PD-1 and PD-L1 Expression Levels as a Potential Biomarker of Chronic Rhinosinusitis and Head and Neck Cancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Patients
- I.
- Control group—102 healthy subjects with deviations of the nasal septum (DSN) with no features of cancers, chronic paranasal sinusitis or inflammatory diseases;
- II.
- Study group—162 patients with CRSwNP;
- III.
- Study group—40 patients with HNC.
2.3. Materials
2.4. Methods
2.4.1. Total RNA Extraction and cDNA Generation
2.4.2. Real-Time PCR
2.4.3. Western Blot Analysis
2.5. Statistics
3. Results
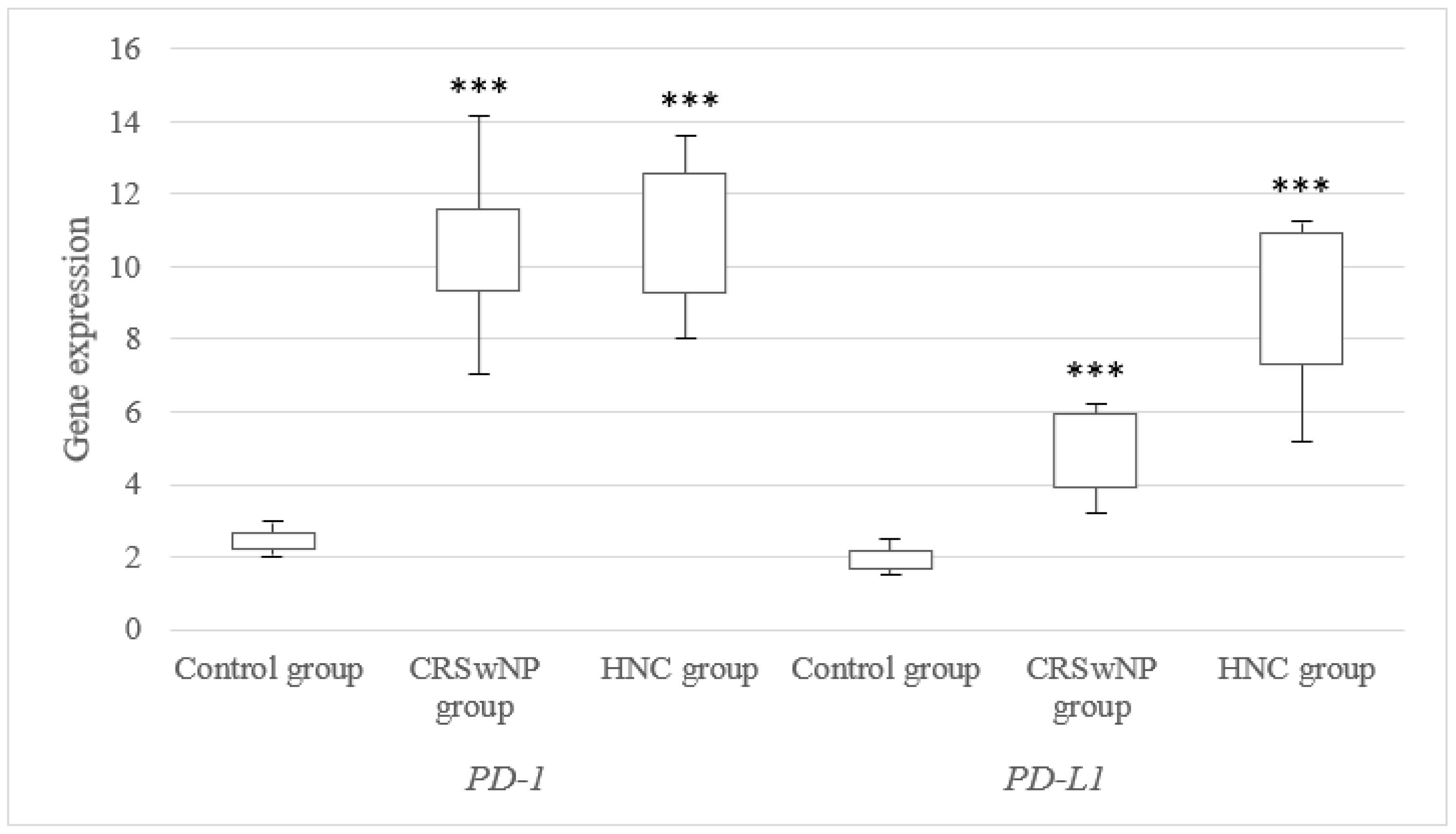
3.1. mRNA Expression Level of the PD-1 and PD-L1 Genes in Tissues from CRSwNP and NHC Patients
3.2. mRNA Expression Level of the PD-1 and PD-L1 Genes in the Different Subgroups of CRSwNP and HNC Patients
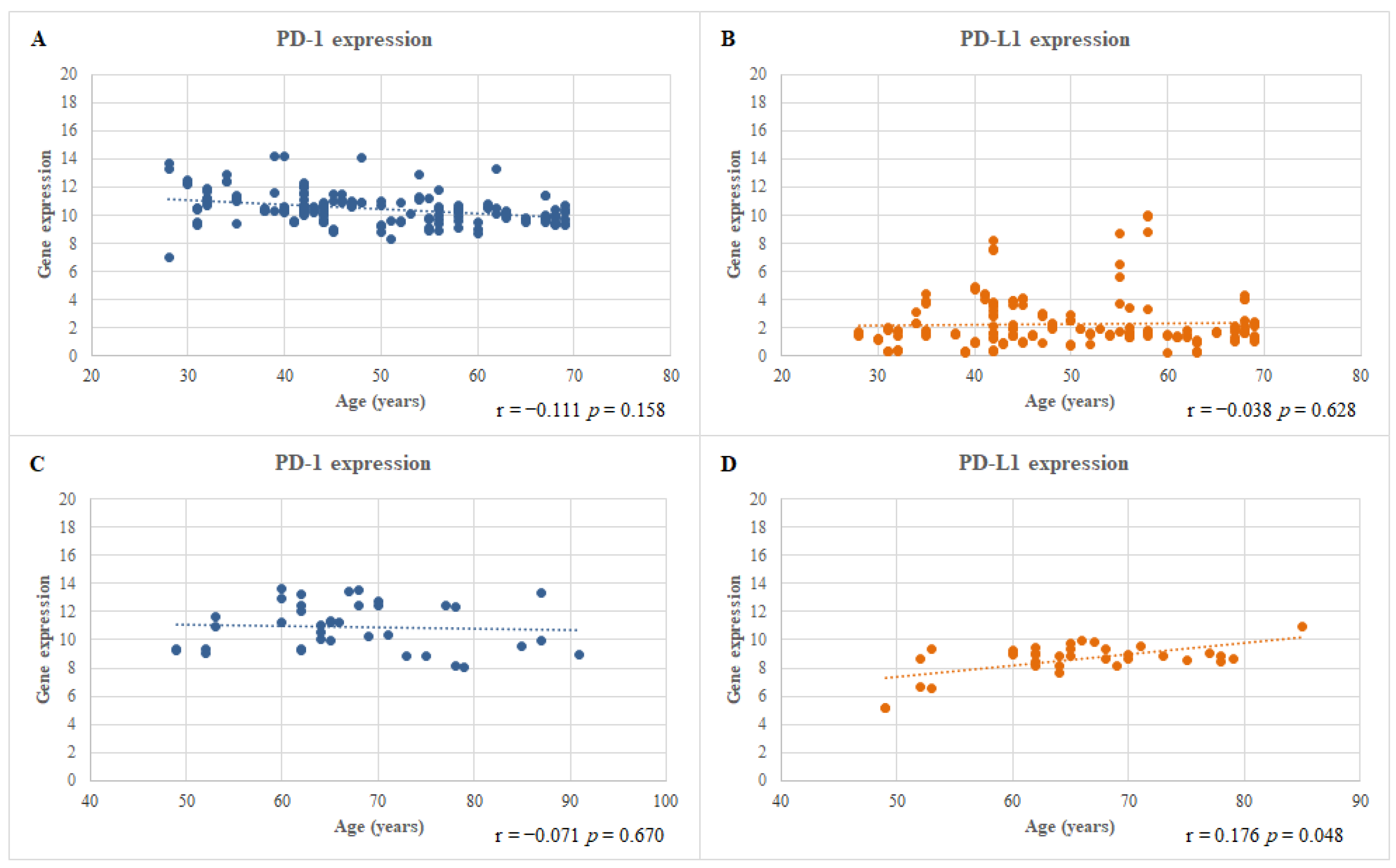
3.3. Correlation between the mRNA Expression Level of the PD-1 and PD-L1 Genes and the Ages of the CRSwNP and HNC Patients
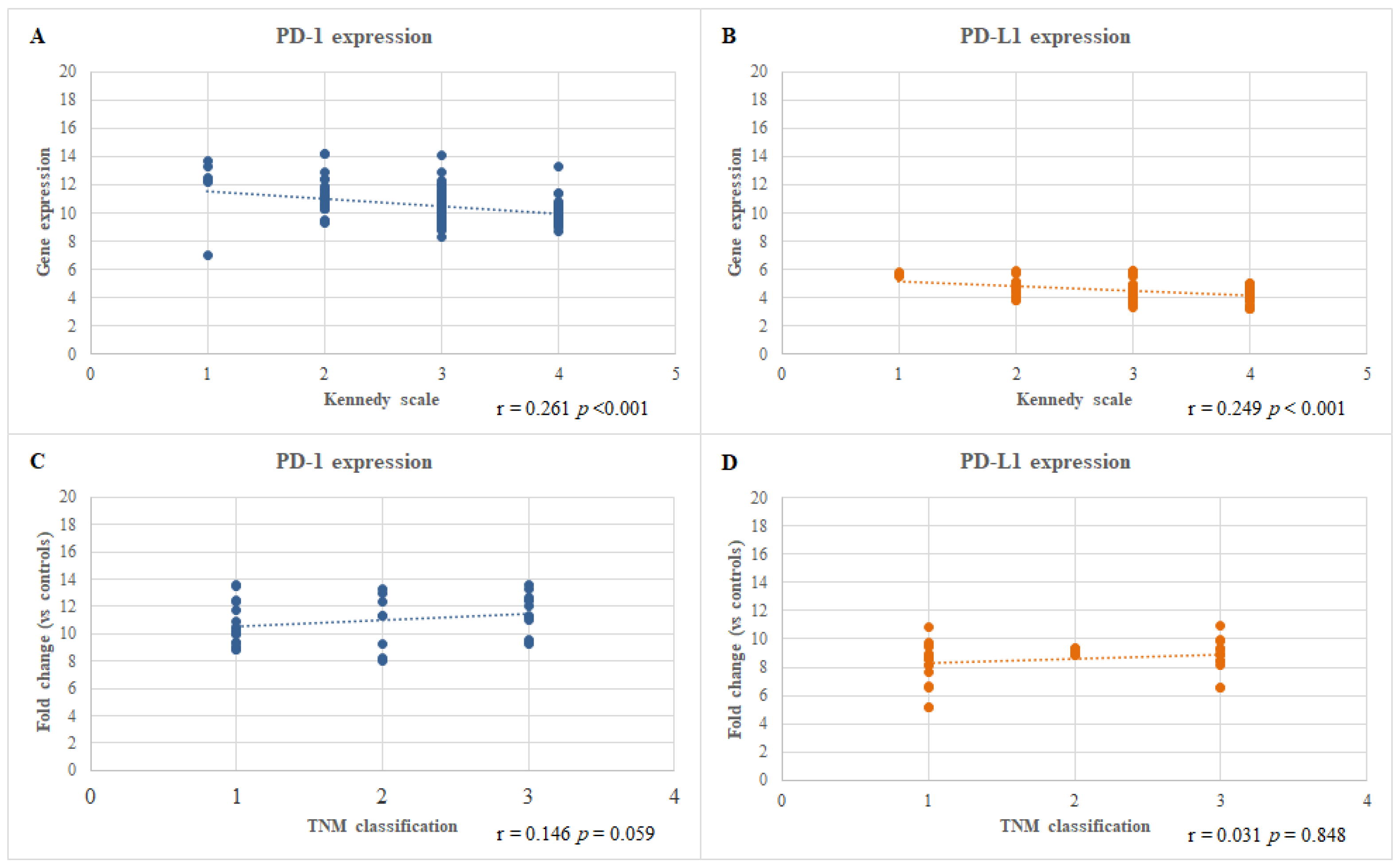
3.4. Correlation between the mRNA Expression Level of the PD-1 and PD-L1 Genes and the Severity of the CRSwNP and HNC Diseases
3.5. Multiple Linear Regression Analyses of PD-1 and PD-L1 mRNA Expression Levels
3.6. PD-1 and PD-L1 Protein Expression Level Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Shurin, M. Cancer as an immune-mediated disease. Immunotargets Ther. 2012, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Rao, X.; Sigdel, K.R. Regulation of inflammation in autoimmune disease. J. Immunol. Res. 2019, 2019, 7403796. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Sedaghat, A.R. Chronic Rhinosinusitis. Am. Fam. Physician 2017, 96, 500–506. [Google Scholar] [PubMed]
- Dietz de Loos, D.; Lourijsen, E.S.; Wildeman, M.A.M.; Freling, N.J.M.; Wolvers, M.D.J.; Reitsma, S.; Fokkens, W.J. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J. Allergy Clin. Immunol. 2019, 143, 1207–1214. [Google Scholar] [CrossRef]
- Hsu, J.; Avila, P.C.; Kern, R.C.; Hayes, M.G.; Schleimer, R.P.; Pinto, J.M. Genetics of chronic rhinosinusitis: State of the field and directions forward. J. Allergy Clin. Immunol. 2013, 131, 977–993.e5. [Google Scholar] [CrossRef]
- Lam, K.; Schleimer, R.; Kern, R.C. The Etiology and Pathogenesis of Chronic Rhinosinusitis: A Review of Current Hypotheses. Curr. Allergy Asthma Rep. 2015, 15, 41. [Google Scholar] [CrossRef]
- Kato, A.; Schleimer, R.P.; Bleier, B.S. Mechanisms and pathogenesis of chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1491–1503. [Google Scholar] [CrossRef]
- Dietz De Loos, D.A.E.; Hopkins, C.; Fokkens, W.J. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope 2013, 123, 57–63. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, A.; Emmanuel, B.; Thomas, K.; Guiang, H. Systematic literature review of the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyposis. Curr. Med. Res. Opin. 2020, 36, 1897–1911. [Google Scholar] [CrossRef]
- Huvenne, W.; van Bruaene, N.; Zhang, N.; van Zele, T.; Patou, J.; Gevaert, P.; Claeys, S.; Van Cauwenberge, P.; Bachert, C. Chronic rhinosinusitis with and without nasal polyps: What is the difference? Curr. Allergy Asthma Rep. 2009, 9, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Tan, B.K. Risk factors for chronic rhinosinusitis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Benninger, M.S. Rhinitis, Sinusitis, and Their Relationships to Allergies. Am. J. Rhinol. 1992, 6, 37–43. [Google Scholar] [CrossRef]
- Han, K.D.; Park, S.H.; Son, S.; Kim, S.H.; Kim, I.; Kim, J.Y.; In, S.M.; Kim, Y.S.; Lee, K.I. Relationship between Chronic Rhinosinusitis and the Incidence of Head and Neck Cancer: A National Population-Based Study. J. Clin. Med. 2022, 11, 5316. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.A.; Wulff-Burchfield, E.; Ghiam, M.; Bond, S.M.; Deng, J. Chronic Systemic Symptoms in Head and Neck Cancer Patients. JNCI Monogr. 2019, 2019, lgz004. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef]
- WHO International Agency for Research on Cancer. Global Cancer Observatory 2018; World Health Organization: Geneva, Switzerland, 2020; pp. 11–12. [Google Scholar]
- Almangush, A.; De Keukeleire, S.; Rottey, S.; Ferdinande, L.; Vermassen, T.; Leivo, I.; Mäkitie, A.A. Tumor-Infiltrating Lymphocytes in Head and Neck Cancer: Ready for Prime Time? Cancers 2022, 14, 1558. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Shinohara, T.; Taniwaki, M.; Ishida, Y.; Kawaichi, M.; Honjo, T. Structure and chromosomal localization of the human PD-1 gene (PDCD1). Genomics 1994, 23, 704–706. [Google Scholar] [CrossRef]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1: PD-L1/2 pathway from discovery to clinical implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef]
- Paterson, D.J.; Jefferies, W.A.; Green, J.R.; Brandon, M.R.; Corthesy, P.; Puklavec, M.; Williams, A.F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol. Immunol. 1987, 24, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Grzywnowicz, M.; Zaleska, J.; Mertens, D.; Tomczak, W.; Wlasiuk, P.; Kosior, K.; Piechnik, A.; Bojarska-Junak, A.; Dmoszynska, A.; Giannopoulos, K. Programmed death-1 and its ligand are novel immunotolerant molecules expressed on leukemic B cells in chronic lymphocytic leukemia. PLoS ONE 2012, 7, e35178. [Google Scholar] [CrossRef]
- Zhang, K.; Kong, X.; Li, Y.; Wang, Z.; Zhang, L.; Xuan, L. PD-1/PD-L1 Inhibitors in Patients With Preexisting Autoimmune Diseases. Front. Pharmacol. 2022, 13, 854967. [Google Scholar] [CrossRef]
- Veluswamy, P.; Wacker, M.; Scherner, M.; Wippermann, J. Delicate role of pd-l1/pd-1 axis in blood vessel inflammatory diseases: Current insight and future significance. Int. J. Mol. Sci. 2020, 21, 8159. [Google Scholar] [CrossRef]
- Ozawa, N.; Yokobori, T.; Osone, K.; Katayama, C.; Suga, K.; Komine, C.; Shibasaki, Y.; Shiraishi, T.; Okada, T.; Kato, R.; et al. PD-L1 upregulation is associated with activation of the DNA double-strand break repair pathway in patients with colitic cancer. Sci. Rep. 2021, 11, 13077. [Google Scholar] [CrossRef]
- Lenouvel, D.; González-Moles, M.Á.; Talbaoui, A.; Ramos-García, P.; González-Ruiz, L.; Ruiz-Ávila, I.; Gil-Montoya, J.A. An update of knowledge on PD-L1 in head and neck cancers: Physiologic, prognostic and therapeutic perspectives. Oral Dis. 2020, 26, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.W. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope 1992, 102, 1–18. [Google Scholar]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mahmood, T.; Yang, P.C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [CrossRef]
- Zhang, L.P.; Lin, L.; Zheng, C.Q.; Shi, G.Y. T-lymphocyte subpopulations and B7-H1/PD-1 expression in nasal polyposis. J. Int. Med. Res. 2010, 38, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Song, J.; Xiong, P.; Cao, P.P.; Liao, B.; Ma, J.; Zhang, Y.N.; Zeng, M.; Liu, Y.; Wang, H.; et al. Disease-specific T-helper cell polarizing function of lesional dendritic cells in different types of chronic rhinosinusitis with nasal polyps. Am. J. Respir. Crit. Care Med. 2014, 190, 628–638. [Google Scholar] [CrossRef]
- Kortekaas Krohn, I.; Bobic, S.; Dooley, J.; Lan, F.; Zhang, N.; Bachert, C.; Steelant, B.; Bullens, D.M.; Liston, A.; Ceuppens, J.L.; et al. Programmed cell death-1 expression correlates with disease severity and IL-5 in chronic rhinosinusitis with nasal polyps. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 985–993. [Google Scholar] [CrossRef]
- Wang, F.; Yang, L.; Xiao, M.; Zhang, Z.; Shen, J.; Anuchapreeda, S.; Tima, S.; Chiampanichayakul, S.; Xiao, Z. PD-L1 regulates cell proliferation and apoptosis in acute myeloid leukemia by activating PI3K-AKT signaling pathway. Sci. Rep. 2022, 12, 11444. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Wu, C.; Huang, W.; Xu, B.; Lian, S.; Wang, L.; Yue, S.; Chen, N.; Zhu, Z. PD-1-Mediated PI3K/Akt/mTOR, Caspase 9/Caspase 3 and ERK Pathways Are Involved in Regulating the Apoptosis and Proliferation of CD4+ and CD8+ T Cells During BVDV Infection in vitro. Front. Immunol. 2020, 11, 467. [Google Scholar] [CrossRef]
- Stutvoet, T.S.; Kol, A.; de Vries, E.G.E.; de Bruyn, M.; Fehrmann, R.S.N.; Terwisscha van Scheltinga, A.G.T.; de Jong, S. MAPK pathway activity plays a key role in PD-L1 expression of lung adenocarcinoma cells. J. Pathol. 2019, 249, 52–64. [Google Scholar] [CrossRef]
- Sumimoto, H.; Takano, A.; Teramoto, K.; Daigo, Y. RAS-MAPK signaling is required for the enhanced PD-L1 expression in human lung cancers. Ann. Oncol. 2016, 27, e0166626. [Google Scholar] [CrossRef]
- Zhao, T.; Li, Y.; Zhang, J.; Zhang, B. PD-L1 expression increased by ifn-γ via jak2-stat1 signaling and predicts a poor survival in colorectal cancer. Oncol. Lett. 2020, 20, 1127–1134. [Google Scholar] [CrossRef]
- Doi, T.; Ishikawa, T.; Okayama, T.; Oka, K.; Mizushima, K.; Yasuda, T.; Sakamoto, N.; Katada, K.; Kamada, K.; Uchiyama, K.; et al. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol. Rep. 2017, 37, 1545–1554. [Google Scholar] [CrossRef]
- Castagnoli, L.; Cancila, V.; Cordoba-Romero, S.L.; Faraci, S.; Talarico, G.; Belmonte, B.; Iorio, M.V.; Milani, M.; Volpari, T.; Chiodoni, C.; et al. WNT signaling modulates PD-L1 expression in the stem cell compartment of triple-negative breast cancer. Oncogene 2019, 38, 4047–4060. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Fu, Y.X. β-Catenin regulates tumor-derived PD-L1. J. Exp. Med. 2020, 217, e20200684. [Google Scholar] [CrossRef]
- Antonangeli, F.; Natalini, A.; Garassino, M.C.; Sica, A.; Santoni, A.; Di Rosa, F. Regulation of PD-L1 Expression by NF-κB in Cancer. Front. Immunol. 2020, 11, 584626. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, J.; Li, R.Y.; Lan, T.; Xiao, C.; Gong, P. PD-LI expression is regulated by NF-κB during EMT signaling in gastric carcinoma. Onco Targets Ther. 2019, 12, 10099–10105. [Google Scholar] [CrossRef] [PubMed]
- Meliante, P.G.; Barbato, C.; Zoccali, F.; Ralli, M.; Greco, A.; de Vincentiis, M.; Colizza, A.; Petrella, C.; Ferraguti, G.; Minni, A.; et al. Programmed Cell Death-Ligand 1 in Head and Neck Squamous Cell Carcinoma: Molecular Insights, Preclinical and Clinical Data, and Therapies. Int. J. Mol. Sci. 2022, 23, 15384. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Wu, Q.; Jiang, L.; Li, S.-C.; He, Q.-J.; Yang, B.; Cao, J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 1–9. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Lu, P.; Liu, C.; Guo, Y.; Yang, Y.; Peng, Y.; Wang, F.; Bo, Z.; Dou, X.; Shi, H.; et al. A combination of PD-1/PD-L1 inhibitors: The prospect of overcoming the weakness of tumor immunotherapy (Review). Mol. Med. Rep. 2021, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Corte, C.M.D.; Liello, R.D.; Viscardi, G.; Sparano, F.; Iacovino, M.L.; Paragliola, F.; Piccolo, A.; Napolitano, S.; Martini, G.; et al. Immunotherapy for head and neck cancer: Present and future. Crit. Rev. Oncol. Hematol. 2022, 174, 103679. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef]
- Kitano, A.; Ono, M.; Yoshida, M.; Noguchi, E.; Shimomura, A.; Shimoi, T.; Kodaira, M.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017, 2, e000150. [Google Scholar] [CrossRef]
- Thangarajah, F.; Morgenstern, B.; Pahmeyer, C.; Schiffmann, L.M.; Puppe, J.; Mallmann, P.; Hamacher, S.; Buettner, R.; Alidousty, C.; Holz, B.; et al. Clinical impact of PD-L1 and PD-1 expression in squamous cell cancer of the vulva. J. Cancer Res. Clin. Oncol. 2019, 145, 1651–1660. [Google Scholar] [CrossRef]
- Saito, H.; Kuroda, H.; Matsunaga, T.; Osaki, T.; Ikeguchi, M. Increased PD-1 expression on CD4+ and CD8+ T cells is involved in immune evasion in gastric cancer. J. Surg. Oncol. 2013, 107, 517–522. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, B.; Chen, X.; Zhan, P.; Zhao, Y.; Zhang, T.; Liu, H.; Afzal, M.Z.; Dermime, S.; Hochwald, S.N.; et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: A meta-analysis of randomized controlled trials. Transl. Lung Cancer Res. 2019, 8, 413–428. [Google Scholar] [CrossRef]
- Shah, M.; Hubbard, R.A.; Mamtani, R.; Marmarelis, M.E.; Hennessy, S. Very high PD-L1 expression as a prognostic indicator of overall survival among patients with advanced non-small cell lung cancer receiving anti-PD-(L)1 monotherapies in routine practice. Pharmacoepidemiol. Drug Saf. 2022, 31, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012, 4, 127ra37. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.H.; Kümmel, A.; Görlich, D.; Mohr, M.; Bröckling, S.; Mikesch, J.H.; Grünewald, I.; Marra, A.; Schultheis, A.M.; Wardelmann, E.; et al. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS ONE 2015, 10, e0136023. [Google Scholar] [CrossRef]
- Droeser, R.A.; Hirt, C.; Viehl, C.T.; Frey, D.M.; Nebiker, C.; Huber, X.; Zlobec, I.; Eppenberger-Castori, S.; Tzankov, A.; Rosso, R.; et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur. J. Cancer 2013, 49, 2233–2242. [Google Scholar] [CrossRef]
- Darb-Esfahani, S.; Kunze, C.A.; Kulbe, H.; Sehouli, J.; Wienert, S.; Lindner, J.; Budczies, J.; Bockmayr, M.; Dietel, M.; Denkert, C.; et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumorinfiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 2016, 7, 1486–1499. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, Y.; Tang, S.; Zhang, G.U.; Lin, Q.; Xu, Y.; Shang, J. High PD-L1 expression is associated with a favorable prognosis in patients with esophageal squamous cell carcinoma undergoing postoperative adjuvant radiotherapy. Oncol. Lett. 2019, 17, 1626–1634. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K.A.; Carvajal, D.E.; Anagnostou, V.K.; Syrigos, K.N.; Sznol, M.; Herbst, R.S.; Gettinger, S.N.; Chen, L.; Rimm, D.L. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Investig. 2014, 94, 107–116. [Google Scholar] [CrossRef]
- Lyu, X.; Zhang, M.; Li, G.; Jiang, Y.; Qiao, Q. PD-1 and PD-L1 Expression Predicts Radiosensitivity and Clinical Outcomes in Head and Neck Cancer and is Associated with HPV Infection. J. Cancer 2019, 10, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Sung, W.W.; Hsieh, M.J.; Tsai, S.C.; Lai, H.W.; Yang, S.M.; Shen, K.H.; Chen, M.K.; Lee, H.; Yeh, K.T.; et al. High PD-L1 Expression Correlates with Metastasis and Poor Prognosis in Oral Squamous Cell Carcinoma. PLoS ONE 2015, 10, e0142656. [Google Scholar] [CrossRef]
- Moratin, J.; Metzger, K.; Safaltin, A.; Herpel, E.; Hoffmann, J.; Freier, K.; Hess, J.; Horn, D. Upregulation of PD-L1 and PD-L2 in neck node metastases of head and neck squamous cell carcinoma. Head Neck 2019, 41, 2484–2491. [Google Scholar] [CrossRef]
- Müller, T.; Braun, M.; Dietrich, D.; Aktekin, S.; Höft, S.; Kristiansen, G.; Göke, F.; Schröck, A.; Brägelmann, J.; Held, S.A.E.; et al. PD-L1: A novel prognostic biomarker in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 52889–52900. [Google Scholar] [CrossRef]
- Balermpas, P.; Rödel, F.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Sak, A.; Stuschke, M.; et al. The PD-1/PD-L1 axis and human papilloma virus in patients with head and neck cancer after adjuvant chemoradiotherapy: A multicentre study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2017, 141, 594–603. [Google Scholar] [CrossRef]
- Vassilakopoulou, M.; Avgeris, M.; Velcheti, V.; Kotoula, V.; Rampias, T.; Chatzopoulos, K.; Perisanidis, C.; Kontos, C.K.; Giotakis, A.I.; Scorilas, A.; et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin. Cancer Res. 2016, 22, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.M.; Vilain, R.E.; Romanes, S.; Yang, J.; Smith, E.; Jones, D.; Scolyer, R.A.; Lee, C.S.; Zhang, M.; Rose, B. PD-L1 expression in tonsillar cancer is associated with human papillomavirus positivity and improved survival: Implications for anti-PD1 clinical trials. Oncotarget 2016, 7, 77010–77020. [Google Scholar] [CrossRef]
- Outh-Gauer, S.; Alt, M.; Le Tourneau, C.; Augustin, J.; Broudin, C.; Gasne, C.; Denize, T.; Mirghani, H.; Fabre, E.; Ménard, M.; et al. Immunotherapy in head and neck cancers: A new challenge for immunologists, pathologists and clinicians. Cancer Treat. Rev. 2018, 65, 54–64. [Google Scholar] [CrossRef]
- Chen, S.W.; Li, S.H.; Shi, D.B.; Jiang, W.M.; Song, M.; Yang, A.K.; Li, Y.D.; Bei, J.X.; Chen, W.K.; Zhang, Q. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int. J. Biol. Markers 2019, 34, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.Y.; Lim, S.H.; Park, K.; Sun, J.M.; Ko, Y.H.; Baek, C.H.; Son, Y.I.; Jeong, H.S.; Ahn, Y.C.; et al. Association Between PD-L1 and HPV Status and the Prognostic Value of PD-L1 in Oropharyngeal Squamous Cell Carcinoma. Cancer Res. Treat. 2016, 48, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef]

| Analyzed Trait | I Group (n = 102) | II Group (n = 162) | III Group (n = 40) |
|---|---|---|---|
| Gender: | |||
| Female (n/%) | 24 (23.5%) | 63 (38.9%) | 4 (10.0%) |
| Male (n/%) | 78 (76.5%) | 99 (61.1%) | 36 (90%) |
| Age (years) ± SD | 52.74 ± 9.77 | 50.11 ± 11.88 | 66.45 ± 10.50 |
| Smoking status “Yes” (n/%) | 20 (19.6%) | 48 (29.6%) | 34 (85.0%) |
| Kennedy scale | N/A | N/A | |
| 1 | N/A | 6 | N/A |
| 2 | N/A | 30 | N/A |
| 3 | N/A | 78 | N/A |
| 4 | N/A | 48 | N/A |
| TNM grading | |||
| T1N0M0 (n/%) | N/A | N/A | 20 (50.0%) |
| T2N0M0 (n/%) | N/A | N/A | 8 (20.0%) |
| T3N0M0 (n/%) | N/A | N/A | 12 (30.0%) |
| Groups | PD-1 Expression | p-Value | PD-L1 Expression | p-Value |
|---|---|---|---|---|
| CRSwNP Patients | ||||
| Control (n = 102) | 2.45 ± 0.22 | Ref. | 1.87 ± 0.19 | Ref. |
| CRSwNP (n = 77) | 5.88 ± 0.58 | <0.001 | 4.31 ± 0.71 | <0.001 |
| CRSwNP with allergy (n = 26) | 14.95 ± 1.12 | <0.001 | 4.49 ± 0.63 | <0.001 |
| CRSwNP with N-ERD (n = 59) | 10.53 ± 3.701,22 | <0.001 | 4.11 ± 0.53 | <0.001 |
| CRSwNP (n = 77) | 5.88 ± 0.58 | Ref. | 4.31 ± 0.71 | Ref. |
| CRSwNP with allergy (n = 26) | 14.95 ± 1.12 | <0.001 | 4.49 ± 0.63 | 0.888 |
| CRSwNP with N-ERD (n = 59) | 10.53 ± 3.70 | 0.051 | 4.11 ± 0.53 | 0.831 |
| HNC Patients | ||||
| Control (n = 102) | 2.45 ± 0.22 | Ref. | 1.87 ± 0.19 | Ref. |
| Nonsmokers (n = 6) | 10.54 ± 1.22 | <0.001 | 5.98 ± 0.87 | <0.001 |
| Smokers (n = 34) | 11.27 ± 1.01 | <0.001 | 11.33 ± 1.02 | <0.001 |
| Nonsmokers (n = 6) | 10.54 ± 1.22 | Ref. | 5.98 ± 0.87 | Ref. |
| Smokers (n = 34) | 11.27 ± 1.01 | 0.770 | 11.33 ± 1.02 | 0.037 |
| CRSwNP Patients | HNC Patients (n = 40) | |||||
|---|---|---|---|---|---|---|
| B ± SE | β | p-Value | B ± SE | β ± SE | p-Value | |
| PD-1 mRNA Expression Level | ||||||
| Age | −0.002 ± 0.017 | −0.022 | 0.900 | −0.007 ± 0.308 | −0.047 | 0.778 |
| Kennedy scale | −0.511 ± 0.251 | −0.360 | 0.043 | N/A | N/A | N/A |
| TNM scale | N/A | N/A | N/A | 0.468 ± 0.308 | 0.249 | 0.137 |
| PD-L1 mRNA Expression Level | ||||||
| Age | 0.013 ± 0.008 | 0.276 | 0.106 | 0.079 ± 0.015 | 0.647 | <0.001 *** |
| Kennedy scale | −0.502 ± 0.123 | −0.694 | <0.001 *** | N/A | N/A | N/A |
| TNM scale | N/A | N/A | N/A | 0.329 ± 0.183 | 0.225 | 0.079 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowska, K.; Kowalski, A.; Merecz-Sadowska, A.; Paprocka-Zjawiona, M.; Sitarek, P.; Kowalczyk, T.; Zielińska-Bliźniewska, H. PD-1 and PD-L1 Expression Levels as a Potential Biomarker of Chronic Rhinosinusitis and Head and Neck Cancers. J. Clin. Med. 2023, 12, 2033. https://doi.org/10.3390/jcm12052033
Malinowska K, Kowalski A, Merecz-Sadowska A, Paprocka-Zjawiona M, Sitarek P, Kowalczyk T, Zielińska-Bliźniewska H. PD-1 and PD-L1 Expression Levels as a Potential Biomarker of Chronic Rhinosinusitis and Head and Neck Cancers. Journal of Clinical Medicine. 2023; 12(5):2033. https://doi.org/10.3390/jcm12052033
Chicago/Turabian StyleMalinowska, Katarzyna, Andrzej Kowalski, Anna Merecz-Sadowska, Milena Paprocka-Zjawiona, Przemysław Sitarek, Tomasz Kowalczyk, and Hanna Zielińska-Bliźniewska. 2023. "PD-1 and PD-L1 Expression Levels as a Potential Biomarker of Chronic Rhinosinusitis and Head and Neck Cancers" Journal of Clinical Medicine 12, no. 5: 2033. https://doi.org/10.3390/jcm12052033
APA StyleMalinowska, K., Kowalski, A., Merecz-Sadowska, A., Paprocka-Zjawiona, M., Sitarek, P., Kowalczyk, T., & Zielińska-Bliźniewska, H. (2023). PD-1 and PD-L1 Expression Levels as a Potential Biomarker of Chronic Rhinosinusitis and Head and Neck Cancers. Journal of Clinical Medicine, 12(5), 2033. https://doi.org/10.3390/jcm12052033










