Prospective Evaluation of Two Cohorts of Non-Operatively Treated Patients with Displaced vs. Minimally and Non-Displaced Distal Radius Fractures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohorts
2.2. Primary Outcome Measure
- Carpal tunnel syndrome and chronic regional pain syndrome;
- Unspecific sensory disturbances;
- Flexor tendon rupture and irritation;
- Extensor tendon rupture and irritation;
- Infection: superficial/deep;
- Vascular compromised (capillary refill ≥2 s).
2.3. Secondary Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Primary Outcome: Complications
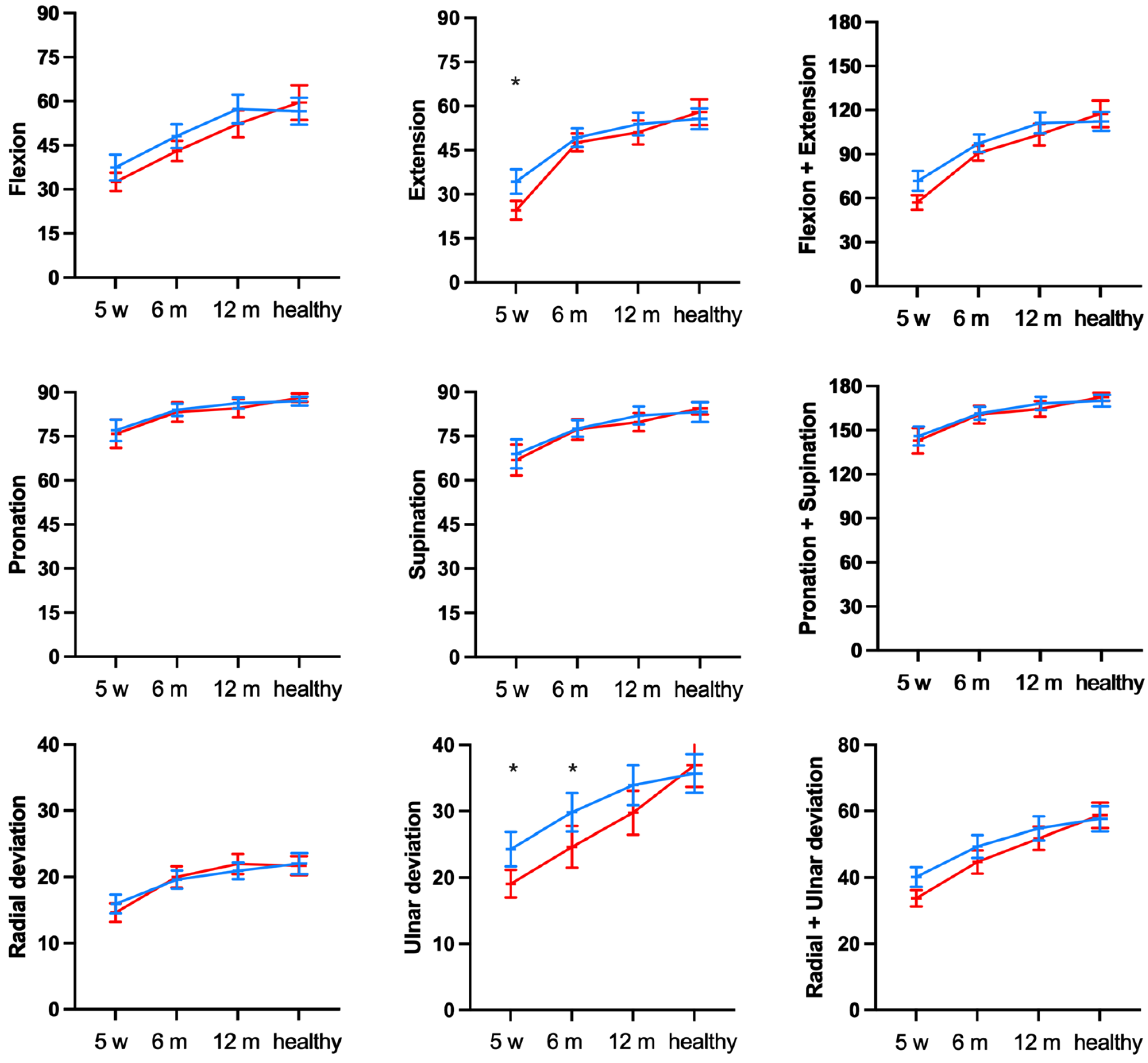
3.2. Secondary Outcomes: Functional Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raudasoja, L.; Aspinen, S.; Vastamäki, H.; Ryhänen, J.; Hulkkonen, S. Epidemiology and Treatment of Distal Radius Fractures in Finland-a Nationwide Register Study. J. Clin. Med. 2022, 11, 2851. [Google Scholar] [CrossRef] [PubMed]
- Driessen, J.H.M.; Hansen, L.; Eriksen, S.A.; van Onzenoort, H.A.W.; Henry, R.M.A.; van den Bergh, J.; Abrahamsen, B.; Vestergaard, P.; de Vries, F. The Epidemiology of Fractures in Denmark in 2011. Osteoporos. Int. 2016, 27, 2017–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundhedsstyrelsen. National Klinisk Retningslinje for Behandling Af Håndledsnære Brud (Distal Radiusfraktur). Available online: https://www.sst.dk/-/media/Udgivelser/2014/NKR-H%C3%A5ndledsn%C3%A6re-underarmsbrud/National-klinisk-retningslinie-for-behandling-af-haandledsnaere-brud.ashx (accessed on 8 February 2023).
- Li, Q.; Ke, C.; Han, S.; Xu, X.; Cong, Y.X.; Shang, K.; Liang, J.D.; Zhang, B.F. Nonoperative Treatment Versus Volar Locking Plate Fixation for Elderly Patients with Distal Radial Fracture: A Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 263. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Li, L.; Yan, H.; Zhou, F.; Gao, W. Safety and Efficacy of Operative Versus Nonsurgical Management of Distal Radius Fractures in Elderly Patients: A Systematic Review and Meta-Analysis. J. Hand Surg. Am. 2016, 41, 404–413. [Google Scholar] [CrossRef]
- Woolnough, T.; Axelrod, D.; Bozzo, A.; Koziarz, A.; Koziarz, F.; Oitment, C.; Gyemi, L.; Gormley, J.; Gouveia, K.; Johal, H. What is the relative effectiveness of the various surgical treatment options for distal radius fractures? A systematic review and network meta-analysis of randomized controlled trials. Clin. Orthop. Relat. Res. 2021, 479, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.R.; Presson, A.P.; McFarland, M.M.; Zhang, C.; Sirniö, K.; Mulders, M.A.M.; Schep, N.W.L.; Tyser, A.R.; Kazmers, N.H. Volar Locked Plating Versus Closed Reduction and Casting for Acute, Displaced Distal Radial Fractures in the Elderly: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Bone Jt. Surg. 2020, 102, 1280–1288. [Google Scholar] [CrossRef]
- Lawson, A.; Na, M.; Naylor, J.M.; Lewin, A.M.; Harris, I.A. Volar Locking Plate Fixation Versus Closed Reduction for Distal Radial Fractures in Adults: A Systematic Review and Meta-Analysis. JBJS Rev. 2021, 9, e20.00022. [Google Scholar] [CrossRef]
- Thorninger, R.; Wæver, D.; Tjørnild, M.; Lind, M.; Rölfing, J.D. VOLCON: A Randomized Controlled Trial Investigating Complications and Functional Outcome of Volar Plating vs. Casting of Unstable Distal Radius Fractures in Patients Older than 65 Years. J. Orthop. Traumatol. 2022, 23, 54. [Google Scholar] [CrossRef]
- American Academy of Orthopaedic Surgeons (AAOS). Management of Distal Radius Fractures Evidence-Based Clinical Practice Guideline. Available online: www.aaos.org/drfcpg (accessed on 8 February 2023).
- The British Society for Surgery of the Hand. Best Practice for Management of Distal Radial Fractures. Available online: https://www.bssh.ac.uk/_userfiles/pages/files/professionals/Radius/Blue%20Book%20DRF%20Final%20Document.pdf (accessed on 8 February 2023).
- Pedersen, J.; Mortensen, S.O.; Rölfing, J.D.; Thorninger, R. A Protocol for a Single-Center, Single-Blinded Randomized-Controlled Trial Investigating Volar Plating Versus Conservative Treatment of Unstable Distal Radius Fractures in Patients Older Than 65 Years. BMC Musculoskelet. Disord. 2019, 20, 309. [Google Scholar] [CrossRef] [Green Version]
- Schønnemann, J.O.; Eggers, J. Validation of the Danish Version of the Quick-Disabilities of Arm, Shoulder and Hand Questionnaire. Dan. Med. J. 2016, 63, A5306. [Google Scholar] [PubMed]
- London, D.A.; Stepan, J.G.; Boyer, M.I.; Calfee, R.P. Performance Characteristics of the Verbal QuickDASH. J. Hand Surg. Am. 2014, 39, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldhahn, J.; Beaton, D.; Ladd, A.; Macdermid, J.; Hoang-Kim, A. Recommendation for Measuring Clinical Outcome in Distal Radius Fractures: A Core Set of Domains for Standardized Reporting in Clinical Practice and Research. Arch. Orthop. Trauma Surg. 2014, 134, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Franchignoni, F.; Vercelli, S.; Giordano, A.; Sartorio, F.; Bravini, E.; Ferriero, G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J. Orthop. Sport. Phys. Ther. 2014, 44, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Institute for Work & Health. The Dash Outcome Measure—Frequently Asked Questions (FAQ): What Is Considered to Be a Clinically Important Change for the Dash/QuickDASH? Available online: https://dash.iwh.on.ca/faq (accessed on 17 February 2023).
- Hansen, A.Ø.; Knygsand-Roenhoej, K.; Ardensø, K. Danish Version of the Patient-Rated Wrist/Hand Evaluation Questionnaire: Translation, Cross-Cultural Adaptation, Test–Retest Reliability and Construct Validity. Hand Ther. 2019, 24, 22–30. [Google Scholar] [CrossRef]
- Walenkamp, M.M.; de Muinck Keizer, R.J.; Goslings, J.C.; Vos, L.M.; Rosenwasser, M.P.; Schep, N.W. The Minimum Clinically Important Difference of the Patient-Rated Wrist Evaluation Score for Patients with Distal Radius Fractures. Clin. Orthop. Relat. Res. 2015, 473, 3235–3241. [Google Scholar] [CrossRef] [Green Version]
- Saving, J.; Wahlgren, S.S.; Olsson, K.; Enocson, A.; Ponzer, S.; Sköldenberg, O.; Wilcke, M.; Navarro, C.M. Nonoperative Treatment Compared with Volar Locking Plate Fixation for Dorsally Displaced Distal Radial Fractures in the Elderly: A Randomized Controlled Trial. J. Bone Jt. Surg. Am. 2019, 101, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.F.; McDonald, C.; Chenier, T.C. Measurement of Grip Strength: Validity and Reliability of the Sphygmomanometer and Jamar Grip Dynamometer. J. Orthop. Sport. Phys. Ther. 1992, 16, 215–219. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, M.G.; Shin, S.J. What Is the Minimum Clinically Important Difference in Grip Strength? Clin. Orthop. Relat. Res. 2014, 472, 2536–2541. [Google Scholar] [CrossRef] [Green Version]
- Cooke, M.E.; Gu, A.; Wessel, L.E.; Koo, A.; Osei, D.A.; Fufa, D.T. Incidence of Carpaæ Tunnel Syndrome after Distal Radius Fracture. J. Hand Surg. Glob. Online 2022, 4, 324–327. [Google Scholar] [CrossRef]
- Arora, R.; Lutz, M.; Deml, C.; Krappinger, D.; Haug, L.; Gabl, M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J. Bone Jt. Surg. Am. 2011, 93, 2146–2153. [Google Scholar] [CrossRef] [Green Version]
- Hassellund, S.S.; Williksen, J.H.; Laane, M.M.; Pripp, A.; Rosales, C.P.; Karlsen, Ø.; Madsen, J.E.; Frihagen, F. Cast immobilization is non-inferior to volar locking plates in relation to QuickDASH after one year in patients aged 65 years and older: A randomized controlled trial of displaced distal radius fractures. Bone Joint J. 2021, 103, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.; Naylor, J.M.; Buchbinder, R.; Ivers, R.; Balogh, Z.J.; Smith, P.; Xuan, W.; Howard, K.; Vafa, A.; Perriman, D.; et al. Surgical plating vs closed reduction for fractures in the distal radius in older patients: A randomized clinical trial. JAMA Surg. 2021, 156, 229–237. [Google Scholar] [PubMed]
- Ochen, Y.; Peek, J.; van der Velde, D.; Beeres, F.J.P.; van Heijl, M.; Groenwold, R.H.H.; Houwert, R.M.; Heng, M. Operative vs nonoperative treatment of distal radius fractures in adults: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e203497. [Google Scholar] [CrossRef] [Green Version]
- Thorninger, R.; Wæver, D.; Pedersen, J.; Tvedegaard-Christensen, J.; Tjørnild, M.; Lind, M.; Rölfing, J.D. Objective Outcome Measures Continue to Improve from 6 to 12 Months after Conservatively Treated Distal Radius Fractures in the Elderly-a Prospective Evaluation of 50 Patients. J. Clin. Med. 2021, 10, 1883. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, S.S.T.; Moazzeni, S.S.; Asgari, S.; Mirbolouk, M.; Azizi, F.; Hadaegh, F. Association between Wrist Circumference and Risk of Any Fracture in Adults: Findings from 15 Years of Follow-up in the Tehran Lipid and Glucose Study. J. Clin. Med. 2022, 11, 7048. [Google Scholar] [CrossRef]
- Oh, C.H.; Kim, J.; Kim, J.; Yoon, S.; Jung, Y.; Lee, H.I.; Choi, J.; Lee, S.; Han, S. The Association of Low Skeletal Muscle Mass with Complex Distal Radius Fracture. J. Clin. Med. 2022, 11, 5581. [Google Scholar] [CrossRef] [PubMed]
- Olech, J.; Konieczny, G.; Tomczyk, L.; Morasiewicz, P. A Randomized Trial Assessing the Muscle Strength and Range of Motion in Elderly Patients Following Distal Radius Fractures Treated with 4- and 6-Week Cast Immobilization. J. Clin. Med. 2021, 10, 5774. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, A.; Vernet, P.; Botero, S.S.; Igeta, Y.; Hidalgo Diaz, J.J.; Liverneaux, P. Conservative Treatment of Distal Fractures after the Age of 65: A Review of Literature. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 1469–1475. [Google Scholar] [CrossRef]
- Shem Tov, L.; Matot, I. Frailty and Anesthesia. Curr. Opin. Anaesthesiol. 2017, 30, 409–417. [Google Scholar] [CrossRef]
- Steinmetz, J.; Rasmussen, L.S. Anesthesia and the Risk of Dementia in the Elderly. La Presse Méd. 2018, 47, e45–e51. [Google Scholar] [CrossRef]
- Moss, N.; Bueno-Cavanillas, A.; Cano-Ibáñez, N.; Khan, K.S. Evidence-Based Medicine Needs Patient and Public Involvement to Remain Relevant: A Proposal for a New Curriculum. Semergen 2022, 49, 101877. [Google Scholar] [CrossRef] [PubMed]
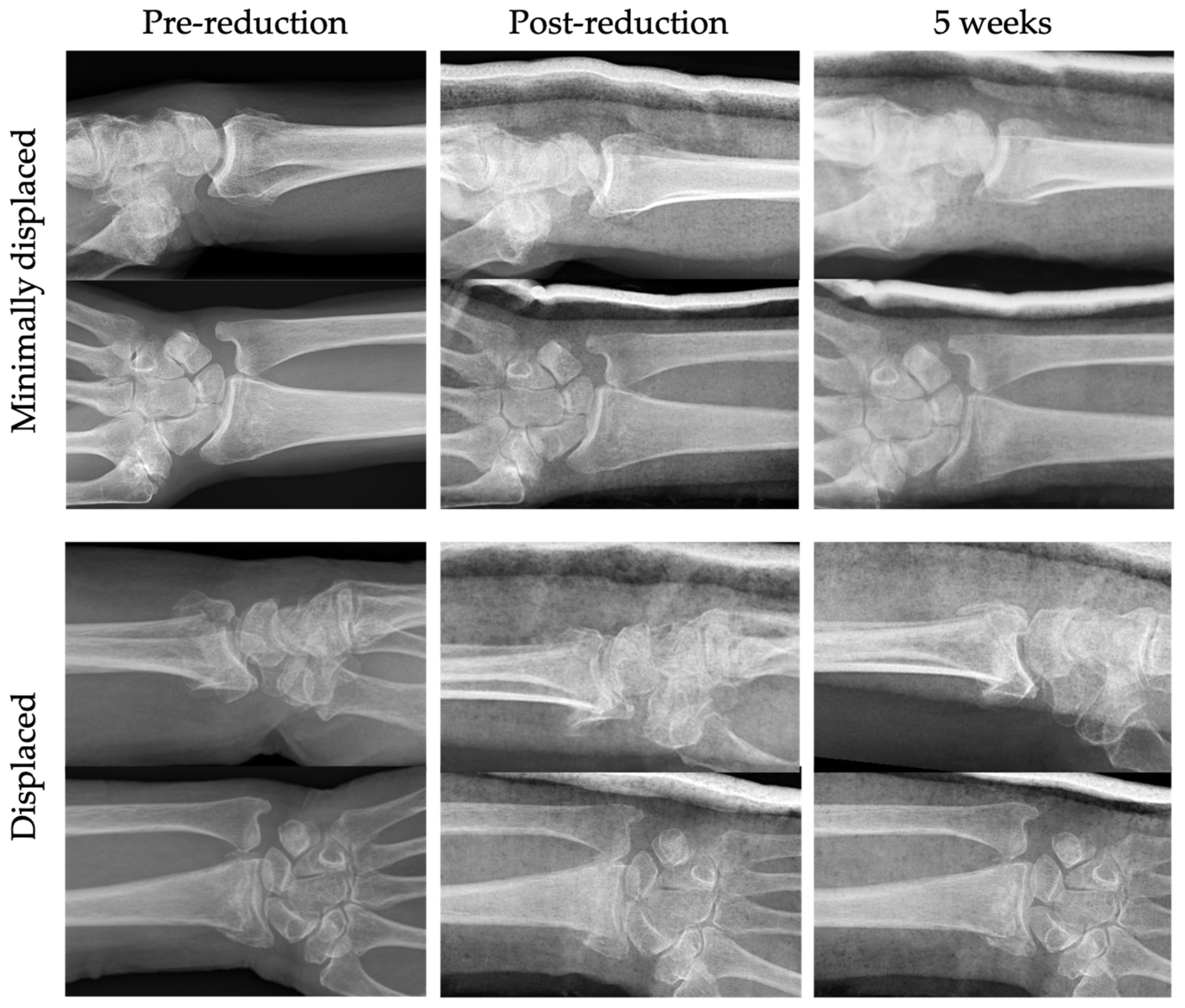


| Displaced | Minimally/Non-Displaced | ||
|---|---|---|---|
| n = 50 | n = 50 | ||
| Sex | |||
| Female | 40 (80%) | 41 (82%) | |
| Male | 10 (20%) | 9 (18%) | |
| Age (years) | |||
| Median (Min., IQR, Max.) | 74 (65, 69–81, 91) | 73 (65, 70–78, 100) | |
| AO/OTA classification | |||
| A1/A2/A3 | 0/22/16 | 0/17/15 | |
| B1/B2/B3 | 0/6/6 | 2/4/4 | |
| C1/C2/C3 | 0/5/1 | 0/2/0 | |
| Fractured side | |||
| Right | 24 (48%) | 18 (36%) | |
| Left | 26 (52%) | 32 (64%) | |
| Hand dominance | |||
| Right | 46 (92%) | 43 (86%) | |
| Left | 1 (2%) | 4 (8%) | |
| Ambidextrous | 1 (2%) | 3 (6%) | |
| Missing data | 2 (4%) | 0 (0%) | |
| Dominant side fractured * | 23 (46%) | 20 (40%) | |
| Working status | |||
| Full-time/part-time work | 1 (2%) | 0 (0%) | |
| Volunteer work | 2 (4%) | 3 (6%) | |
| Retired | 45 (90%) | 47 (94%) | |
| Missing data | 2 (4%) | 0 (0%) | |
| Smoking status | |||
| Non-smoker | 37 (74%) | 41 (82%) | |
| Smoker | 9 (18%) | 9 (18%) | |
| Missing data | 4 (8%) | 0 (0%) | |
| Alcohol consumption ** | |||
| <7/14 units/week | 38 (76%) | 44 (88%) | |
| >7/14 units/week | 8 (16%) | 6 (12%) | |
| Missing data | 4 (8%) | 0 (0%) | |
| ASA | |||
| ASA class 1 | 13 (26%) | 16 (32%) | |
| ASA class 2 | 30 (60%) | 25 (50%) | |
| ASA class 3 | 6 (12%) | 9 (18%) | |
| ASA class 4–5 | 0 (0%) | 0 (0%) | |
| Missing data | 1 (2%) | 0 (0%) | |
| Comorbidities | |||
| Hypertension | 23 (46%) | 22 (44%) | |
| Diabetes | 6 (12%) | 3 (6%) | |
| Osteoporosis | 3 (6%) | 7 (14%) | |
| Depression | 4 (8%) | 9 (18%) | |
| Medications | |||
| 0 | 8 (16%) | 5 (10%) | |
| 1–4 | 26 (52%) | 38 (76%) | |
| ≥5 | 16 (32%) | 7 (14%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorninger, R.; Wæver, D.; Tjørnild, M.; Lind, M.; Rölfing, J.D. Prospective Evaluation of Two Cohorts of Non-Operatively Treated Patients with Displaced vs. Minimally and Non-Displaced Distal Radius Fractures. J. Clin. Med. 2023, 12, 2076. https://doi.org/10.3390/jcm12052076
Thorninger R, Wæver D, Tjørnild M, Lind M, Rölfing JD. Prospective Evaluation of Two Cohorts of Non-Operatively Treated Patients with Displaced vs. Minimally and Non-Displaced Distal Radius Fractures. Journal of Clinical Medicine. 2023; 12(5):2076. https://doi.org/10.3390/jcm12052076
Chicago/Turabian StyleThorninger, Rikke, Daniel Wæver, Michael Tjørnild, Martin Lind, and Jan Duedal Rölfing. 2023. "Prospective Evaluation of Two Cohorts of Non-Operatively Treated Patients with Displaced vs. Minimally and Non-Displaced Distal Radius Fractures" Journal of Clinical Medicine 12, no. 5: 2076. https://doi.org/10.3390/jcm12052076
APA StyleThorninger, R., Wæver, D., Tjørnild, M., Lind, M., & Rölfing, J. D. (2023). Prospective Evaluation of Two Cohorts of Non-Operatively Treated Patients with Displaced vs. Minimally and Non-Displaced Distal Radius Fractures. Journal of Clinical Medicine, 12(5), 2076. https://doi.org/10.3390/jcm12052076





