Stage IV Colorectal Cancer Management and Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Diagnosis of Stage IV Colorectal Cancer
4. Treatment Strategies for Stage IV Colorectal Cancer
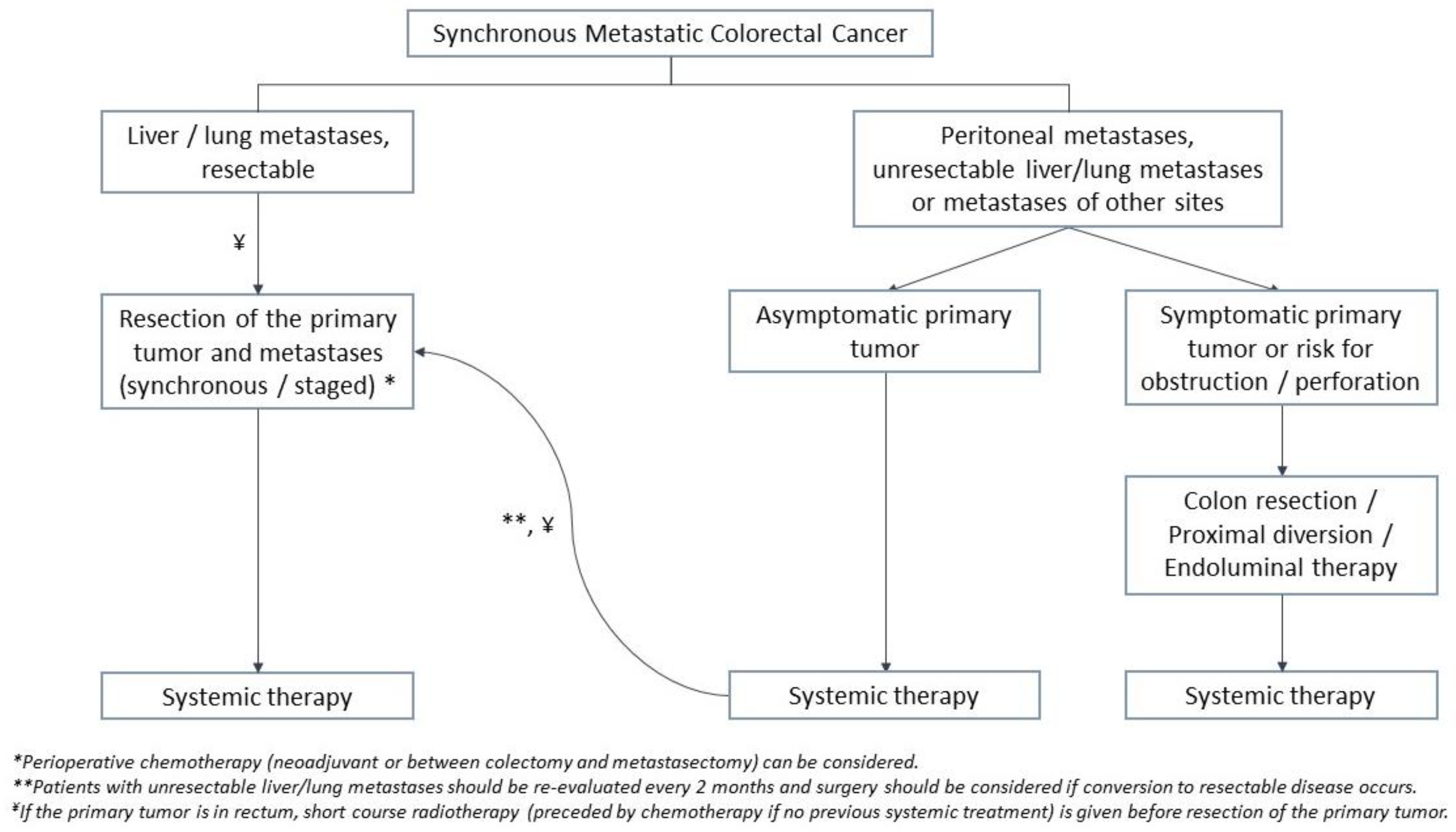
4.1. Treatment of Synchronous Metastases
4.1.1. Management of the Primary Tumor in the Setting of Unresectable Disease
4.1.2. Liver Metastases
4.1.3. Lung Metastases
4.1.4. Peritoneal Metastases
4.1.5. Other Metastases
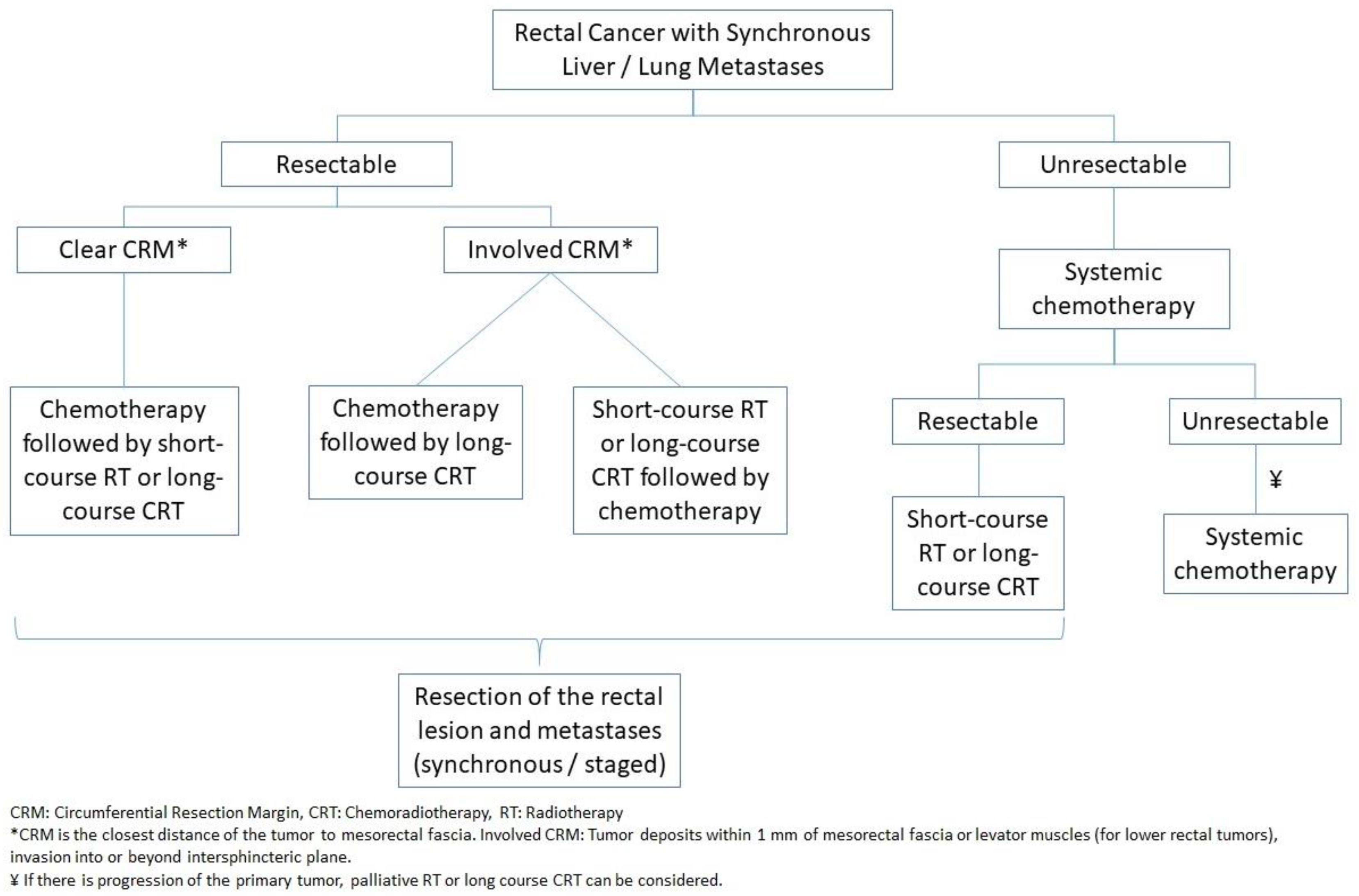
4.1.6. Rectal Cancer
4.2. Treatment of Metachronous Metastases
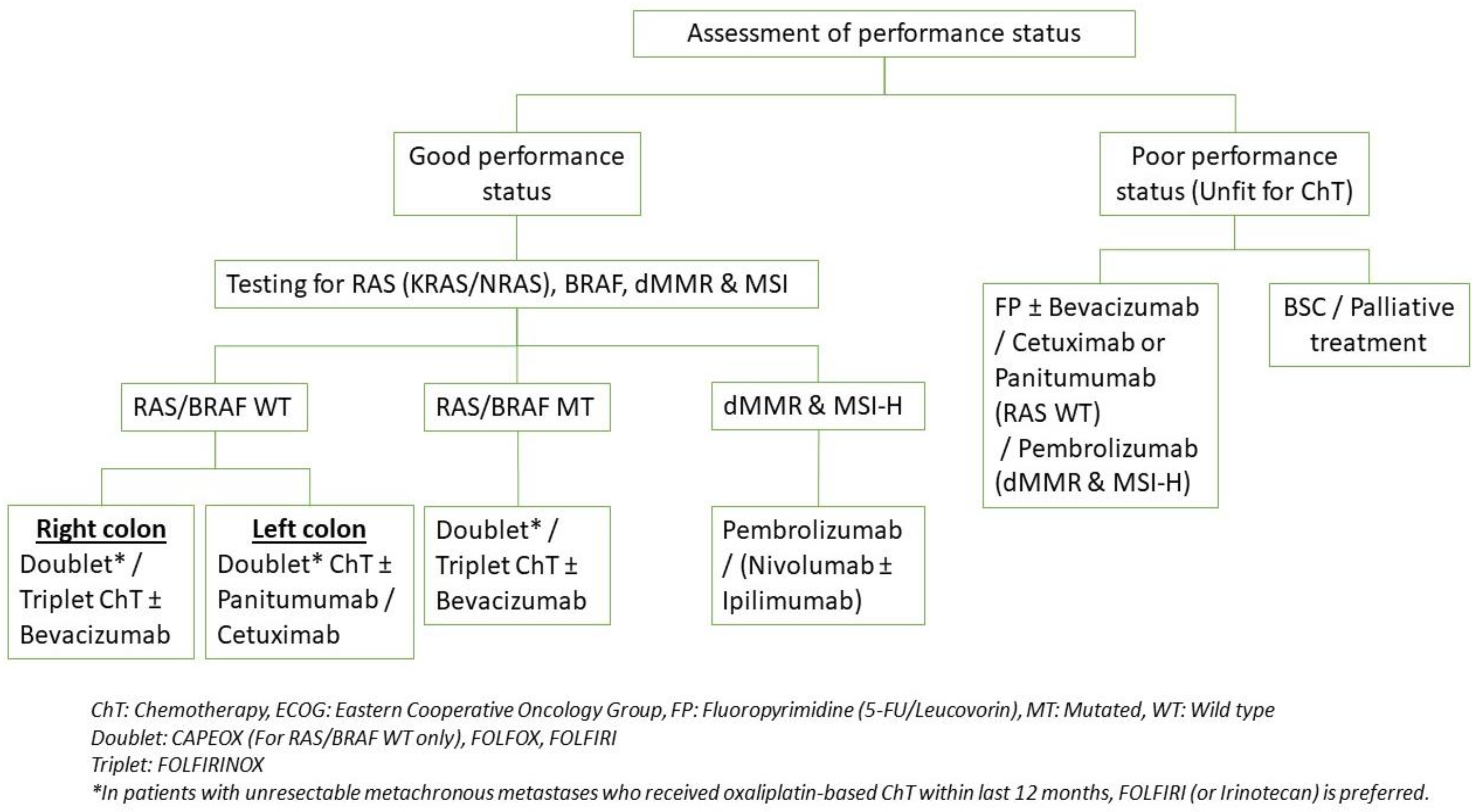
4.3. Systemic Therapy
4.3.1. Molecular Profiling
4.3.2. Maintenance Therapy
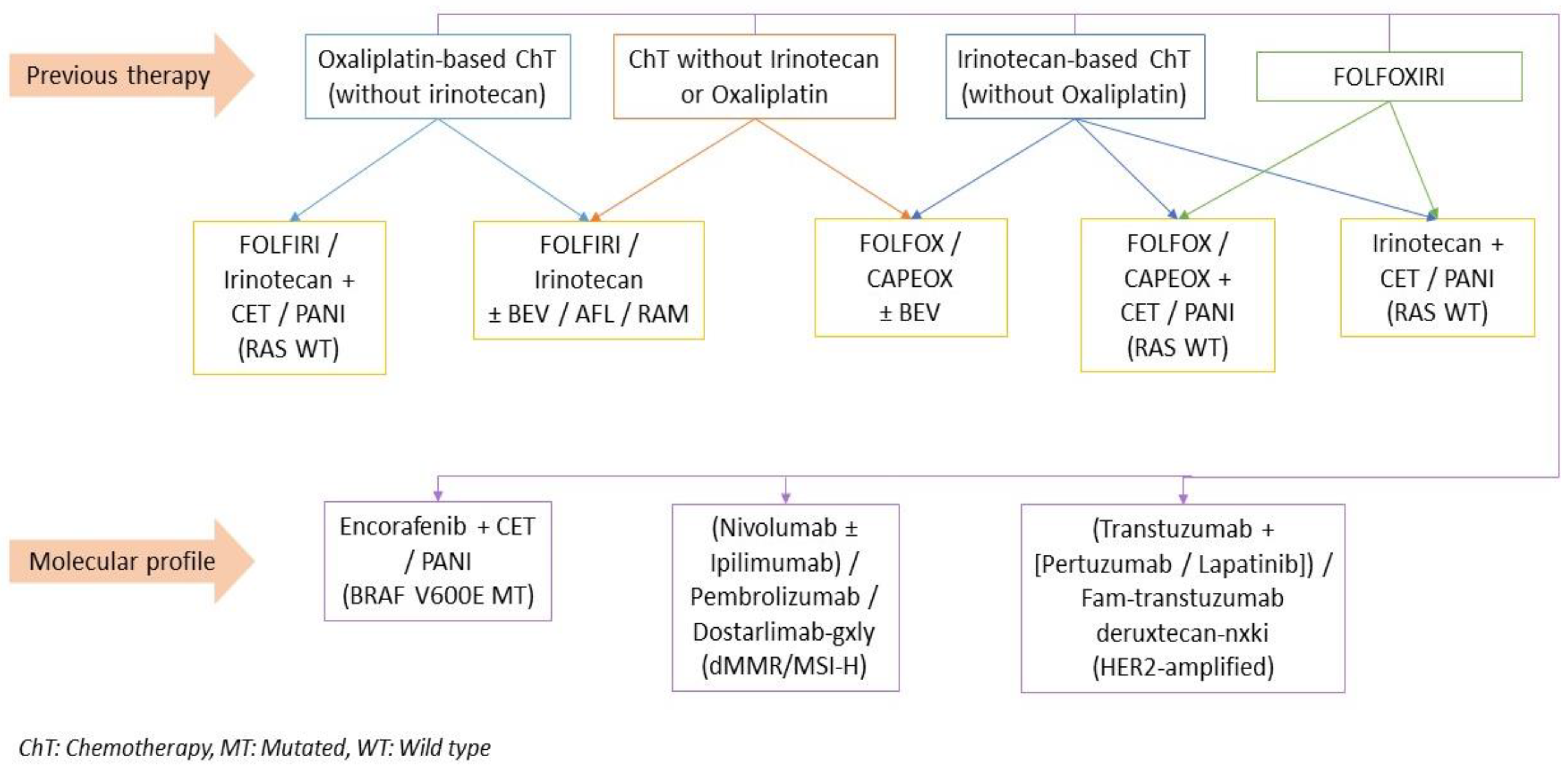
4.3.3. Second-Line and Subsequent Therapy
5. Surveillance
6. Future Direction of Stage IV Management
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2022, 72, 338–344. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute—SEER Program. Colorectal Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 7 January 2023).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Väyrynen, V.; Wirta, E.; Seppälä, T.; Sihvo, E.; Mecklin, J.; Vasala, K.; Kellokumpu, I. Incidence and management of patients with colorectal cancer and synchronous and metachronous colorectal metastases: A population-based study. BJS Open 2020, 4, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Elferink, M.A.G.; de Jong, K.P.; Klaase, J.M.; Siemerink, E.J.; de Wilt, J.H.W. Metachronous metastases from colorectal cancer: A population-based study in North-East Netherlands. Int. J. Color. Dis. 2014, 30, 205–212. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, N.P.M.; Van Der Kruijssen, D.E.W.; Hugen, N.; De Hingh, I.H.J.T.; Nagtegaal, I.; Verhoeven, R.; Koopman, M.; De Wilt, J.H.W. The Impact of Primary Tumor Location in Synchronous Metastatic Colorectal Cancer: Differences in Metastatic Sites and Survival. Ann. Surg. Oncol. 2019, 27, 1580–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakedis, J.; Schmidt, C.R. Surgical Treatment of Metastatic Colorectal Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 377–399. [Google Scholar] [CrossRef]
- Van Der Geest, L.G.M.; Lam-Boer, J.; Koopman, M.; Verhoef, C.; Elferink, M.A.G.; De Wilt, J.H.W. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis 2015, 32, 457–465. [Google Scholar] [CrossRef]
- Lee, R.M.; Cardona, K.; Russell, M.C. Historical perspective: Two decades of progress in treating metastatic colorectal cancer. J. Surg. Oncol. 2019, 119, 549–563. [Google Scholar] [CrossRef]
- Razenberg, L.; van Gestel, Y.; Creemers, G.-J.; Verwaal, V.; Lemmens, V.; de Hingh, I. Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur. J. Surg. Oncol. (EJSO) 2015, 41, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Simkens, G.A.; Van Oudheusden, T.R.; Nieboer, D.; Steyerberg, E.W.; Rutten, H.J.; Luyer, M.D.; Nienhuijs, S.W.; De Hingh, I.H. Development of a Prognostic Nomogram for Patients with Peritoneally Metastasized Colorectal Cancer Treated with Cytoreductive Surgery and HIPEC. Ann. Surg. Oncol. 2016, 23, 4214–4221. [Google Scholar] [CrossRef]
- Jawed, I.; Wilkerson, J.; Prasad, V.; Duffy, A.G.; Fojo, T. Colorectal Cancer Survival Gains and Novel Treatment Regimens: A Systematic Review and Analysis. JAMA Oncol. 2015, 1, 787–795. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.Q.; Taylor, J.P.; Stem, M.; Almaazmi, H.; Efron, J.E.; Atallah, C.; Safar, B. Aggressive Multimodal Treatment and Metastatic Colorectal Cancer Survival. J. Am. Coll. Surg. 2020, 230, 689–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Breugom, A.; Bastiaannet, E.; Guren, M.; Kørner, H.; Boelens, P.; Dekker, F.; Kapiteijn, E.; Gelderblom, H.; Larsen, I.; Liefers, G.; et al. Treatment strategies and overall survival for incurable metastatic colorectal cancer—A EURECCA international comparison including 21,196 patients from the Netherlands and Norway. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Eisterer, W.; Prager, G. Chemotherapy, Still an Option in the Twenty-First Century in Metastatic Colorectal Cancer? Cardiovasc. Interv. Radiol. 2019, 42, 1213–1220. [Google Scholar] [CrossRef]
- Bhimani, N.; Wong, G.; Molloy, C.; Pavlakis, N.; Diakos, C.; Clarke, S.; Dieng, M.; Hugh, T. Cost of treating metastatic colorectal cancer: A systematic review. Public Health 2022, 211, 97–104. [Google Scholar] [CrossRef]
- Benson, A.B.; Al-Hawary, M.M.; Azad, N.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; Garrido-Laguna, I.; et al. NCCN Guidelines Version 2.2022 Colon Cancer Continue NCCN Guidelines Panel Disclosures. 2022. Available online: https://www.nccn.org/home/member- (accessed on 7 January 2023).
- Benson, A.B.; Al-Hawary, M.M.; Azad, N.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; Garrido-Laguna, I.; et al. NCCN Guidelines Version 3.2022 Rectal Cancer Continue NCCN Guidelines Panel Disclosures; 2022. Available online: https://www.nccn.org/home/member- (accessed on 7 January 2023).
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 10–32. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1. [Google Scholar] [CrossRef] [Green Version]
- Hedrick, T.L. The ASCRS Manual of Colon and Rectal Surgery, 4th ed.; Steele, S.R., Hull, T.L., Hyman, N., Maykel, J.A., Read, T.E., Whitlow, C.B., Eds.; Springer: Cham, Switzerland, 2022; p. 547. [Google Scholar]
- Lakemeyer, L.; Sander, S.; Wittau, M.; Henne-Bruns, D.; Kornmann, M.; Lemke, J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases 2021, 9, 21. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, Z.; Wu, J.; Li, Z.; Wu, Y. Prognostic value of pretreatment serum carbohydrate antigen 19-9 level in patients with colorectal cancer: A meta-analysis. PLoS ONE 2017, 12, e0188139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balthazar, E.; Megibow, A.; Hulnick, D.; Naidich, D. Carcinoma of the colon: Detection and preoperative staging by CT. Am. J. Roentgenol. 1988, 150, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niekel, M.C.; Bipat, S.; Stoker, J. Diagnostic Imaging of Colorectal Liver Metastases with CT, MR Imaging, FDG PET, and/or FDG PET/CT: A Meta-Analysis of Prospective Studies Including Patients Who Have Not Previously Undergone Treatment 1. Radiology 2010, 257, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cai, Y.-Z.; Xu, G.-H. Diagnostic Accuracy of MRI for Assessment of T Category and Circumferential Resection Margin Involvement in Patients with Rectal Cancer. Dis. Colon Rectum 2016, 59, 789–799. [Google Scholar] [CrossRef]
- Taylor, F.G.; Quirke, P.; Heald, R.J.; Moran, B.J.; Blomqvist, L.; Swift, I.R.; Sebag-Montefiore, D.; Tekkis, P.; Brown, G. Preoperative Magnetic Resonance Imaging Assessment of Circumferential Resection Margin Predicts Disease-Free Survival and Local Recurrence: 5-Year Follow-Up Results of the MERCURY Study. J. Clin. Oncol. 2014, 32, 34–43. [Google Scholar] [CrossRef]
- Faletti, R.; Gatti, M.; Arezzo, A.; Stola, S.; Benedini, M.C.; Bergamasco, L.; Morino, M.; Fonio, P. Preoperative staging of rectal cancer using magnetic resonance imaging: Comparison with pathological staging. Int. J. Clin. Rev. 2018, 73, 13–19. [Google Scholar] [CrossRef]
- Ashraf, S.; Hompes, R.; Slater, A.; Lindsey, I.; Bach, S.; Mortensen, N.J.; Cunningham, C.; on behalf of the Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Color. Dis. 2011, 14, 821–826. [Google Scholar] [CrossRef]
- Bipat, S.; Glas, A.S.; Slors, F.J.M.; Zwinderman, A.H.; Bossuyt, P.M.M.; Stoker, J. Rectal Cancer: Local Staging and Assessment of Lymph Node Involvement with Endoluminal US, CT, and MR Imaging—A Meta-Analysis. Radiology 2004, 232, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sadahiro, S.; Suzuki, T.; Okada, K.; Saito, G. Comparisons of Rigid Proctoscopy, Flexible Colonoscopy, and Digital Rectal Examination for Determining the Localization of Rectal Cancers. Dis. Colon Rectum 2018, 61, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Mischel, A.-M.; Rosielle, D.A. Eastern Cooperative Oncology Group Performance Status #434. J. Palliat. Med. 2022, 25, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Corvino, F.; Giurazza, F.; Cangiano, G.; Silvestre, M.; Cavaglià, E.; de Magistris, G.; Amodio, F.; Corvino, A.; Niola, R. Endovascular Treatment of Peripheral Vascular Blowout Syndrome in End-Stage Malignancies. Ann. Vasc. Surg. 2019, 58, 382.e1–382.e5. [Google Scholar] [CrossRef] [PubMed]
- Huntress, L.A.; Kogan, S.; Nagarsheth, K.; Nassiri, N. Palliative Endovascular Techniques for Management of Peripheral Vascular Blowout Syndrome in End-Stage Malignancies. Vasc. Endovasc. Surg. 2017, 51, 394–399. [Google Scholar] [CrossRef]
- Cameron, M.G.; Kersten, C.; Vistad, I.; Fosså, S.; Guren, M.G. Palliative pelvic radiotherapy of symptomatic incurable rectal cancer—A systematic review. Acta Oncol. 2013, 53, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Cirocchi, R.; Trastulli, S.; Abraha, I.; Vettoretto, N.; Boselli, C.; Montedori, A.; Parisi, A.; Noya, G.; Platell, C. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable Stage IV colorectal cancer. Cochrane Database Syst. Rev. 2012, 8, CD008997. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.-Y.; Kim, J.-E.; Yoo, N.; Cho, H.-M.; Kim, H.; An, H.-J.; Kye, B.-H. Survival Outcomes after Elective or Emergency Surgery for Synchronous Stage IV Colorectal Cancer. Biomedicines 2022, 10, 3114. [Google Scholar] [CrossRef] [PubMed]
- Fiori, E.; Lamazza, A.; Schillaci, A.; Femia, S.; DeMasi, E.; DeCesare, A.; Sterpetti, A.V. Palliative management for patients with subacute obstruction and stage IV unresectable rectosigmoid cancer: Colostomy versus endoscopic stenting: Final results of a prospective randomized trial. Am. J. Surg. 2012, 204, 321–326. [Google Scholar] [CrossRef]
- Gianotti, L.; Tamini, N.; Nespoli, L.; Rota, M.; Bolzonaro, E.; Frego, R.; Redaelli, A.; Antolini, L.; Ardito, A.; Nespoli, A.; et al. A prospective evaluation of short-term and long-term results from colonic stenting for palliation or as a bridge to elective operation versus immediate surgery for large-bowel obstruction. Surg. Endosc. 2012, 27, 832–842. [Google Scholar] [CrossRef]
- Urgorri, A.S.; Saperas, E.; Castella, E.O.; Román, M.P.; Pons, F.R.; Priego, L.B.; Cusco, J.M.D.; Sánchez, M.P.; Caserras, X.B.; Álvarez-González, M.A. Colonic stent vs surgical resection of the primary tumor. Effect on survival from stage-IV obstructive colorectal cancer. Rev. Esp. Enferm. Dig. 2020, 112, 694–700. [Google Scholar] [CrossRef]
- Daniels, M.; Merkel, S.; Agaimy, A.; Hohenberger, W. Treatment of perforated colon carcinomas—Outcomes of radical surgery. Int. J. Color. Dis. 2015, 30, 1505–1513. [Google Scholar] [CrossRef]
- Bs, J.K.L.; Huber, K.E.; DiPetrillo, T.A.; Wazer, D.E.; Leonard, K.L. Patterns of care of radiation therapy in patients with stage IV rectal cancer: A Surveillance, Epidemiology, and End Results analysis of patients from 2004 to 2009. Cancer 2013, 120, 731–737. [Google Scholar] [CrossRef]
- Horn, S.R.; Stoltzfus, K.C.; Lehrer, E.J.; Dawson, L.A.; Tchelebi, L.; Gusani, N.J.; Sharma, N.K.; Chen, H.; Trifiletti, D.M.; Zaorsky, N.G. Epidemiology of liver metastases. Cancer Epidemiol. 2020, 67, 101760. [Google Scholar] [CrossRef]
- Akgül, Ö.; Çetinkaya, E.; Ersöz, Ş.; Tez, M. Role of surgery in colorectal cancer liver metastases. World J. Gastroenterol. 2014, 20, 6113. [Google Scholar] [CrossRef]
- Nordlinger, B.; Van Cutsem, E.; Rougier, P.; Köhne, C.-H.; Ychou, M.; Sobrero, A.; Adam, R.; Arvidsson, D.; Carrato, A.; Georgoulias, V.; et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur. J. Cancer 2007, 43, 2037–2045. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.J.; Kim, S.-G.; Lee, K.-W.; Cho, S.H.; Kim, T.W.; Baek, J.Y.; Park, Y.S.; Hong, S.; Chu, C.W.; Beom, S.-H.; et al. A Randomized Phase II Study of Perioperative Chemotherapy Plus Bevacizumab Versus Postoperative Chemotherapy Plus Bevacizumab in Patients With Upfront Resectable Hepatic Colorectal Metastases. Clin. Color. Cancer 2020, 19, e140–e150. [Google Scholar] [CrossRef]
- Park, E.J.; Baik, S.H. Recent Advance in the Surgical Treatment of Metastatic Colorectal Cancer-An English Version. J. Anus Rectum Colon 2022, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ghiasloo, M.; Pavlenko, D.; Verhaeghe, M.; Van Langenhove, Z.; Uyttebroek, O.; Berardi, G.; Troisi, R.I.; Ceelen, W. Surgical treatment of stage IV colorectal cancer with synchronous liver metastases: A systematic review and network meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 1203–1213. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, N.; Dunn, K.B. Metastasectomy for Stage IV Colorectal Cancer. Dis. Colon Rectum 2010, 53, 1080–1092. [Google Scholar] [CrossRef]
- de Jong, M.C.; Pulitano, C.; Ribero, D.; Strub, J.; Mentha, G.; Schulick, R.D.; Choti, M.A.; Aldrighetti, L.; Capussotti, L.; Pawlik, T.M. Rates and Patterns of Recurrence Following Curative Intent Surgery for Colorectal Liver Metastasis: An international multi-institutional analysis of 1669 patients. Ann. Surg. 2009, 250, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.; Raab, H.-R.; Weitz, J.; Lordick, F.; Hartmann, J.; Stoehlmacher-Williams, J.; Lang, H.; Trarbach, T.; et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann. Oncol. 2014, 25, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Warner, S.; Ito, K.; Raoof, M.; Wu, G.X.; Lu, W.P.; Kessler, J.; Kim, J.Y.; Fong, Y. Cytoreduction for colorectal metastases: Liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr. Probl. Surg. 2018, 55, 330–379. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Li, H.; Jia, G.-Q.; Fang, D.; Tang, Y.-Y.; Xie, J.; Chen, K.-F.; Chen, Z.-Y. Parenchymal-sparing versus extended hepatectomy for colorectal liver metastases: A systematic review and meta-analysis. Cancer Med. 2019, 8, 6165–6175. [Google Scholar] [CrossRef] [Green Version]
- Moris, D.; Ronnekleiv-Kelly, S.; Rahnemai-Azar, A.A.; Felekouras, E.; Dillhoff, M.; Schmidt, C.; Pawlik, T.M. Parenchymal-Sparing Versus Anatomic Liver Resection for Colorectal Liver Metastases: A Systematic Review. J. Gastrointest. Surg. 2017, 21, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Donadon, M.; Marconi, M.; Botea, F.; Palmisano, A.; Del Fabbro, D.; Procopio, F.; Montorsi, M. Systematic Extended Right Posterior Sectionectomy. Ann. Surg. 2008, 247, 603–611. [Google Scholar] [CrossRef]
- Chouillard, E.; Cherqui, D.; Tayar, C.; Brunetti, F.; Fagniez, P.-L. Anatomical Bi- and Trisegmentectomies as Alternatives to Extensive Liver Resections. Ann. Surg. 2003, 238, 29–34. [Google Scholar] [CrossRef]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Sasaki, K.; Ijzermans, J.N.M.; van Vugt, J.L.A.; Pawlik, T.M.; Choti, M.A.; Cameron, J.L.; He, J.; et al. Anatomical Resections Improve Disease-free Survival in Patients With KRAS-mutated Colorectal Liver Metastases. Ann. Surg. 2017, 266, 641–649. [Google Scholar] [CrossRef]
- Keck, J.; Gaedcke, J.; Ghadimi, M.; Lorf, T. Surgical Therapy in Patients with Colorectal Liver Metastases. Digestion 2022, 103, 245–252. [Google Scholar] [CrossRef]
- Mentha, G.; Majno, P.E.; Andres, A.; Rubbia-Brandt, L.; Morel, P.; Roth, A.D. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br. J. Surg. 2006, 93, 872–878. [Google Scholar] [CrossRef]
- Frühling, P.; Strömberg, C.; Isaksson, B.; Urdzik, J. A comparison of the simultaneous, liver-first, and colorectal-first strategies for surgical treatment of synchronous colorectal liver metastases at two major liver-surgery institutions in Sweden. HPB 2022, 25, 26–36. [Google Scholar] [CrossRef]
- Boudjema, K.; Locher, C.; Sabbagh, C.; Ortega-Deballon, P.; Heyd, B.; Bachellier, P.; Métairie, S.; Paye, F.; Bourlier, P.; Adam, R.; et al. Simultaneous Versus Delayed Resection for Initially Resectable Synchronous Colorectal Cancer Liver Metastases: A Prospective, Open-label, Randomized, Controlled Trial. Ann. Surg. 2021, 273, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Song, L.; Tang, J.-Y.; Sun, W.-P.; Li, Z. Safety and long-term prognosis of simultaneous versus staged resection in synchronous colorectal cancer with liver metastasis: A systematic review and meta-analysis. Eur. J. Med. Res. 2022, 27, 297. [Google Scholar] [CrossRef]
- Adam, R.; Miller, R.; Pitombo, M.; Wicherts, D.A.; de Haas, R.J.; Bitsakou, G.; Aloia, T. Two-stage Hepatectomy Approach for Initially Unresectable Colorectal Hepatic Metastases. Surg. Oncol. Clin. N. Am. 2007, 16, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Jaeck, D.; Oussoultzoglou, E.; Rosso, E.; Greget, M.; Weber, J.-C.; Bachellier, P. A Two-Stage Hepatectomy Procedure Combined With Portal Vein Embolization to Achieve Curative Resection for Initially Unresectable Multiple and Bilobar Colorectal Liver Metastases. Ann. Surg. 2004, 240, 1037–1051. [Google Scholar] [CrossRef]
- Del Basso, C.; Gaillard, M.; Lainas, P.; Zervaki, S.; Perlemuter, G.; Chagué, P.; Rocher, L.; Voican, C.S.; Dagher, I.; Tranchart, H. Current strategies to induce liver remnant hypertrophy before major liver resection. World J. Hepatol. 2021, 13, 1629–1641. [Google Scholar] [CrossRef]
- Imai, K.; Benitez, C.C.; Allard, M.-A.; Vibert, E.; Cunha, A.S.; Cherqui, D.; Castaing, D.; Bismuth, H.; Baba, H.; Adam, R. Failure to Achieve a 2-Stage Hepatectomy for Colorectal Liver Metastases: How to Prevent It? Ann. Surg. 2015, 262, 772–779. [Google Scholar] [CrossRef]
- Brouquet, A.; Abdalla, E.K.; Kopetz, S.; Garrett, C.R.; Overman, M.J.; Eng, C.; Andreou, A.; Loyer, E.M.; Madoff, D.C.; Curley, S.A.; et al. High Survival Rate After Two-Stage Resection of Advanced Colorectal Liver Metastases: Response-Based Selection and Complete Resection Define Outcome. J. Clin. Oncol. 2011, 29, 1083–1090. [Google Scholar] [CrossRef] [Green Version]
- Yi, F.; Zhang, W.; Feng, L. Efficacy and safety of different options for liver regeneration of future liver remnant in patients with liver malignancies: A systematic review and network meta-analysis. World J. Surg. Oncol. 2022, 20, 399. [Google Scholar] [CrossRef]
- Moris, D.; Ronnekleiv-Kelly, S.; Kostakis, I.D.; Tsilimigras, D.I.; Beal, E.W.; Papalampros, A.; Dimitroulis, D.; Felekouras, E.; Pawlik, T.M. Operative Results and Oncologic Outcomes of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Versus Two-Stage Hepatectomy (TSH) in Patients with Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. World J. Surg. 2017, 42, 806–815. [Google Scholar] [CrossRef]
- Sandström, P.; Røsok, B.I.; Sparrelid, E.; Larsen, P.N.; Larsson, A.L.; Lindell, G.; Schultz, N.A.; Bjørnbeth, B.A.; Isaksson, B.; Rizell, M.; et al. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann. Surg. 2018, 267, 833–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yang, Z.; Zhang, S.; Wang, W.; Zheng, S. Conventional Two-Stage Hepatectomy or Associating Liver Partitioning and Portal Vein Ligation for Staged Hepatectomy for Colorectal Liver Metastases? A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Czigany, Z.; Sharmeen, S.; Van Der Kroft, G.; Strnad, P.; Ulmer, T.F.; Isfort, P.; Bruners, P.; Lurje, G.; Neumann, U.P. ALPPS versus two-stage hepatectomy for colorectal liver metastases—A comparative retrospective cohort study. World J. Surg. Oncol. 2020, 18, 140. [Google Scholar] [CrossRef] [PubMed]
- Vico, T.D.; Castro, P.G.; Navarro, L.A.; Sánchez, A.S.; Góngora, L.M.; Orón, E.M.M.; Ibáñez, J.M.; Alonso, N.T.; Arrillaga, I.G.-P.; Trancón, J.E.G. Two stage hepatectomy (TSH) versus ALPPS for initially unresectable colorectal liver metastases: A systematic review and meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2023, 49, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, M.R.; Puijk, R.S.; Van Tilborg, A.A.J.M.; Henningsen, K.H.; Fernandez, L.G.; Neyt, M.; Heymans, J.; Frankema, J.S.; De Jong, K.P.; Richel, D.J.; et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2018, 41, 1189–1204. [Google Scholar] [CrossRef] [Green Version]
- Engstrand, J.; Nilsson, H.; Jansson, A.; Isaksson, B.; Freedman, J.; Lundell, L.; Jonas, E. A multiple microwave ablation strategy in patients with initially unresectable colorectal cancer liver metastases—A safety and feasibility study of a new concept. Eur. J. Surg. Oncol. (EJSO) 2014, 40, 1488–1493. [Google Scholar] [CrossRef]
- Di Martino, M.; Rompianesi, G.; Mora-Guzmán, I.; Martín-Pérez, E.; Montalti, R.; Troisi, R.I. Systematic review and meta-analysis of local ablative therapies for resectable colorectal liver metastases. Eur. J. Surg. Oncol. (EJSO) 2019, 46, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, S.; Marmorino, F.; Furbetta, N.; Carullo, M.; Gianardi, D.; Palmeri, M.; Di Franco, G.; Comandatore, A.; Moretto, R.; Cecilia, E.; et al. Surgery combined with intra-operative microwaves ablation for the management of colorectal cancer liver metastasis: A case-matched analysis and evaluation of recurrences. Front. Oncol. 2022, 12, 1023301. [Google Scholar] [CrossRef]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B.; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef]
- Olson, R.; Jiang, W.; Liu, M.; Bergman, A.; Schellenberg, D.; Mou, B.; Alexander, A.; Carolan, H.; Hsu, F.; Miller, S.; et al. Treatment With Stereotactic Ablative Radiotherapy for Up to 5 Oligometastases in Patients With Cancer: Primary Toxic Effect Results of the Nonrandomized Phase 2 SABR-5 Clinical Trial. JAMA Oncol. 2022, 8, 1644. [Google Scholar] [CrossRef]
- Mühlbacher, F.; Huk, I.; Steininger, R.; Gnant, M.; Götzinger, P.; Wamser, P.; Banhegyi, C.; Piza, F. Is orthotopic liver transplantation a feasible treatment for secondary cancer of the liver? Transplant Proc. 1991, 23 Pt 2, 1567–1568. [Google Scholar] [PubMed]
- Hagness, M.; Foss, A.; Line, P.-D.; Scholz, T.; Jørgensen, P.F.; Fosby, B.; Boberg, K.M.; Mathisen, Ø.; Gladhaug, I.P.; Egge, T.S.; et al. Liver Transplantation for Nonresectable Liver Metastases From Colorectal Cancer. Ann. Surg. 2013, 257, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Syversveen, T.; Solheim, J.M.; Solberg, S.; Grut, H.; Bjørnbeth, B.A.; Hagness, M.; Line, P.-D. Survival Following Liver Transplantation for Patients With Nonresectable Liver-only Colorectal Metastases. Ann. Surg. 2020, 271, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Mitry, E.; Guiu, B.; Cosconea, S.; Jooste, V.; Faivre, J.; Bouvier, A.-M. Epidemiology, management and prognosis of colorectal cancer with lung metastases: A 30-year population-based study. Gut 2010, 59, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Meimarakis, G.; Spelsberg, F.; Angele, M.; Preissler, G.; Fertmann, J.; Crispin, A.; Reu, S.; Kalaitzis, N.; Stemmler, M.; Giessen, C.; et al. Resection of Pulmonary Metastases from Colon and Rectal Cancer: Factors to Predict Survival Differ Regarding to the Origin of the Primary Tumor. Ann. Surg. Oncol. 2014, 21, 2563–2572. [Google Scholar] [CrossRef]
- Li, J.; Yuan, Y.; Yang, F.; Wang, Y.; Zhu, X.; Wang, Z.; Zheng, S.; Wan, D.; He, J.; Wang, J.; et al. Expert consensus on multidisciplinary therapy of colorectal cancer with lung metastases (2019 edition). J. Hematol. Oncol. 2019, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Nanji, S.; Karim, S.; Tang, E.; Brennan, K.; McGuire, A.; Pramesh, C.; Booth, C.M. Pulmonary Metastasectomy for Colorectal Cancer: Predictors of Survival in Routine Surgical Practice. Ann. Thorac. Surg. 2018, 105, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Handy, J.R.; Bremner, R.M.; Crocenzi, T.S.; Detterbeck, F.C.; Fernando, H.C.; Fidias, P.M.; Firestone, S.; Johnstone, C.A.; Lanuti, M.; Litle, V.R.; et al. Expert Consensus Document on Pulmonary Metastasectomy. Ann. Thorac. Surg. 2019, 107, 631–649. [Google Scholar] [CrossRef] [Green Version]
- Mangiameli, G.; Cioffi, U.; Alloisio, M.; Testori, A. Pulmonary Metastases: Surgical Principles, Surgical Indications, and Innovations; Sergi, C.M., Ed.; Exon Publications: Brisbane, Australia, 3 May 2022. [Google Scholar]
- Cerfolio, R.J.; Mccarty, T.; Bryant, A. Non-imaged pulmonary nodules discovered during thoracotomy for metastasectomy by lung palpation. Eur. J. Cardio-Thorac.Surg. 2009, 35, 786–791. [Google Scholar] [CrossRef]
- Hamaji, M.; Cassivi, S.D.; Shen, K.R.; Allen, M.S.; Nichols, F.C.; Deschamps, C.; Wigle, D.A. Is Lymph Node Dissection Required in Pulmonary Metastasectomy for Colorectal Adenocarcinoma? Ann. Thorac. Surg. 2012, 94, 1796–1800. [Google Scholar] [CrossRef]
- Wolf, F.J.; Grand, D.J.; Machan, J.T.; DiPetrillo, T.A.; Mayo-Smith, W.W.; Dupuy, D.E. Microwave Ablation of Lung Malignancies: Effectiveness, CT Findings, and Safety in 50 Patients. Radiology 2008, 247, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Tselikas, L.; Yazbeck, C.; Kattan, J. Systemic Versus Local Therapies for Colorectal Cancer Pulmonary Metastasis: What to Choose and When? J. Gastrointest. Cancer 2016, 47, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Flood, M.; Das, A.; Soucisse, M.; Kong, J.; Ramsay, R.; Michael, M.; Loveday, B.; Warrier, S.; Heriot, A. Synchronous Liver Resection, Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Liver and Peritoneal Metastases: A Systematic Review and Meta-analysis. Dis. Colon Rectum 2021, 64, 754–764. [Google Scholar] [CrossRef]
- Vassos, N.; Piso, P. Metastatic Colorectal Cancer to the Peritoneum: Current Treatment Options. Curr. Treat. Options Oncol. 2018, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Xie, X.; Min, T.; Sun, T.; Wang, H.; Zhang, Y.; Dang, C.; Zhang, H. Development of the Peritoneal Metastasis: A Review of Back-Grounds, Mechanisms, Treatments and Prospects. J. Clin. Med. 2022, 12, 103. [Google Scholar] [CrossRef]
- Sato, H.; Kotake, K.; Sugihara, K.; Takahashi, H.; Maeda, K.; Uyama, I. Clinicopathological Factors Associated with Recurrence and Prognosis after R0 Resection for Stage IV Colorectal Cancer with Peritoneal Metastasis. Dig. Surg. 2016, 33, 382–391. [Google Scholar] [CrossRef]
- Verwaal, V.J.; Van Ruth, S.; De Bree, E.; Van Slooten, G.W.; Van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized Trial of Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy Versus Systemic Chemotherapy and Palliative Surgery in Patients With Peritoneal Carcinomatosis of Colorectal Cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Cashin, P.; Mahteme, H.; Spång, N.; Syk, I.; Frödin, J.; Torkzad, M.; Glimelius, B.; Graf, W. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomised trial. Eur. J. Cancer 2016, 53, 155–162. [Google Scholar] [CrossRef]
- Narasimhan, V.; Tan, S.; Kong, J.; Pham, T.; Michael, M.; Ramsay, R.; Warrier, S.; Heriot, A. Prognostic factors influencing survival in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for isolated colorectal peritoneal metastases: A systematic review and meta-analysis. Color. Dis. 2020, 22, 1482–1495. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Brind’Amour, A.; Dubé, P.; Tremblay, J.; Soucisse, M.; Mack, L.; Bouchard-Fortier, A.; McCart, J.; Govindarajan, A.; Bischof, D.; Haase, E.; et al. Canadian Guidelines on the Management of Colorectal Peritoneal Metastases. Curr. Oncol. 2020, 27, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, S.; Cavallaro, G.; La Rovere, F.; Usai, V.; Siragusa, L.; Izzo, P.; Izzo, L.; Fassari, A.; Izzo, S.; Franceschilli, M.; et al. Synchronous liver and peritoneal metastases from colorectal cancer: Is cytoreductive surgery and hyperthermic intraperitoneal chemotherapy combined with liver resection a feasible option? Front. Surg. 2022, 9, 1006591. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Zhang, X.; Shen, Z.; Cai, J.; Tan, Y.; Weng, J.; Rong, Y.; Lin, X. Clinical outcomes of curative treatment for colorectal liver metastases combined with cytoreductive surgery and intraperitoneal chemotherapy for peritoneal metastases: A systematic review and meta-analysis of current evidence. Int. J. Hyperth. 2020, 37, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Shah, R.H.; Vakiani, E.; Nash, G.M.; Skottowe, H.P.; Yaeger, R.; Cercek, A.; Lincoln, A.; Tran, C.; Segal, N.H.; et al. Clinical and genetic determinants of ovarian metastases from colorectal cancer. Cancer 2016, 123, 1134–1143. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Liu, Z.; Yang, J.; Sun, J.; Wang, P. The clinicopathological characteristics, prognosis, and CT features of ovary metastasis from colorectal carcinoma. Transl. Cancer Res. 2021, 10, 3248–3258. [Google Scholar] [CrossRef]
- Van der Meer, R.; de Hingh, I.H.J.T.; Bloemen, J.G.; Janssen, L.; Roumen, R.M.H. Role Of Ovarian Metastases In Colorectal Cancer (ROMIC): A Dutch study protocol to evaluate the effect of prophylactic salpingo-oophorectomy in postmenopausal women. BMC Women’s Health 2022, 22, 441. [Google Scholar] [CrossRef]
- Li, X.; Hu, W.; Sun, H.; Gou, H. Survival outcome and prognostic factors for colorectal cancer with synchronous bone metastasis: A population-based study. Clin. Exp. Metastasis 2021, 38, 89–95. [Google Scholar] [CrossRef]
- Tsao, M.N.; Xu, W.; Wong, R.K.; Lloyd, N.; Laperriere, N.; Sahgal, A.; Rakovitch, E.; Chow, E. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst. Rev. 2018, 1, CD003869. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, X.; Yan, Y.; Yang, J.; Li, S.; Du, X. Effect of radical lymphadenectomy in colorectal cancer with para-aortic lymph node metastasis: A systematic review and meta-analysis. BMC Surg. 2022, 22, 181. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.-S.; Kim, B.H. Is There a Role for Perioperative Pelvic Radiotherapy in Surgically Resected Stage IV Rectal Cancer? A Propensity Score-matched Analysis. Am. J. Clin. Oncol. 2021, 44, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Frykholm, G.J.; Glimelius, B.; Påhlman, L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: Final treatment results of a randomized trial and an evaluation of late secondary effects. Dis. Colon Rectum 1993, 36, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renz, P.; Wegner, R.E.; Hasan, S.; Brookover, R.; Finley, G.; Monga, D.; Raj, M.; McCormick, J.; Kirichenko, A. Survival Outcomes After Surgical Management of the Primary Tumor With and Without Radiotherapy for Metastatic Rectal Adenocarcinoma: A National Cancer Database (NCDB) Analysis. Clin. Color. Cancer 2019, 18, e237–e243. [Google Scholar] [CrossRef] [PubMed]
- Custers, P.A.; Hupkens, B.J.P.; Grotenhuis, B.A.; Kuhlmann, K.F.D.; Breukink, S.O.; Beets, G.L.; Melenhorst, J.; Beets-Tan, R.G.H.; Buijsen, J.; Festen, S.; et al. Selected stage IV rectal cancer patients managed by the watch-and-wait approach after pelvic radiotherapy: A good alternative to total mesorectal excision surgery? Color. Dis. 2022, 24, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, A.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- van Gestel, Y.R.; de Hingh, I.H.; van Herk-Sukel, M.P.; van Erning, F.N.; Beerepoot, L.V.; Wijsman, J.H.; Slooter, G.D.; Rutten, H.J.; Creemers, G.-J.M.; Lemmens, V.E. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol. 2014, 38, 448–454. [Google Scholar] [CrossRef]
- Reboux, N.; Jooste, V.; Goungounga, J.; Robaszkiewicz, M.; Nousbaum, J.-B.; Bouvier, A.-M. Incidence and Survival in Synchronous and Metachronous Liver Metastases from Colorectal Cancer. JAMA Netw. Open 2022, 5, e2236666. [Google Scholar] [CrossRef]
- Guo, Y.; Xiong, B.-H.; Zhang, T.; Cheng, Y.; Ma, L. XELOX vs. FOLFOX in metastatic colorectal cancer: An updated meta-analysis. Cancer Investig. 2016, 34, 94–104. [Google Scholar] [CrossRef]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Colucci, G.; Gebbia, V.; Paoletti, G.; Giuliani, F.; Caruso, M.; Gebbia, N.; Cartenì, G.; Agostara, B.; Pezzella, G.; Manzione, L.; et al. Phase III Randomized Trial of FOLFIRI Versus FOLFOX4 in the Treatment of Advanced Colorectal Cancer: A Multicenter Study of the Gruppo Oncologico Dell’Italia Meridionale. J. Clin. Oncol. 2005, 23, 4866–4875. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Shi, M.; Shen, X.; Yang, C.; Yang, L.; Zhang, J. Capecitabine Plus Irinotecan Versus 5-FU/Leucovorin Plus Irinotecan in the Treatment of Colorectal Cancer: A Meta-analysis. Clin. Color. Cancer 2013, 13, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration. U.S. Food & Drug Administration. Package Insert. AVASTIN® (bevacizumab) Injection, for Intravenous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125085s225lbl.pdf (accessed on 27 January 2023).
- Cunningham, D.; Lang, I.; Marcuello, E.; Lorusso, V.; Ocvirk, J.; Shin, D.B.; Jonker, D.; Osborne, S.; Andre, N.; Waterkamp, D.; et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial. Lancet Oncol. 2013, 14, 1077–1085. [Google Scholar] [CrossRef]
- Kabbinavar, F.F.; Schulz, J.; McCleod, M.; Patel, T.; Hamm, J.T.; Hecht, J.R.; Mass, R.; Perrou, B.; Nelson, B.; Novotny, W.F. Addition of Bevacizumab to Bolus Fluorouracil and Leucovorin in First-Line Metastatic Colorectal Cancer: Results of a Randomized Phase II Trial. J. Clin. Oncol. 2005, 23, 3697–3705. [Google Scholar] [CrossRef] [PubMed]
- Buchler, T.; Pavlík, T.; Melichar, B.; Bortlíček, Z.; Usiakova, Z.; Dušek, L.; Kiss, I.; Kohoutek, M.; Benešová, V.; Vyzula, R.; et al. Bevacizumab with 5-fluorouracil, leucovorin, and oxaliplatin versus bevacizumab with capecitabine and oxaliplatin for metastatic colorectal carcinoma: Results of a large registry-based cohort analysis. BMC Cancer 2014, 14, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltz, L.B.; Clarke, S.; Diaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.-S.; Rivera, F.; et al. Bevacizumab in Combination With Oxaliplatin-Based Chemotherapy As First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef] [Green Version]
- Hochster, H.S.; Hart, L.L.; Ramanathan, R.K.; Childs, B.H.; Hainsworth, J.D.; Cohn, A.L.; Wong, L.; Fehrenbacher, L.; Abubakr, Y.; Saif, M.W.; et al. Safety and Efficacy of Oxaliplatin and Fluoropyrimidine Regimens With or Without Bevacizumab As First-Line Treatment of Metastatic Colorectal Cancer: Results of the TREE Study. J. Clin. Oncol. 2008, 26, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Morse, M.A.; Hurwitz, H.I.; Bendell, J.C.; Gan, T.J.; Hill, S.E.; Clary, B.M. Addition of Bevacizumab to Irinotecan- and Oxaliplatin-Based Preoperative Chemotherapy Regimens Does Not Increase Morbidity after Resection of Colorectal Liver Metastases. J. Am. Coll. Surg. 2008, 206, 96–106. [Google Scholar] [CrossRef]
- Scappaticci, F.A.; Fehrenbacher, L.; Cartwright, T.; Hainsworth, J.D.; Heim, W.; Berlin, J.; Kabbinavar, F.; Novotny, W.; Sarkar, S.; Hurwitz, H. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J. Surg. Oncol. 2005, 91, 173–180. [Google Scholar] [CrossRef]
- Cremolini, C.; Antoniotti, C.; Stein, A.; Bendell, J.; Gruenberger, T.; Rossini, D.; Masi, G.; Ongaro, E.; Hurwitz, H.; Falcone, A.; et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J. Clin. Oncol. 2020, 38, 3314–3324. [Google Scholar] [CrossRef]
- Cremolini, C.; Rossini, D.; Lonardi, S.; Antoniotti, C.; Pietrantonio, F.; Marmorino, F.; Antonuzzo, L.; Boccaccino, A.; Randon, G.; Giommoni, E.; et al. Modified FOLFOXIRI plus panitumumab (mFOLFOXIRI/PAN) versus mFOLFOX6/PAN as initial treatment of patients with unresectable RAS and BRAF wild-type metastatic colorectal cancer (mCRC): Results of the phase III randomized TRIPLETE study by GONO. J. Clin. Oncol. 2022, 40, LBA3505. [Google Scholar] [CrossRef]
- Venderbosch, S.; Nagtegaal, I.D.; Maughan, T.S.; Smith, C.G.; Cheadle, J.P.; Fisher, D.; Kaplan, R.; Quirke, P.; Seymour, M.T.; Richman, S.D.; et al. Mismatch Repair Status and BRAF Mutation Status in Metastatic Colorectal Cancer Patients: A Pooled Analysis of the CAIRO, CAIRO2, COIN, and FOCUS Studies. Clin. Cancer Res. 2014, 20, 5322–5330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenz, H.-J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- A Diaz, L.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Patelli, G.; Tosi, F.; Amatu, A.; Mauri, G.; Curaba, A.; Patanè, D.; Pani, A.; Scaglione, F.; Siena, S.; Sartore-Bianchi, A. Strategies to tackle RAS-mutated metastatic colorectal cancer. ESMO Open 2021, 6, 100156. [Google Scholar] [CrossRef]
- Rowland, A.; Dias, M.M.; Wiese, M.D.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Meta-analysis comparing the efficacy of anti-EGFR monoclonal antibody therapy between KRAS G13D and other KRAS mutant metastatic colorectal cancer tumours. Eur. J. Cancer 2016, 55, 122–130. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R. Panitumumab–FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Brulé, S.; Jonker, D.; Karapetis, C.; O’Callaghan, C.; Moore, M.; Wong, R.; Tebbutt, N.; Underhill, C.; Yip, D.; Zalcberg, J.; et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur. J. Cancer 2015, 51, 1405–1414. [Google Scholar] [CrossRef]
- Moretto, R.; Cremolini, C.; Rossini, D.; Pietrantonio, F.; Battaglin, F.; Mennitto, A.; Bergamo, F.; Loupakis, F.; Marmorino, F.; Berenato, R.; et al. Location of Primary Tumor and Benefit From Anti-Epidermal Growth Factor Receptor Monoclonal Antibodies in Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer. Oncology 2016, 21, 988–994. [Google Scholar] [CrossRef] [Green Version]
- Venook, A.P.; Niedzwiecki, D.; Innocenti, F.; Fruth, B.; Greene, C.; O’Neil, B.H.; Shaw, J.E.; Atkins, J.N.; Horvath, L.E.; Polite, B.N.; et al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2016, 34, 3504. [Google Scholar] [CrossRef]
- Alavi, K.M.; Poylin, V.M.; Davids, J.S.M.; Patel, S.V.M.; Felder, S.M.; Valente, M.A.D.; Paquette, I.M.M.; Feingold, D.L.M. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colonic Volvulus and Acute Colonic Pseudo-Obstruction. Dis. Colon Rectum 2021, 64, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Rowland, A.; Dias, M.M.; Wiese, M.; Kichenadasse, G.; McKinnon, R.; Karapetis, C.; Sorich, M. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br. J. Cancer 2015, 112, 1888–1894. [Google Scholar] [CrossRef] [Green Version]
- Pietrantonio, F.; Petrelli, F.; Coinu, A.; Di Bartolomeo, M.; Borgonovo, K.; Maggi, C.; Cabiddu, M.; Iacovelli, R.; Bossi, I.; Lonati, V.; et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur. J. Cancer 2015, 51, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Cutsem, E.V.; Desai, J. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E–Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Köhne, C.-H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab Plus Irinotecan, Fluorouracil, and Leucovorin As First-Line Treatment for Metastatic Colorectal Cancer: Updated Analysis of Overall Survival According to Tumor KRAS and BRAF Mutation Status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.S.; Fakih, M.; Ali, S.M.; Elvin, J.A.; Schrock, A.B.; Suh, J.; Vergilio, J.-A.; Ramkissoon, S.; Severson, E.; Daniel, S.; et al. Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer 2018, 124, 1358–1373. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Y.; Zheng, Z.-X.; Sun, Y.; Bai, Y.-H.; Shi, Y.-F.; Zhou, L.-X.; Yao, Y.-F.; Wu, A.-W.; Cao, D.-F. Significance of HER2 protein expression and HER2 gene amplification in colorectal adenocarcinomas. World J. Gastrointest. Oncol. 2019, 11, 335–347. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Pro-files: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol. 2018, 6, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Gatalica, Z.; Xiu, J.; Swensen, J.; Vranic, S. Molecular characterization of cancers with NTRK gene fusions. Mod. Pathol. 2019, 32, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Mountjoy, L.J.; Firwana, B.; Liu, A.J.; Almader-Douglas, D.; Mody, K.; Hubbard, J.; Borad, M.; Ahn, D.H.; Murad, M.H.; et al. The Role of Maintenance Strategies in Metastatic Colorectal Cancer. JAMA Oncol. 2020, 6, e194489. [Google Scholar] [CrossRef]
- Chibaudel, B.; Maindrault-Goebel, F.; Lledo, G.; Mineur, L.; André, T.; Bennamoun, M.; Mabro, M.; Artru, P.; Carola, E.; Flesch, M.; et al. Can Chemotherapy Be Discontinued in Unresectable Metastatic Colorectal Cancer? The GERCOR OPTIMOX2 Study. J. Clin. Oncol. 2009, 27, 5727–5733. [Google Scholar] [CrossRef]
- Hegewisch-Becker, S.; Graeven, U.; A Lerchenmüller, C.; Killing, B.; Depenbusch, R.; Steffens, C.-C.; Al-Batran, S.-E.; Lange, T.; Dietrich, G.; Stoehlmacher, J.; et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 1355–1369. [Google Scholar] [CrossRef]
- Koeberle, D.; Betticher, D.C.; von Moos, R.; Dietrich, D.; Brauchli, P.; Baertschi, D.; Matter, K.; Winterhalder, R.; Borner, M.; Anchisi, S.; et al. Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: A randomized phase III non-inferiority trial (SAKK 41/06). Ann. Oncol. 2015, 26, 709–714. [Google Scholar] [CrossRef]
- Simkens, L.H.J.; van Tinteren, H.; May, A.; Tije, A.J.T.; Creemers, G.-J.M.; Loosveld, O.J.L.; E de Jongh, F.; Erdkamp, F.L.G.; Erjavec, Z.; E van der Torren, A.M.; et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): A phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015, 385, 1843–1852. [Google Scholar] [CrossRef]
- Luo, H.; Li, Y.; Wang, W.; Wang, Z.; Yuan, X.; Ma, D.; Wang, F.; Zhang, D.; Lin, D.; Lin, Y.; et al. Single-agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer: Randomized clinical trial of efficacy and safety. Ann. Oncol. 2016, 27, 1074–1081. [Google Scholar] [CrossRef]
- Aparicio, T.; Ghiringhelli, F.; Boige, V.; Le Malicot, K.; Taieb, J.; Bouché, O.; Phelip, J.-M.; François, E.; Borel, C.; Faroux, R.; et al. Bevacizumab Maintenance Versus No Maintenance During Chemotherapy-Free Intervals in Metastatic Colorectal Cancer: A Randomized Phase III Trial (PRODIGE 9). J. Clin. Oncol. 2018, 36, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Rubio, E.; Gómez-España, A.; Massutí, B.; Sastre, J.; Abad, A.; Valladares, M.; Rivera, F.; Safont, M.J.; de Prado, P.M.; Gallén, M.; et al. First-Line XELOX Plus Bevacizumab Followed by XELOX Plus Bevacizumab or Single-Agent Bevacizumab as Maintenance Therapy in Patients with Metastatic Colorectal Cancer: The Phase III MACRO TTD Study. Oncology 2012, 17, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, S.; Uslu, R.; Dane, F.; Yilmaz, U.; Zengin, N.; Buyukunal, E.; Buyukberber, S.; Camci, C.; Sencan, O.; Kilickap, S.; et al. Bevacizumab + Capecitabine as Maintenance Therapy after Initial Bevacizumab + XELOX Treatment in Previously Untreated Patients with Metastatic Colorectal Cancer: Phase III ‘Stop and Go’ Study Results—A Turkish Oncology Group Trial. Oncology 2013, 85, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Hagman, H.; Frödin, J.-E.; Berglund, Å.; Sundberg, J.; Vestermark, L.; Albertsson, M.; Fernebro, E.; Johnsson, A. A randomized study of KRAS-guided maintenance therapy with bevacizumab, erlotinib or metronomic capecitabine after first-line induction treatment of metastatic colorectal cancer: The Nordic ACT2 trial. Ann. Oncol. 2016, 27, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Antoniotti, C.; Lonardi, S.; Aprile, G.; Bergamo, F.; Masi, G.; Grande, R.; Tonini, G.; Mescoli, C.; Cardellino, G.G.; et al. Activity and Safety of Cetuximab Plus Modified FOLFOXIRI Followed by Maintenance With Cetuximab or Bevacizumab for RAS and BRAF Wild-type Metastatic Colorectal Cancer. JAMA Oncol. 2018, 4, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrantonio, F.; Morano, F.; Corallo, S.; Miceli, R.; Lonardi, S.; Raimondi, A.; Cremolini, C.; Rimassa, L.; Bergamo, F.; Sartore-Bianchi, A.; et al. Maintenance Therapy With Panitumumab Alone vs Panitumumab Plus Fluorouracil-Leucovorin in Patients With RAS Wild-Type Metastatic Colorectal Cancer. JAMA Oncol. 2019, 5, 1268–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modest, D.P.; Karthaus, M.; Fruehauf, S.; Graeven, U.; Müller, L.; König, A.O.; von Weikersthal, L.F.; Caca, K.; Kretzschmar, A.; Goekkurt, E.; et al. Panitumumab Plus Fluorouracil and Folinic Acid Versus Fluorouracil and Folinic Acid Alone as Maintenance Therapy in RAS Wild-Type Metastatic Colorectal Cancer: The Randomized PANAMA Trial (AIO KRK 0212). J. Clin. Oncol. 2022, 40, 72–82. [Google Scholar] [CrossRef]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B., III. Bevacizumab in Combination with Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for Previously Treated Metastatic Colorectal Cancer: Results From the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of Aflibercept to Fluorouracil, Leucovorin, and Irinotecan Improves Survival in a Phase III Randomized Trial in Patients With Metastatic Colorectal Cancer Previously Treated With an Oxaliplatin-Based Regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef] [Green Version]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef] [Green Version]
- Sargent, D.; Sobrero, A.; Grothey, A.; O’Connell, M.J.; Buyse, M.; André, T.; Zheng, Y.; Green, E.; Labianca, R.; O’Callaghan, C.; et al. Evidence for Cure by Adjuvant Therapy in Colon Cancer: Observations Based on Individual Patient Data From 20,898 Patients on 18 Randomized Trials. J. Clin. Oncol. 2009, 27, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.I.; Lim, S.-B.; Yoon, Y.S.; Kim, C.W.; Yu, C.S.; Kim, T.W.; Kim, J.H.; Kim, J.C. Comparison of recurrence patterns between ≤5 years and >5 years after curative operations in colorectal cancer patients. J. Surg. Oncol. 2013, 108, 9–13. [Google Scholar] [CrossRef]
- Butte, J.M.; Gönen, M.; Allen, P.J.; Kingham, T.P.; Sofocleous, C.T.; DeMatteo, R.P.; Fong, Y.; Kemeny, N.E.; Jarnagin, W.R.; D’Angelica, M.I. Recurrence After Partial Hepatectomy for Metastatic Colorectal Cancer: Potentially Curative Role of Salvage Repeat Resection. Ann. Surg. Oncol. 2015, 22, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.; Zwaal, C.; Asmis, T.; Cho, C.; Galica, J.; Ginty, A.; Govindarajan, A. An Evidence-Based Guideline for Surveillance of Patients after Curative Treatment for Colon and Rectal Cancer. Curr. Oncol. 2022, 29, 724–740. [Google Scholar] [CrossRef]
- Verberne, C.J.; Zhan, Z.; Heuvel, E.R.V.D.; Oppers, F.; de Jong, A.M.; Grossmann, I.; Klaase, J.M.; de Bock, G.H.; Wiggers, T. Survival analysis of the CEAwatch multicentre clustered randomized trial. Br. J. Surg. 2017, 104, 1069–1077. [Google Scholar] [CrossRef]
- Desch, C.E.; Benson, A.B.; Somerfield, M.R.; Flynn, P.J.; Krause, C.; Loprinzi, C.L.; Minsky, B.D.; Pfister, D.G.; Virgo, K.S.; Petrelli, N.J. Colorectal Cancer Surveillance: 2005 Update of an American Society of Clinical Oncology Practice Guideline. J. Clin. Oncol. 2005, 23, 8512–8519. [Google Scholar] [CrossRef]
- Hardiman, K.M.M.; Felder, S.I.M.; Friedman, G.M.; Migaly, J.M.; Paquette, I.M.M.; Feingold, D.L.M. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Surveillance and Survivorship Care of Patients After Curative Treatment of Colon and Rectal Cancer. Dis. Colon Rectum 2021, 64, 517–533. [Google Scholar] [CrossRef]
- Hyder, O.; Dodson, R.M.; Mayo, S.C.; Schneider, E.B.; Weiss, M.J.; Herman, J.M.; Wolfgang, C.L.; Pawlik, T.M. Post-treatment surveillance of patients with colorectal cancer with surgically treated liver metastases. Surgery 2013, 154, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Sobhani, I.; Itti, E.; Luciani, A.; Baumgaertner, I.; Layese, R.; André, T.; Ducreux, M.; Gornet, J.-M.; Goujon, G.; Aparicio, T.; et al. Colorectal cancer (CRC) monitoring by 6-monthly 18FDG-PET/CT: An open-label multicentre randomised trial. Ann. Oncol. 2018, 29, 931–937. [Google Scholar] [CrossRef]
- Kotani, D.; Oki, E.; Nakamura, Y.; Yukami, H.; Mishima, S.; Bando, H.; Shirasu, H.; Yamazaki, K.; Watanabe, J.; Kotaka, M.; et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023, 29, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hervieu, C.; Christou, N.; Battu, S.; Mathonnet, M. The Role of Cancer Stem Cells in Colorectal Cancer: From the Basics to Novel Clinical Trials. Cancers 2021, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.; Wickremesekera, S.K.; Peng, L.; Tan, S.T.; Itinteang, T. Cancer stem cells in colorectal cancer: A review. J. Clin. Pathol. 2017, 71, 110–116. [Google Scholar] [CrossRef]
- Nenkov, M.; Ma, Y.; Gaßler, N.; Chen, Y. Metabolic Reprogramming of Colorectal Cancer Cells and the Microenvironment: Implication for Therapy. Int. J. Mol. Sci. 2021, 22, 6262. [Google Scholar] [CrossRef] [PubMed]


| Resectability of Metastases | Treatment Goal | Treatment Strategy |
|---|---|---|
| R0-resectable liver or lung metastases | Cure/No evidence of disease | R0 resection of the primary tumor and all metastases Possible better long-term outcomes with perioperative ChT Adjuvant therapy/Observation |
| Potentially resectable metastatic disease | Cure/No evidence of disease | Doublet/Triplet ChT ± targeted therapy R0 resection after conversion to resectable disease Adjuvant therapy/Observation |
| Unlikely to ever become resectable disease | Symptom control Prolonged survival Better QoL | Doublet/Triplet ChT ± targeted therapy Surgery in the presence of (or high-risk for) primary tumor-related symptoms |
| Maximum tumor diameter > 5.5 cm |
| CEA levels > 80 µg/L |
| Time from primary cancer surgery to liver transplantation < 2 years |
| Progression under chemotherapy |
| ECOG performance status < 2 |
| No major comorbidities (medically fit for surgery) |
| None-mild symptoms |
| Stable disease (no tumor progression) under chemotherapy |
| No extra-abdominal metastases * |
| Completeness of cytoreduction (CC score 0–1) possible |
| Peritoneal cancer index < 20 |
| Patient’s motivation and informed consent |
| Study (Trial Name) | Induction Chemotherapy | Maintenance Therapy | Outcomes | Results | Significance |
|---|---|---|---|---|---|
| Chibaudel 2009 [162] (GERCOR OPTIMOX2) | mFOLFOX7 | FP vs. No treatment | PFS OS | 8.6 vs. 6.6 months, HR 0.61 23.8 vs. 19.5 months, HR 0.88 | p = 0.0017 p = NS |
| Hegewisch-Becker 2015 [163] (AIO 0207 #) | CAPOX/FOLFOX + BEVA | FP + BEVA vs. BEVA vs. No treatment | PFS OS Grade 3–4 AEs | 6.3 vs. 4.6 vs. 3.5 months 20.2 vs. 21.9 vs. 23.1 30.4% vs. 24.3% vs. 13.3% | p < 0.0001 p = NS N/A * |
| Koeberle 2015 [164] (SAKK 41/06) | FP/FOLFOX/FOLFIRI + BEVA | BEVA vs. No treatment | PFS OS Grade 3–4 AEs | 9.5 vs. 8.5 months, HR 0.75 25.4 vs. 23.8 months, HR 0.83 6.1% vs. 0.8% | p = 0.025 p = NS N/A * |
| Simkens 2015 [165] (CAIRO3) | CAPOX + BEVA | CAP/BEVA vs. No treatment | PFS OS Grade 3–4 AEs | 8.5 vs. 4.1 months, HR 0.4 25.9 vs. 22.4 months, HR 0.83 60% vs. 34% | p < 0.0001 p = 0.06 p < 0.0001 |
| Luo 2016 [166] | CAPOX/FOLFOX | CAP vs. No treatment | PFS OS Grade 3–4 AEs | 6.4 vs. 3.4 months, HR 0.54 25.6 vs. 23.3 months, HR 0.85 41.9% vs. 22.4% | p < 0.001 p = NS N/A * |
| Aparicio 2018 [167] (PRODIGE 9) | FOLFIRI + BEVA | BEVA vs. No treatment | PFS PFS rate (12 months) OS | 9.2 vs. 8.9 months, HR 0.91 30.2% vs. 21% 21.7 vs. 22 months, HR 1.11 | p = NS p = NS p = NS |
| Dìaz-Rubio 2012 [168] (MACRO TTD) | CAPOX + BEVA | BEVA vs. Continuation of ChT | PFS OS Grade 3–4 AEs | 9.7 vs. 10.4 months, HR 1.10 20 vs. 23.2 months, HR 1.05 55% vs. 47% | p = NS p = NS N/A * |
| Yalcin 2013 [169] (Stop and Go) | CAPOX + BEVA | CAP + BEVA vs. Continuation of ChT | PFS OS Grade 3–4 AEs | 11.0 vs. 8.3 months, HR 0.6 23.8 vs. 20.2 months 34.4% vs. 48.4% | p = 0.002 p = NS p = NS |
| Hagman 2016 [170] (Nordic ACT2) | CAPOX/FOLFOX or CAPIRI/FOLFIRI ± BEVA | BEVA vs. BEVA + ERLO (KRAS WT) | PFS PFS rate (3 months) OS Grade 3–4 AEs | 3.6 vs. 5.7 months, HR 0.93 64.7% vs. 63.6% 30.7 vs. 20.6 months, HR 0.58 25.7% vs. 58.2% | p = NS p = NS p = 0.051 N/A * |
| BEVA vs. CAP (KRAS MT) | PFS PFS rate (3 months) OS Grade 3–4 AEs | 3.9 vs. 3.7 months, HR 1.19 75% vs. 66.7% 26.4 vs. 28.0 months, HR 1.57 20.6% vs. 15.2% | p = NS p = NS p = NS N/A * | ||
| Cremolini 2018 [171] | mFOLFOXIRI + CET | CET vs. BEVA (KRAS WT) | PFS OS Grade 3–4 AEs | 13.3 vs. 10.8 months, HR 0.73 37.5 vs. 37 months, HR 0.98 25% vs. 8% | p = NS p = NS N/A * |
| Pietrantonio 2019 [172] | FOLFOX + PANI | FP + PANI vs. PANI | PFS rate (10 months) OS rate (18 months) Grade 3–4 AEs | 59.9% vs. 49% 66.4% vs. 62.4% 42.4% vs. 20.3% | p = 0.01 p = NS N/A * |
| Modest 2021 [173] (PANAMA) | FOLFOX + PANI | FP + PANI vs. FP | PFS OS | 8.8 vs. 5.7 months, HR 0.72 28.7 vs. 25.7 months, HR 0.84 | p = 0.014 p = NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez Dominguez, O.; Yilmaz, S.; Steele, S.R. Stage IV Colorectal Cancer Management and Treatment. J. Clin. Med. 2023, 12, 2072. https://doi.org/10.3390/jcm12052072
Hernandez Dominguez O, Yilmaz S, Steele SR. Stage IV Colorectal Cancer Management and Treatment. Journal of Clinical Medicine. 2023; 12(5):2072. https://doi.org/10.3390/jcm12052072
Chicago/Turabian StyleHernandez Dominguez, Oscar, Sumeyye Yilmaz, and Scott R. Steele. 2023. "Stage IV Colorectal Cancer Management and Treatment" Journal of Clinical Medicine 12, no. 5: 2072. https://doi.org/10.3390/jcm12052072
APA StyleHernandez Dominguez, O., Yilmaz, S., & Steele, S. R. (2023). Stage IV Colorectal Cancer Management and Treatment. Journal of Clinical Medicine, 12(5), 2072. https://doi.org/10.3390/jcm12052072






