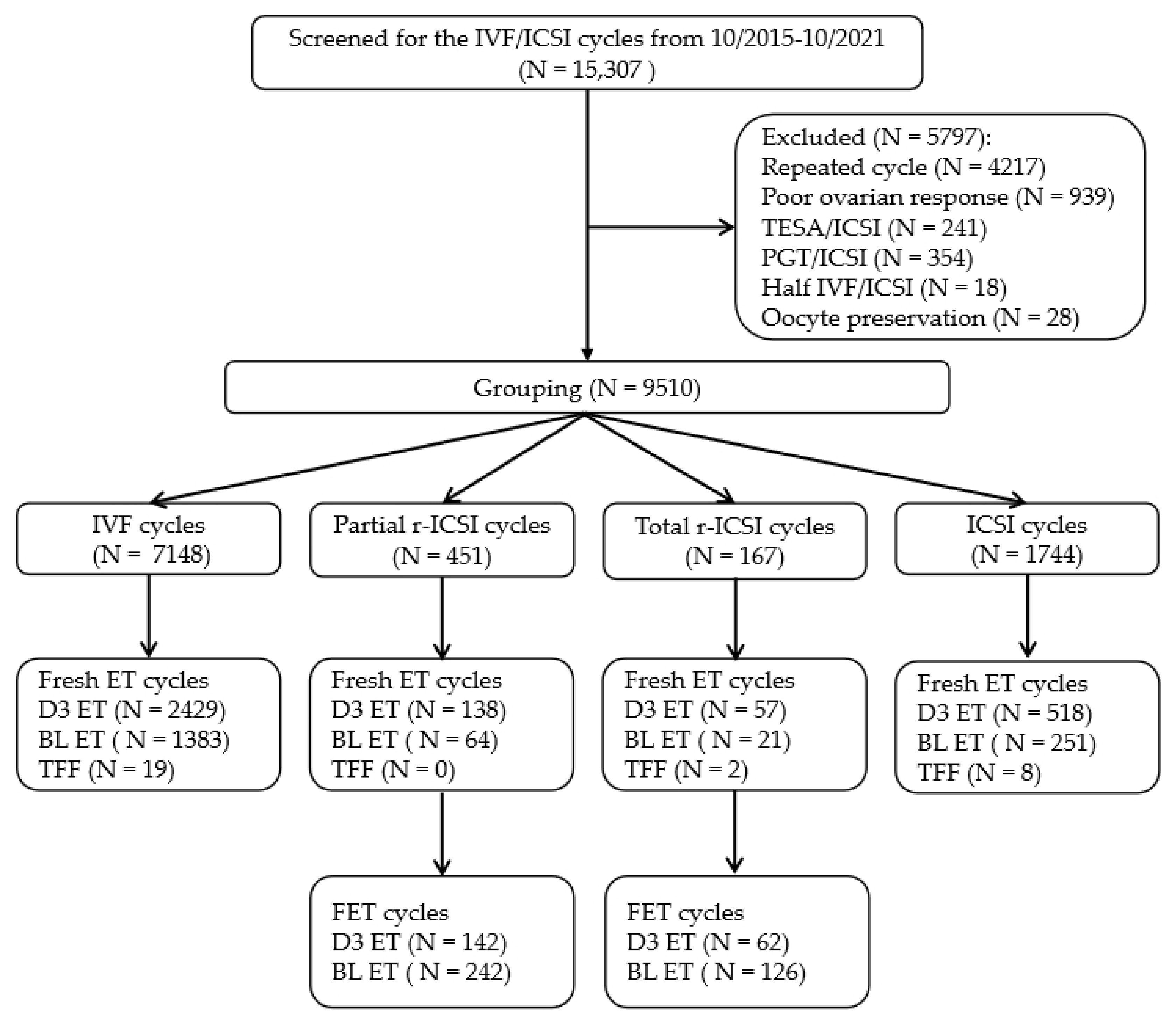

| Short-Term IVF | Partial r-ICSI | Total r-ICSI | ICSI | p-Value | |
|---|---|---|---|---|---|
| Cycles | 7148 | 451 | 167 | 1744 | |
| Maternal age (years) | 31.61 ± 3.94 a | 30.9 ± 4.00 b | 31.92 ± 4.06 a | 31.45 ± 4.44 a | <0.01 |
| Paternal age (years) | 33.68 ± 4.82 a | 33.74 ± 4.85 a | 33.88 ± 4.75 a | 34.49 ± 5.08 b | <0.01 |
| BMI (kg/m2) | 22.19 ± 3.94 | 22.1 ± 4.01 | 22.37 ± 4.06 | 22.08 ± 4.44 | NS |
| AMH (ng/mL) | 3.89 ± 2.85 a | 4.55 ± 2.89 b | 4.10 ± 4.63 a | 3.78 ± 2.61 a | <0.01 |
| Infertility duration | 3.24 ± 2.41 a | 3.78 ± 2.28 b | 4.28 ± 3.17 b | 3.44 ± 2.51 a | <0.01 |
| Infertility type, n (%) | <0.01 | ||||
| Primary infertility | 3419 (47.83) | 296 (65.63) | 121(72.46) | 999 (57.28) | |
| Secondary infertility | 3729 (52.17) | 155 (34.37) | 46 (27.54) | 745 (42.72) | |
| Infertility diagnosis, n (%) | <0.01 | ||||
| Tubal factor | 4475 (62.60) | 194 (43.02) | 78 (46.71) | / | |
| Ovulation dysfunction | 1302 (18.21) | 107 (23.73) | 30 (17.96) | / | |
| Endometriosis | 823 (11.51) | 45 (9.98) | 11 (6.59) | / | |
| Male factor | 128 (1.79) | 21 (4.66) | 4 (2.4) | 1744 | |
| Unexplained infertility | 420 (5.88) | 84 (18.58) | 44 (26.35) | / | |
| COH protocols, n (%) | NS | ||||
| Follicular-phase GnRHa | 3064 (42.87) | 195 (43.24) | 69 (41.32) | 731 (41.92) | |
| Luteal-phase GnRHa | 1943 (27.18) | 112 (24.83) | 44 (26.35) | 444 (25.46) | |
| GnRH antagonist | 1991 (27.85) | 123 (27.27) | 42 (25.15) | 460 (26.38) | |
| PPOS | 420 (5.88) | 21 (4.66) | 12 (7.19) | 109 (6.25) | |
| Total Gn dosage (IU) | 2105 ± 757 | 2012 ± 710 | 2115 ± 724 | 2131 ± 717 | NS |
| Gn stimulation days | 10.64 ± 2.2 | 10.63 ± 2.25 | 10.67 ± 2.21 | 10.64 ± 2.19 | NS |
| Estradiol on hCG day (pg/mL) | 3428 ± 2194 a | 3729 ± 2129 b | 3163 ± 1683 a | 3326 ± 1828 a | <0.01 |
| Progesterone on hCG day (ng/mL) | 1.02 ± 0.53 | 1.08 ± 0.53 | 1.07 ± 0.66 | 1.05 ± 0.62 | 0.06 |
| LH on hCG day (IU/L) | 1.64 ± 1.77 | 1.5 ± 2.6 | 1.55 ± 1.62 | 1.65 ± 1.7 | NS |
| Short-Term IVF | Partial r-ICSI | Total r-ICSI | ICSI | p Value | |
|---|---|---|---|---|---|
| Cycles | 7148 | 451 | 167 | 1744 | |
| No. of oocyte retrieval | 14.23 ± 7.26 a | 15.27 ± 6.54 b | 12.45 ± 5.79 c | 14.03 ± 6.89 a | <0.001 |
| 2PN rate | 62.03 ± 20.48 a | 57.75 ± 18.63 b | 65.31 ± 23.09 a | 72.61 ± 20.31 c# | <0.001 |
| ≥3PN rate | 8.94 ± 10.26 a | 3.15 ± 5.88 b | 2.19 ± 4.12 c | 1.50 ± 6.23 c | <0.001 |
| 1PN rate | 3.58 ± 6.23 | 3.72 ± 4.78 | 3.99 ± 6.79 | 3.26 ± 6.15 | NS |
| No. of TQE on D3 | 4.58 ± 4.07 a | 4.03 ± 3.38 b | 4.50 ± 3.18 ab | 4.13 ± 3.57 b | <0.001 |
| Rate of TQE on D3 | 57.59 ± 28.34 a | 52.02 ± 30.25 b | 55.93 ± 30.63 ab | 52.92 ± 31.48 b | <0.001 |
| BL rate | 58.63 ± 25.31 a | 55.18 ± 25.91 b | 58.85 ± 24.08 ab | 55.51 ± 27.72 b | <0.001 |
| Top-quality BL rate | 48.51 ± 24.06 a | 45.1 ± 23.34 b | 47.47 ± 25.07 ab | 44.68 ± 25.79 b | <0.001 |
| No. of D5-BL | 2.44 ± 2.26 a | 1.61 ± 1.77 b | 1.52 ± 1.85 b | 1.98 ± 2.14 d | <0.001 |
| No. of D6-BL | 0.83 ± 1.34 a | 1.23 ± 1.31b | 1.46 ± 1.38 b | 0.89 ± 1.33 a | <0.001 |
| Fresh embryo transfers, n (%) | 3812 (53.33) a | 202 (44.79) ab | 78 (46.71) ab | 769 (44.09) b | <0.001 |
| Cleavage-stage ET | 2429 (63.72) | 138 (68.32) | 57 (73.08) | 518 (67.36) | NS |
| Blastocyst ET | 1383 (36.28) | 64 (31.68) | 21 (26.92) | 251 (32.64) | |
| Freeze-all cycle | 3165 (44.28) a | 236 (52.33) b | 79 (47.31) ab | 897 (51.43) b | <0.001 |
| TFF cycle | 19 (0.27) | 0 (/) | 2 (1.20) | 8 (0.46) | NS |
| Cycle without available embryo, n (%) | 152 (2.13) a | 13 (2.88) a | 8 (4.79) b | 70 (4.01) b | <0.001 |
| Short-Term IVF | Partial r-ICSI | Total r-ICSI | ICSI | p-Value | |
|---|---|---|---|---|---|
| Fresh cleavage-stage ETs | 2429 | 138 | 57 | 518 | |
| No. of embryos transferred | 1.84 ± 0.37 | 1.86 ± 0.33 | 1.89 ± 0.36 | 1.86 ± 0.33 | NS |
| Clinical pregnancy rate (% per ET) | 51.96 (1262/2429) | 55.80 (77/138) | 64.91 (37/57) | 50.58 (262/518) | NS |
| Implantation rate (% per ET embryos) | 34.08 (1640/4812) | 35.53 (97/273) | 43.36 (49/113) | 32.20 (332/1031) | NS |
| Pregnancy loss rate (% per CP) | 14.42 (182/1262) | 6.49 (5/77) | 8.16 (4/37) | 16.41 (43/262) | NS |
| Ectopic pregnancy rate (% per CP) | 2.06 (26/1262) | 1.30 (1/77) | 3.51 (2/37) | 1.53 (4/262) | NS |
| Live birth rate (% per ET) | 43.31 (1052/2429) | 51.45 (71/138) | 54.39 (31/57) | 41.51 (215/518) | NS |
| Preterm birth rate (% per LB) | 21.67 (228/1052) | 18.31 (13/71) | 22.58 (7/31) | 18.14 (39/215) | NS |
| C-section rate (% per LB) | 78.99 (831/1052) | 67.61 (48/71) | 77.42 (24/31) | 75.81 (163/215) | NS |
| Twin birth rate (% per LB) | 30.99 (326/1052) | 25.35 (18/71) | 32.26 (10/31) | 22.79 (49/215) | NS |
| Sex ratio (Male: female) | 1.15 a (738:640) | 0.89 b (42:47) | 0.41 b (12:29) | 0.83 b (120:144) | 0.002 |
| Mean birth weight, g | 3043 ± 716 | 3025 ± 685 | 3051 ± 692 | 3029 ± 722 | NS |
| LBW (% per neonate) | 4.21 (58/1378) | 4.49 (4/89) | 4.88 (2/41) | 4.55 (12/264) | NS |
| VLBW (% per neonate) | 0.58 (8/1378) | / (0/89) | 0 (0/41) | 0.758 (2/264) | NS |
| Short-Term IVF | Partial r-ICSI | Total r-ICSI | ICSI | p-Value | |
|---|---|---|---|---|---|
| Blastocyst ETs | 1383 | 64 | 21 | 251 | |
| No. of embryos transferred | 1.33 ± 0.47 | 1.41 ± 0.48 | 1.38 ± 0.48 | 1.35 ± 0.42 | NS |
| Clinical pregnancy rate (% per ET) | 59.22 a (819/1383) | 26.56 b (17/64) | 33.33 b (7/21) | 58.96 a (148/251) | <0.001 |
| Implantation rate (% per ET) | 45.08 a (929/2061) | 21.11 b (19/90) | 31.25 b (10/32) | 49.47 a (187/378) | <0.001 |
| Pregnancy loss rate (% per CP) | 8.67 (71/819) | 11.76 (2/17) | 28.57 (2/7) | 9.46 (14/148) | NS |
| Ectopic pregnancy rate (% per CP) | 2.08 (17/819) | / (0/17) | / (0/7) | 2.03 (3/148) | NS |
| Live birth rate (% per ET) | 50.69 a (701/1383) | 23.43 b (15/64) | 23.81 b (5/21) | 51.79 a (130/251) | <0.001 |
| Preterm birth rate (% per LB) | 8.13 a (57/701) | / ab (0/15) | 20.00 ab (1/5) | 14.62 b (19/130) | 0.05 |
| C-section rate (% per LB) | 75.61 (530/701) | 80.00 (12/15) | 80.00 (4/5) | 75.38 (98/130) | NS |
| Twin birth rate (% per LB) | 18.54 (130/701) | 6.67 (1/15) | 20.00 (1/5) | 26.92 (35/130) | NS |
| Sex ratio (Male:female) | 1.16 (447:384) | 1.29 (9:7) | / (6:0) | 1.04 (84:81) | NS |
| Mean birth weight, g | 3128 ± 746 | 3105 ± 728 | 3119 ± 732 | 3095 ± 743 | NS |
| LBW (% per neonates) | 4.09 (34/831) | 6.25 (1/16) | 0 (0/6) | 6.06 (10/165) | NS |
| VLBW (% per neonates) | 0.48 (4/831) | / (0/16) | 0 (0/6) | 0.61 (1/165) | NS |
| Partial r-ICSI | Total r-ICSI | p-Value | |||
|---|---|---|---|---|---|
| Cleavage-Stage | Blastocyst | Cleavage-Stage | Blastocyst | ||
| Total cycles of FET with transfers | 142 | 242 | 62 | 126 | |
| Age at transfer (years) | 32.22 ± 4.16 | 31.14 ±4.08 | 32.26 ± 4.34 | 31.75 ± 4.14 | NS |
| Number of FET transfers, n (%) | NS | ||||
| 1st cycle of FET | 103 (72.54) | 200 (82.64) | 46 (74.19) | 108 (85.71) | |
| 2nd cycle of FET | 30 (21.13) | 35 (14.46) | 14 (22.58) | 16 (12.70) | |
| 3rd cycle of FET | 9 (6.34) | 6 (2.48) | 2 (3.23) | 2 (1.59) | |
| 4th cycle of FET | 0 (/) | 1 (0.41) | 0 (/) | 0 (/) | |
| Endometrial preparation, n (%) | |||||
| Natural cycle | 37 (26.06) | 60 (24.79) | 14 (22.58) | 35 (27.78) | |
| HRT cycle | 64 (45.07) | 103 (42.56) | 28 (45.16) | 48 (38.10) | |
| GnRHa + HRT cycle | 41 (28.87) | 79 (32.64) | 18 (29.03) | 43 (34.13) | |
| No. of embryos transferred | 1.88 ± 0.39 a | 1.32 ± 0.45 b | 1.87 ± 0.38 a | 1.36 ± 0.44 b | <0.001 |
| Implantation rate (% per ET embryos) | 30.58 a (85/278) | 49.42 b (170/344) | 32.77 a (39/119) | 51.12 b (91/178) | <0.001 |
| Clinical pregnancy rate (% per ET) | 50.00 (71/142) | 57.02 (138/242) | 53.23 (33/62) | 58.73 (74/126) | NS |
| Pregnancy loss rate (% per CP) | 4.22 (3/71) | 10.14 (14/138) | 6.06 (2/33) | 9.46 (7/74) | NS |
| Ectopic pregnancy rate (% per CP) | 1.41 (1/71) | 2.17 (3/138) | 3.03 (1/33) | 1.35 (1/74) | NS |
| Live birth rate (% per ET) | 46.48 (66/142) | 50.00 (121/242) | 48.39 (30/62) | 52.38 (66/126) | NS |
| Preterm birth rate (% per LB) | 7.58 (5/66) | 9.09 (11/121) | 10.00 (3/30) | 10.61 (7/66) | NS |
| C-section rate (% per LB) | 77.27 (51/66) | 67.77 (82/121) | 73.33 (22/30) | 75.76 (50/66) | NS |
| Twin birth rate (% per LB) | 21.21 (14/66) | 23.14 (28/121) | 23.33 (7/30) | 25.76 (17/66) | NS |
| Sex ratio (Male:female) | 0.86 (37:43) | 0.99 (74:75) | 0.94 (18:19) | 1.05 (42:40) | NS |
| Mean birth weight, g | 3089 ± 695 | 3125 ± 713 | 3105 ± 709 | 3133 ± 724 | NS |
| LBW (% per neonates) | 3.75 (3/80) | 4.70 (7/149) | 2.70 (7/37) | 3.66 (3/82) | NS |
| VLBW (% per neonates) | 1.25 (1/80) | 0.67 (1/149) | / (0/37) | / (0/82) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhou, H.; Liu, X.; Zhao, J.; Qi, Q.; Xie, Q. Cycle Characteristics and Pregnancy Outcomes of Early Rescue Intracytoplasmic Sperm Injection Cycles in Normal and Hyper-Ovarian Response Women: A Six-Year Retrospective Study. J. Clin. Med. 2023, 12, 1993. https://doi.org/10.3390/jcm12051993
Chen L, Zhou H, Liu X, Zhao J, Qi Q, Xie Q. Cycle Characteristics and Pregnancy Outcomes of Early Rescue Intracytoplasmic Sperm Injection Cycles in Normal and Hyper-Ovarian Response Women: A Six-Year Retrospective Study. Journal of Clinical Medicine. 2023; 12(5):1993. https://doi.org/10.3390/jcm12051993
Chicago/Turabian StyleChen, Liang, Hanjing Zhou, Xueli Liu, Jing Zhao, Qianrong Qi, and Qingzhen Xie. 2023. "Cycle Characteristics and Pregnancy Outcomes of Early Rescue Intracytoplasmic Sperm Injection Cycles in Normal and Hyper-Ovarian Response Women: A Six-Year Retrospective Study" Journal of Clinical Medicine 12, no. 5: 1993. https://doi.org/10.3390/jcm12051993





