Neurokinin-1 Receptor Antagonists as a Potential Novel Therapeutic Option for Osteosarcoma Patients
Abstract
:1. Introduction
2. SP/NK-1R System in Osteosarcoma
3. NK-1R Antagonists as Anticancer Drugs: A Therapeutic Approach against Osteosarcoma: Mechanistic Insights
3.1. Role of NK-1R Antagonists as Antiproliferative and Proapoptotic Agents in Osteosarcoma
3.2. Role of NK-1R Antagonists as Antiangiogenic Agents in Osteosarcoma
3.3. Role of NK-1R Antagonists as Agents That Counteract the Warburg Effect in Osteosarcoma
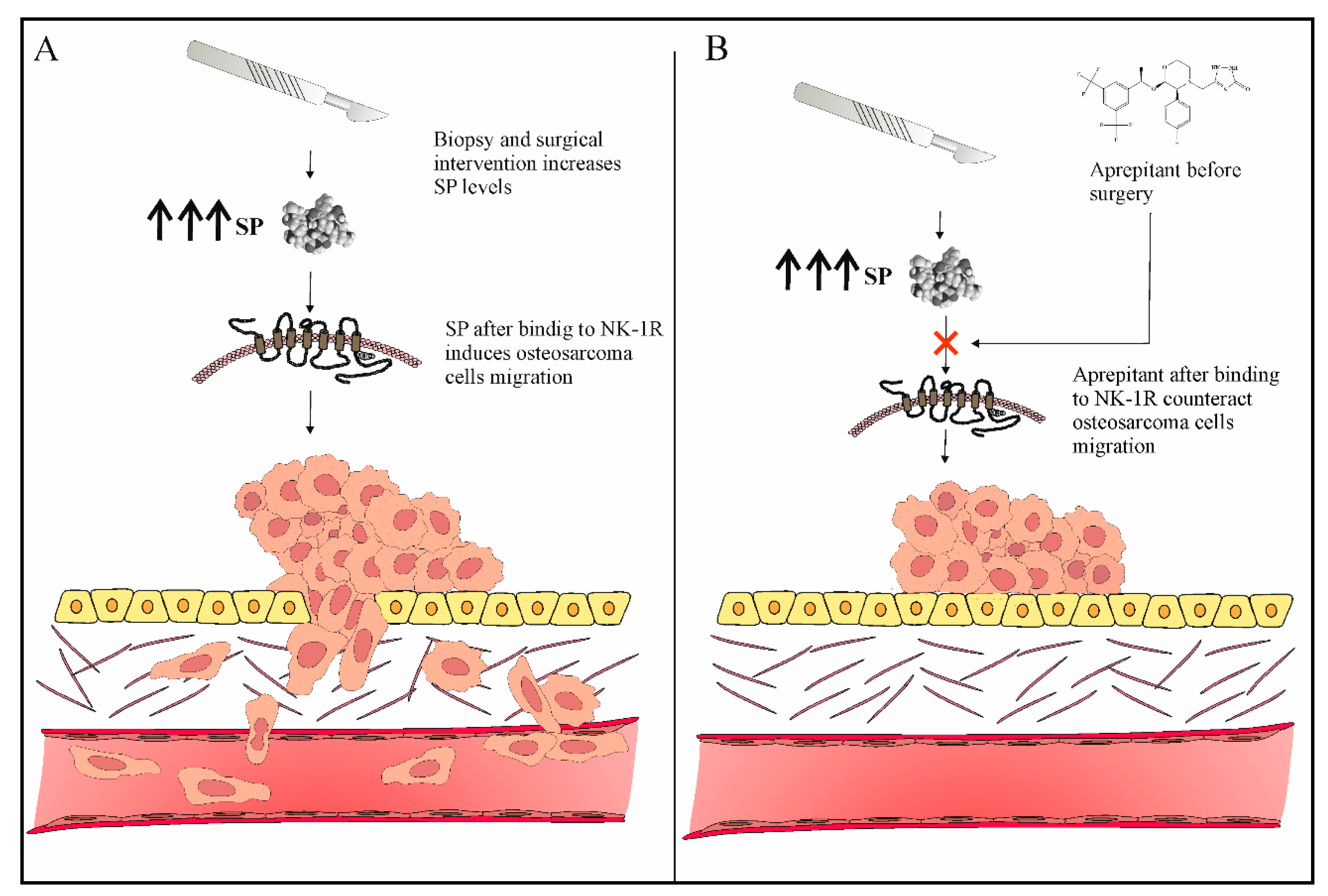
3.4. Role of NK-1R Antagonists as Antimetastatic Agents in Osteosarcoma
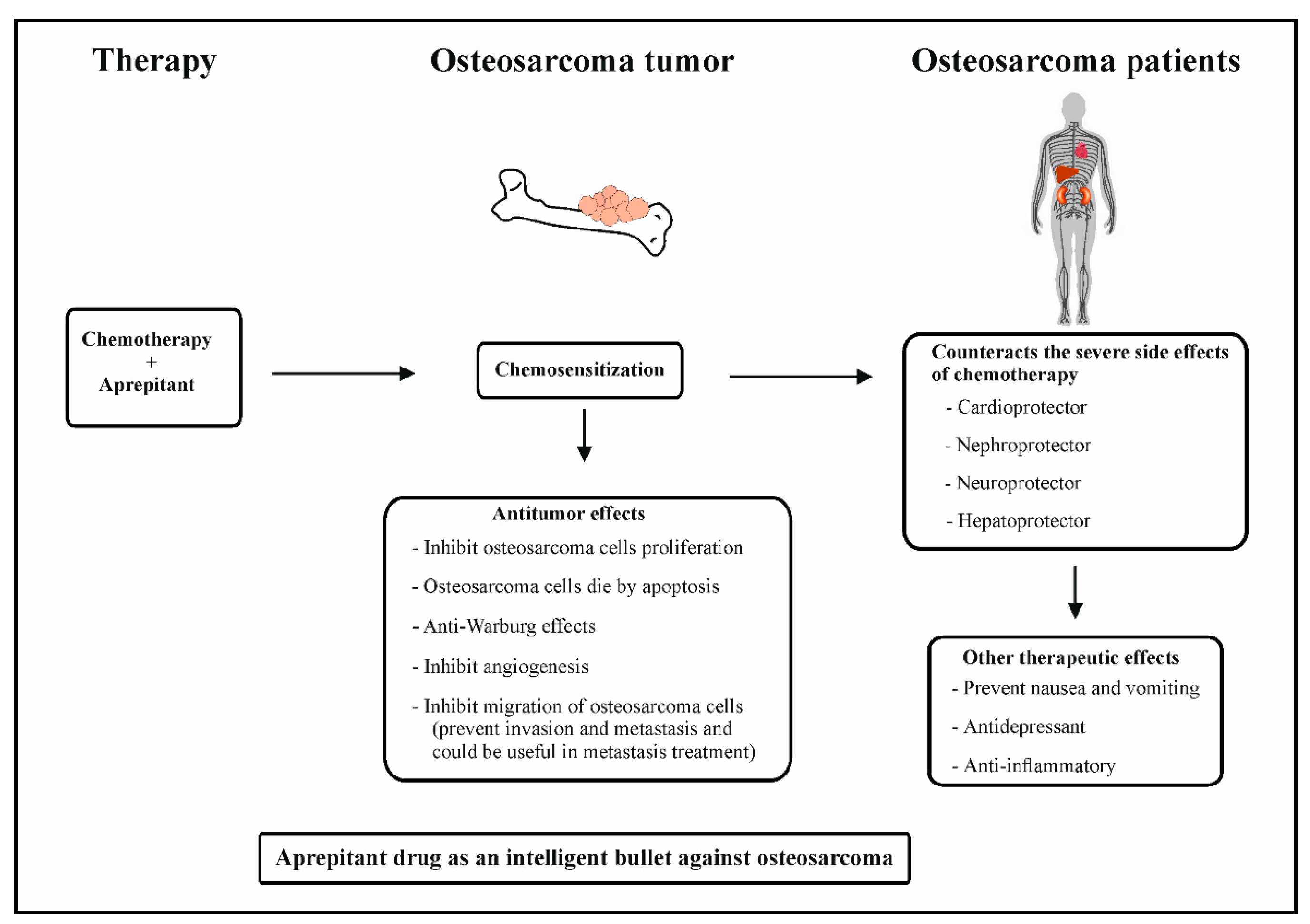
4. Combination of Chemotherapy and Aprepitant in Osteosarcoma Therapy
5. Safety and Efficacy
6. NK-1R Antagonists as Intelligent Drugs in Osteosarcoma Therapy
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wittig, J.C.; Bickels, J.; Priebat, D.; Jelinek, J.; Kellar-Graney, K.; Shmookler, B.; Malawer, M.M. Osteosarcoma: A multidisciplinary approach to diagnosis and treatment. Am. Fam. Physician 2002, 65, 1123–1132. [Google Scholar] [PubMed]
- Odri, G.A.; Tchicaya-Bouanga, J.; Yoon, D.J.Y.; Modrowski, D. Metastatic Progression of Osteosarcomas: A Review of Current Knowledge of Environmental versus Oncogenic Drivers. Cancers 2022, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Wurtz, L.D.; Collier, C.D. Ninety Percent or Greater Tumor Necrosis Is Associated with Survival and Social Determinants of Health in Patients with Osteosarcoma in the National Cancer Database. Clin. Orthop. Relat. Res. 2022, 481, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.; Coveñas, R.; Muñoz, M. Combination Therapy of Chemotherapy or Radiotherapy and the Neurokinin-1 Receptor Antagonist Aprepitant: A New Antitumor Strategy? Curr. Med. Chem. 2023, 30, 1798–1812. [Google Scholar] [CrossRef]
- Muñoz, M.; Berger, M.; Rosso, M.; Gonzalez-Ortega, A.; Carranza, A.; Coveñas, R. Antitumor activity of neurokinin-1 receptor antagonists in MG-63 human osteosarcoma xenografts. Int. J. Oncol. 2013, 44, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, M.; Rosso, M.; Robles-Frías, M.J.; Salinas-Martín, M.V.; Coveñas, R. Immunolocalization of the neurokinin-1 receptor: A new target in the treatment of human malignant melanoma. Lab. Investig. 2010, 90, 1259–121269. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; Rosso, M.; Pérez, A.; Covenas, R.; Rosso, R.; Zamarriego, C.; Soult, J.A.; Montero, I. Antitumoral Action of the Neurokinin-1-Receptor Antagonist L-733,060 and Mitogenic Action of Substance P on Human Retinoblastoma Cell Lines. Investig. Opthalmology Vis. Sci. 2005, 46, 2567–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, M.; Neth, O.; Ilmer, M.; Garnier, A.; Salinas-Martín, M.V.; de Agustin Asencio, J.C.; von Schweinitz, D.; Kappler, R.; Muñoz, M. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J. Hepatol. 2014, 60, 985–994. [Google Scholar] [CrossRef]
- Akazawa, T.; Kwatra, S.G.; Goldsmith, L.E.; Richardson, M.D.; Cox, E.A.; Sampson, J.H.; Kwatra, M.M. A constitutively active form of neurokinin 1 receptor and neurokinin 1 receptor-mediated apoptosis in glioblastomas. J. Neurochem. 2009, 109, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- Kolorz, J.; Demir, S.; Gottschlich, A.; Beirith, I.; Ilmer, M.; Lüthy, D.; Walz, C.; Dorostkar, M.M.; Magg, T.; Hauck, F.; et al. The Neurokinin-1 Receptor Is a Target in Pediatric Rhabdoid Tumors. Curr. Oncol. 2021, 29, 94–110. [Google Scholar] [CrossRef]
- Molinos-Quintana, A.; Trujillo-Hacha, P.; Piruat, J.I.; Bejarano-García, J.A.; García-Guerrero, E.; Pérez-Simón, J.A.; Muñoz, M. Human acute myeloid leukemia cells express Neurokinin-1 receptor, which is involved in the antileukemic effect of Neurokinin-1 receptor antagonists. Investig. New Drugs 2019, 37, 17–26. [Google Scholar] [CrossRef]
- Muñoz, M.; González-Ortega, A.; Coveñas, R. The NK-1 receptor is expressed in human leukemia and is involved in the antitumor action of aprepitant and other NK-1 receptor antagonists on acute lymphoblastic leukemia cell lines. Investig. New Drugs 2012, 30, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Alsaeed, M.A.; Ebrahimi, S.; Alalikhan, A.; Hashemi, S.F.; Hashemy, S.I. The Potential In Vitro Inhibitory Effects of Neurokinin-1 Receptor (NK-1R) Antagonist, Aprepitant, in Osteosarcoma Cell Migration and Metastasis. BioMed Res. Int. 2022, 2022, 8082608. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Coveñas, R. The Neurokinin-1 Receptor Antagonist Aprepitant: An Intelligent Bullet against Cancer? Cancers 2020, 12, 2682. [Google Scholar] [CrossRef]
- Muñoz, M.F.; Argüelles, S.; Rosso, M.; Medina, R.; Coveñas, R.; Ayala, A.; Muñoz, M. The Neurokinin-1 Receptor Is Essential for the Viability of Human Glioma Cells: A Possible Target for Treating Glioblastoma. BioMed Res. Int. 2022, 2022, 6291504. [Google Scholar] [CrossRef]
- Skidgel, R.A.; Engelbrecht, S.; Johnson, A.R.; Erdös, E.G. Hydrolysis of substance P and neurotensin by converting enzyme and neutral endopeptidase. Peptides 1984, 5, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Nausch, I.; Heymann, E. Substance P in Human Plasma Is Degraded by Dipeptidyl Peptidase IV, Not by Cholinesterase. J. Neurochem. 1985, 44, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, M.; Shen, R.; Nanus, D.M. Involvement of neutral endopeptidase in neoplastic progression. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2005, 1751, 52–59. [Google Scholar] [CrossRef]
- Cutrona, G.; Tasso, P.; Dono, M.; Roncella, S.; Ulivi, M.; Carpaneto, E.M.; Fontana, V.; Comis, M.; Morabito, F.; Spinelli, M.; et al. CD10 is a marker for cycling cells with propensity to apoptosis in childhood ALL. Br. J. Cancer 2002, 86, 1776–1785. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Sharma, J.; Sikarwar, P.; Kakkar, A.K. Dipeptidyl peptidase 4 (DPP-4) inhibitors and the risk of lung cancer: Current evidence and future directions. Expert Rev. Clin. Pharmacol. 2023, 16, 39–47. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Mo, X.; Chen, G.; Lin, L. Synergistic relationship between dipeptidyl peptidase IV and neutral endopeptidase expression and the combined prognostic significance in osteosarcoma patients. Med. Oncol. 2013, 30, 608. [Google Scholar] [CrossRef] [PubMed]
- Davoodian, M.; Boroumand, N.; Mehrabi Bahar, M.; Jafarian, A.H.; Asadi, M.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in breast cancer. Mol. Biol. Rep. 2019, 46, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Zhou, J.; Wen, L.; Zhu, Y.; Luo, Y.; Wang, W. The relationship between the expression of Ki-67 and the prognosis of osteosarcoma. BMC Cancer 2021, 21, 210. [Google Scholar] [CrossRef] [PubMed]
- Hennig, I.M.; Laissue, J.A.; Horisberger, U.; Reubi, J.-C. Substance-P receptors in human primary neoplasms: Tumoral and vascular localization. Int. J. Cancer 1995, 61, 786–792. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Pacini, M.; Geppetti, P.; Alessandri, G.; Maggi, C.A. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc. Res. 1990, 40, 264–278. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Fan, J.; Mei, J.; Zhang, M.-Z.; Yuan, F.; Li, S.-Z.; Yu, G.-R.; Chen, L.-H.; Tang, Q.; Xian, C.J. Clinicopathological significance of glucose transporter protein-1 overexpression in human osteosarcoma. Oncol. Lett. 2017, 14, 2439–2445. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Zhou, J.-Q.; Yu, G.-R.; Lu, D.-D. Glucose Transporter Protein 1–Targeted RNA Interference Inhibits Growth and Invasion of the Osteosarcoma Cell Line MG63 In Vitro. Cancer Biother. Radiopharm. 2010, 25, 521–527. [Google Scholar] [CrossRef]
- Jian, F.; Yuan, F.; Jiong, M.; Zhu, X.-Z.; Yu, G.-R.; Lu, D.-D. Silencing of Glucose Transporter Protein-1 by RNA Interference Inhibits Human Osteosarcoma Mg63 Cells Growth in vivo. Technol. Cancer Res. Treat. 2014, 14, 243–248. [Google Scholar] [CrossRef]
- Maschek, G.; Savaraj, N.; Priebe, W.; Braunschweiger, P.; Hamilton, K.; Tidmarsh, G.F.; De Young, L.R.; Lampidis, T.J. 2-Deoxy-d-glucose Increases the Efficacy of Adriamycin and Paclitaxel in Human Osteosarcoma and Non-Small Cell Lung Cancers In Vivo. Cancer Res. 2004, 64, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 2013, 48, 1–9. [Google Scholar] [CrossRef]
- Medrano, S.; Gruenstein, E.; Dimlich, R.V. Substance P receptors on human astrocytoma cells are linked to glycogen breakdown. Neurosci. Lett. 1994, 167, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ji, T.; Guo, W. Anti-angiogenesis target therapy for advanced osteosarcoma (Review). Oncol. Rep. 2017, 38, 625–636. [Google Scholar] [CrossRef] [Green Version]
- Kumta, S.M.; Huang, L.; Cheng, Y.Y.; Chow, L.T.; Lee, K.M.; Zheng, M.H. Expression of VEGF and MMP-9 in giant cell tumor of bone and other osteolytic lesions. Life Sci. 2003, 73, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef] [Green Version]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef]
- Loughran, C.F.; Keeling, C.R. Seeding of tumour cells following breast biopsy: A literature review. Br. J. Radiol. 2011, 84, 869–874. [Google Scholar] [CrossRef]
- Gharaee, N.; Pourali, L.; Jafarian, A.H.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in endometrial cancer. Mol. Biol. Rep. 2018, 45, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.; Kasembeli, M.; Bharadwaj, U.; Engineer, N.; Eckols, K.T.; Tweardy, D.J. Substance P Receptor Signaling Mediates Doxorubicin-Induced Cardiomyocyte Apoptosis and Triple-Negative Breast Cancer Chemoresistance. BioMed Res. Int. 2016, 2016, 1959270. [Google Scholar] [CrossRef] [Green Version]
- Legi, A.; Rodriguez, E.; Eckols, T.K.; Mistry, C.; Robinson, P. Substance P Antagonism Prevents Chemotherapy-Induced Cardiotoxicity. Cancers 2021, 13, 1732. [Google Scholar] [CrossRef]
- Un, H.; Ugan, R.A.; Kose, D.; Bayir, Y.; Cadirci, E.; Selli, J.; Halici, Z. A novel effect of Aprepitant: Protection for cisplatin-induced nephrotoxicity and hepatotoxicity. Eur. J. Pharmacol. 2020, 880, 173168. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Pei, G.; Zhao, Z.; Kim, S.T.; German, A.; Robinson, P. Substance P Antagonism as a Novel Therapeutic Option to Enhance Efficacy of Cisplatin in Triple Negative Breast Cancer and Protect PC12 Cells against Cisplatin-Induced Oxidative Stress and Apoptosis. Cancers 2021, 13, 3871. [Google Scholar] [CrossRef] [PubMed]
- Coveñas, R.; Muñoz, M. Involvement of the Substance P/Neurokinin-1 Receptor System in Cancer. Cancers 2022, 14, 3539. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Coveñas, R. Safety of neurokinin-1 receptor antagonists. Expert Opin. Drug Saf. 2013, 12, 673–685. [Google Scholar] [CrossRef]
- Yang, M.-J.; Xu, H.-R.; Li, H.; Chen, W.-L.; Yuan, F.; Li, X.-N. Comparison of Pharmacokinetics of Aprepitant in Healthy Chinese and Caucasian Subjects. Drug Des. Dev. Ther. 2020, 14, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.K.; Kohli, A. Aprepitant. [Updated 2022 Sep 22]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Muñoz, M.; Rosso, M. The NK-1 receptor antagonist aprepitant as a broad spectrum antitumor drug. Investig. New Drugs 2010, 28, 187–193. [Google Scholar] [CrossRef]
- Okumura, L.M.; da Silva Ries, S.A.; Meneses, C.F.; Michalowski, M.B.; Ferreira, M.A.P.; Moreira, L.B. Adverse events associated with aprepitant pediatric bone cancer patients. J. Oncol. Pharm. Pract. 2019, 25, 735–738. [Google Scholar] [CrossRef]
- Tebas, P.; Tuluc, F.; Barrett, J.S.; Wagner, W.; Kim, D.; Zhao, H.; Gonin, R.; Korelitz, J.; Douglas, S.D. A Randomized, Placebo Controlled, Double Masked Phase IB Study Evaluating the Safety and Antiviral Activity of Aprepitant, a Neurokinin-1 Receptor Antagonist in HIV-1 Infected Adults. PLoS ONE 2011, 6, e24180. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.K.; Cote, G.M.; Choy, E.; Yang, P.; Harmon, D.; Schwab, J.; Nielsen, G.P.; Chebib, I.; Ferrone, S.; Wang, X.; et al. Programmed Cell Death Ligand 1 Expression in Osteosarcoma. Cancer Immunol. Res. 2014, 2, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Kramer, M.S.; Cutler, N.; Feighner, J.; Shrivastava, R.; Carman, J.; Sramek, J.J.; Reines, S.A.; Liu, G.; Snavely, D.; Wyatt-Knowles, E.; et al. Distinct Mechanism for Antidepressant Activity by Blockade of Central Substance P Receptors. Science 1998, 281, 1640–1645. [Google Scholar] [CrossRef]
- Giagnuolo, G.; Buffardi, S.; Rossi, F.; Petruzziello, F.; Tortora, C.; Buffardi, I.; Marra, N.; Beneduce, G.; Menna, G.; Parasole, R. Single center experience on efficacy and safety of Aprepitant for preventing chemotherapy-induced nausea and vomiting (CINV) in pediatric Hodgkin Lymphoma. PLoS ONE 2019, 14, e0215295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kose, D.; Un, H.; Ugan, R.A.; Halici, Z.; Cadirci, E.; Tastan, T.B.; Kahramanlar, A. Aprepitant: An antiemetic drug, contributes to the prevention of acute lung injury with its anti-inflammatory and antioxidant properties. J. Pharm. Pharmacol. 2021, 73, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Spitsin, S.; Tebas, P.; Barrett, J.S.; Pappa, V.; Kim, D.; Taylor, D.; Evans, D.L.; Douglas, S.D. Antiinflammatory effects of aprepitant coadministration with cART regimen containing ritonavir in HIV-infected adults. JCI Insight 2017, 2, e95893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, P.; Rosso, M.; Muñoz, M. Neurokinin-1 Receptor Antagonists as a Potential Novel Therapeutic Option for Osteosarcoma Patients. J. Clin. Med. 2023, 12, 2135. https://doi.org/10.3390/jcm12062135
Robinson P, Rosso M, Muñoz M. Neurokinin-1 Receptor Antagonists as a Potential Novel Therapeutic Option for Osteosarcoma Patients. Journal of Clinical Medicine. 2023; 12(6):2135. https://doi.org/10.3390/jcm12062135
Chicago/Turabian StyleRobinson, Prema, Marisa Rosso, and Miguel Muñoz. 2023. "Neurokinin-1 Receptor Antagonists as a Potential Novel Therapeutic Option for Osteosarcoma Patients" Journal of Clinical Medicine 12, no. 6: 2135. https://doi.org/10.3390/jcm12062135
APA StyleRobinson, P., Rosso, M., & Muñoz, M. (2023). Neurokinin-1 Receptor Antagonists as a Potential Novel Therapeutic Option for Osteosarcoma Patients. Journal of Clinical Medicine, 12(6), 2135. https://doi.org/10.3390/jcm12062135







