Commissural Alignment and Coronary Access after Transcatheter Aortic Valve Replacement
Abstract
:1. Introduction
1.1. Prevalence of Coronary Artery Disease in Patients Undergoing TAVR
1.2. CAG and PCI after TAVR
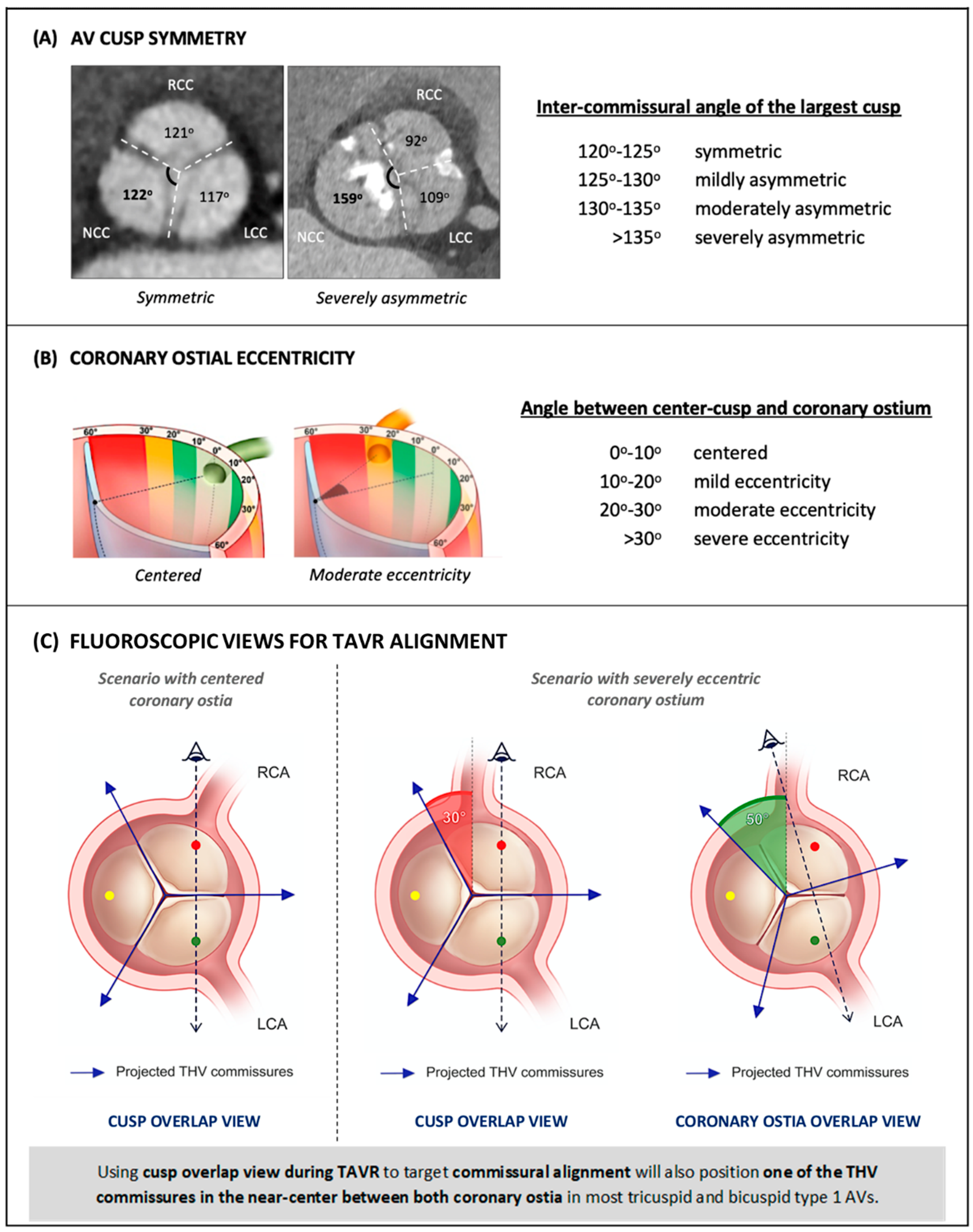
1.3. Difficulties in Coronary Access after TAVR in Relation to Valve Design and Implantation Technique
2. Delivery System Technologies and Impact on Commissure Orientation
2.1. Patient-Specific Implantation Techniques to Obtain Neo-Commissural Alignment
2.1.1. Self-Expanding THV Platforms
2.1.2. Balloon-Expandable SAPIEN 3 Platform
3. Commissural vs. Coronary Ostial Alignment
4. Possible Implications of Commissural Alignment beyond Coronary Access: Lights and Shadows
4.1. Commissural Alignment and Aortic Regurgitation
4.2. Commissural Alignment and Valve Thrombosis
4.3. Commissural Alignment and Structural Valve Deterioration
4.4. Commissural Alignment and THV-in-THV
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goel, S.S.; Ige, M.; Tuzcu, E.M.; Ellis, S.G.; Stewart, W.J.; Svensson, L.G.; Lytle, B.W.; Kapadia, S.R. Severe aortic stenosis and coronary artery disease--implications for management in the transcatheter aortic valve replacement era: A comprehensive review. J. Am. Coll. Cardiol. 2013, 62, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snow, T.M.; Ludman, P.; Banya, W.; DeBelder, M.; MacCarthy, P.M.; Davies, S.W.; Di Mario, C.; Moat, N.E. Management of concomitant coronary artery disease in patients undergoing transcatheter aortic valve implantation: The United Kingdom tavi registry. Int. J. Cardiol. 2015, 199, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Beyersdorf, F.; Bauer, T.; Freemantle, N.; Walther, T.; Frerker, C.; Herrmann, E.; Bleiziffer, S.; Möllmann, H.; Landwehr, S.; Ensminger, S.; et al. Five-year outcome in 18 010 patients from the german aortic valve registry. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2021, 60, 1139–1146. [Google Scholar] [CrossRef]
- Huczek, Z.; Zbroński, K.; Grodecki, K.; Scisło, P.; Rymuza, B.; Kochman, J.; Dąbrowski, M.; Witkowski, A.; Wojakowski, W.; Parma, R.; et al. Concomitant coronary artery disease and its management in patients referred to transcatheter aortic valve implantation: Insights from the pol-tavi registry. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2018, 91, 115–123. [Google Scholar] [CrossRef]
- Faroux, L.; Guimaraes, L.; Wintzer-Wehekind, J.; Junquera, L.; Ferreira-Neto, A.N.; Del Val, D.; Muntané-Carol, G.; Mohammadi, S.; Paradis, J.M.; Rodés-Cabau, J. Coronary artery disease and transcatheter aortic valve replacement: Jacc state-of-the-art review. J. Am. Coll. Cardiol. 2019, 74, 362–372. [Google Scholar] [CrossRef]
- Faroux, L.; Munoz-Garcia, E.; Serra, V.; Alperi, A.; Nombela-Franco, L.; Fischer, Q.; Veiga, G.; Donaint, P.; Asmarats, L.; Vilalta, V.; et al. Acute coronary syndrome following transcatheter aortic valve replacement. Circulation. Cardiovasc. Interv. 2020, 13, e008620. [Google Scholar] [CrossRef] [PubMed]
- Mentias, A.; Desai, M.Y.; Saad, M.; Horwitz, P.A.; Rossen, J.D.; Panaich, S.; Elbadawi, A.; Abbott, J.D.; Sorajja, P.; Jneid, H.; et al. Incidence and outcomes of acute coronary syndrome after transcatheter aortic valve replacement. JACC. Cardiovasc. Interv. 2020, 13, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Vilalta, V.; Asmarats, L.; Ferreira-Neto, A.N.; Maes, F.; de Freitas Campos Guimarães, L.; Couture, T.; Paradis, J.M.; Mohammadi, S.; Dumont, E.; Kalavrouziotis, D.; et al. Incidence, clinical characteristics, and impact of acute coronary syndrome following transcatheter aortic valve replacement. JACC. Cardiovasc. Interv. 2018, 11, 2523–2533. [Google Scholar] [CrossRef]
- Jørgensen, T.H.; Thyregod, H.G.H.; Ihlemann, N.; Nissen, H.; Petursson, P.; Kjeldsen, B.J.; Steinbrüchel, D.A.; Olsen, P.S.; Søndergaard, L. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. Surgical aortic valve replacement. Eur. Heart J. 2021, 42, 2912–2919. [Google Scholar] [CrossRef]
- Faroux, L.; Lhermusier, T.; Vincent, F.; Nombela-Franco, L.; Tchétché, D.; Barbanti, M.; Abdel-Wahab, M.; Windecker, S.; Auffret, V.; Campanha-Borges, D.C.; et al. St-segment elevation myocardial infarction following transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2021, 77, 2187–2199. [Google Scholar] [CrossRef]
- Ochiai, T.; Chakravarty, T.; Yoon, S.H.; Kaewkes, D.; Flint, N.; Patel, V.; Mahani, S.; Tiwana, R.; Sekhon, N.; Nakamura, M.; et al. Coronary access after tavr. JACC Cardiovasc. Interv. 2020, 13, 693–705. [Google Scholar] [CrossRef]
- Yudi, M.B.; Sharma, S.K.; Tang, G.H.L.; Kini, A. Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2018, 71, 1360–1378. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Costa, G.; Picci, A.; Criscione, E.; Reddavid, C.; Valvo, R.; Todaro, D.; Deste, W.; Condorelli, A.; Scalia, M.; et al. Coronary cannulation after transcatheter aortic valve replacement: The re-access study. JACC Cardiovasc. Interv. 2020, 13, 2542–2555. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Zaid, S.; Fuchs, A.; Yamabe, T.; Yazdchi, F.; Gupta, E.; Ahmad, H.; Kofoed, K.F.; Goldberg, J.B.; Undemir, C.; et al. Alignment of transcatheter aortic-valve neo-commissures (align tavr): Impact on final valve orientation and coronary artery overlap. JACC Cardiovasc. Interv. 2020, 13, 1030–1042. [Google Scholar] [CrossRef]
- Bieliauskas, G.; Wong, I.; Bajoras, V.; Wang, X.; Kofoed, K.F.; De Backer, O.; Søndergaard, L. Patient-specific implantation technique to obtain neo-commissural alignment with self-expanding transcatheter aortic valves. JACC Cardiovasc. Interv. 2021, 14, 2097–2108. [Google Scholar] [CrossRef]
- Kitamura, M.; Wilde, J.; Dumpies, O.; Gutberlet, M.; Gohmann, R.; Shibata, M.; Noack, T.; Thiele, H.; Holzhey, D.; Abdel-Wahab, M. Patient-specific neocommissural alignment of the evolut valve: A pilot study in transcatheter aortic valve-in-valve replacement. JACC Cardiovasc. Interv. 2021, 14, 934–936. [Google Scholar] [CrossRef]
- De Marco, F.; Casenghi, M.; Spagnolo, P.; Popolo Rubbio, A.; Brambilla, N.; Testa, L.; Bedogni, F. A patient-specific algorithm to achieve commissural alignment with acurate neo: The sextant technique. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 98, E847–E854. [Google Scholar] [CrossRef]
- Buono, A.; Messina, A.; Bettari, L.; Pero, G.; Cuccia, C.; Ielasi, A.; Bieliauskas, G.; Maffeo, D. Commissural alignment with the novel Hydra transcatheter heart valve during aortic valve replacement. EuroIntervention 2022, 18, 822–823. [Google Scholar] [CrossRef]
- Spilias, N.; Sabbak, N.; Harb, S.C.; Yun, J.J.; Vargo, P.R.; Unai, S.; Puri, R.; Reed, G.W.; Krishnaswamy, A.; Kapadia, S.R. A novel method of assessing commissural alignment for the sapien 3 transcatheter aortic valve. JACC Cardiovasc. Interv. 2021, 14, 1269–1272. [Google Scholar] [CrossRef]
- Tarantini, G.; Nai Fovino, L.; Scotti, A.; Massussi, M.; Cardaioli, F.; Rodinò, G.; Benedetti, A.; Boiago, M.; Matsuda, Y.; Continisio, S.; et al. Coronary access after transcatheter aortic valve replacement with commissural alignment: The align-access study. Circulation. Cardiovasc. Interv. 2022, 15, e011045. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.L.; Amat-Santos, I.J.; De Backer, O.; Avvedimento, M.; Redondo, A.; Barbanti, M.; Costa, G.; Tchétché, D.; Eltchaninoff, H.; Kim, W.K.; et al. Rationale, definitions, techniques, and outcomes of commissural alignment in tavr: From the align-tavr consortium. JACC Cardiovasc. Interv. 2022, 15, 1497–1518. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; De Backer, O.; Bieliauskas, G.; Wong, I.; Bajoras, V.; Xiong, T.Y.; Zhang, Y.; Kofoed, K.F.; Chen, M.; Sondergaard, L. Cusp symmetry and coronary ostial eccentricity and its impact on coronary access following tavr. JACC Cardiovasc. Interv. 2022, 15, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.; Baladrón Zorita, C.; Tchétché, D.; Santos-Martinez, S.; Delgado-Arana, J.R.; Barrero, A.; Gutiérrez, H.; Serrador Frutos, A.; Ybarra Falcón, C.; Gómez, M.G.; et al. Commissural versus coronary optimized alignment during transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2022, 15, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Curzen, N.; Bressloff, N.W. The impact of imperfect frame deployment and rotational orientation on stress within the prosthetic leaflets during transcatheter aortic valve implantation. J. Biomech. 2017, 53, 22–28. [Google Scholar] [CrossRef]
- Fuchs, A.; Kofoed, K.F.; Yoon, S.H.; Schaffner, Y.; Bieliauskas, G.; Thyregod, H.G.; Makkar, R.; Søndergaard, L.; De Backer, O.; Bapat, V. Commissural alignment of bioprosthetic aortic valve and native aortic valve following surgical and transcatheter aortic valve replacement and its impact on valvular function and coronary filling. JACC Cardiovasc. Interv. 2018, 11, 1733–1743. [Google Scholar] [CrossRef]
- Raschpichler, M.; Flint, N.; Yoon, S.H.; Kaewkes, D.; Patel, C.; Singh, C.; Patel, V.; Kashif, M.; Borger, M.A.; Chakravarty, T.; et al. Commissural alignment after balloon-expandable transcatheter aortic valve replacement is associated with improved hemodynamic outcomes. JACC Cardiovasc. Interv. 2022, 15, 1126–1136. [Google Scholar] [CrossRef]
- Cahill, T.J.; Kirtane, A.J.; Leon, M.; Kodali, S.K. Subclinical leaflet thrombosis and anticoagulation after transcatheter aortic valve replacement: A review. JAMA Cardiol. 2022, 7, 866–872. [Google Scholar] [CrossRef]
- Khan, J.M.; Rogers, T.; Weissman, G.; Torguson, R.; Rodriguez-Weisson, F.J.; Chezar-Azerrad, C.; Greenspun, B.; Gupta, N.; Medvedofsky, D.; Zhang, C.; et al. Anatomical characteristics associated with hypoattenuated leaflet thickening in low-risk patients undergoing transcatheter aortic valve replacement. Cardiovasc. Revascula. Med. Incl. Mol. Interv. 2021, 27, 1–6. [Google Scholar] [CrossRef]
- Fukui, M.; Bapat, V.N.; Garcia, S.; Dworak, M.W.; Hashimoto, G.; Sato, H.; Gössl, M.; Enriquez-Sarano, M.; Lesser, J.R.; Cavalcante, J.L.; et al. Deformation of transcatheter aortic valve prostheses: Implications for hypoattenuating leaflet thickening and clinical outcomes. Circulation 2022, 146, 480–493. [Google Scholar] [CrossRef]
- Ochiai, T.; Oakley, L.; Sekhon, N.; Komatsu, I.; Flint, N.; Kaewkes, D.; Yoon, S.H.; Raschpichler, M.; Patel, V.; Tiwana, R.; et al. Risk of coronary obstruction due to sinus sequestration in redo transcatheter aortic valve replacement. JACC. Cardiovasc. Interv. 2020, 13, 2617–2627. [Google Scholar] [CrossRef]


| Factors Restraining Coronary Access | |
|---|---|
| Native anatomy |
|
| |
| |
| |
| |
| |
| THV design |
|
| |
| |
| |
| Procedural characteristics |
|
| |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quagliana, A.; Montarello, N.J.; Willemen, Y.; Bække, P.S.; Jørgensen, T.H.; De Backer, O.; Sondergaard, L. Commissural Alignment and Coronary Access after Transcatheter Aortic Valve Replacement. J. Clin. Med. 2023, 12, 2136. https://doi.org/10.3390/jcm12062136
Quagliana A, Montarello NJ, Willemen Y, Bække PS, Jørgensen TH, De Backer O, Sondergaard L. Commissural Alignment and Coronary Access after Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine. 2023; 12(6):2136. https://doi.org/10.3390/jcm12062136
Chicago/Turabian StyleQuagliana, Angelo, Nicholas J. Montarello, Yannick Willemen, Pernille S. Bække, Troels H. Jørgensen, Ole De Backer, and Lars Sondergaard. 2023. "Commissural Alignment and Coronary Access after Transcatheter Aortic Valve Replacement" Journal of Clinical Medicine 12, no. 6: 2136. https://doi.org/10.3390/jcm12062136
APA StyleQuagliana, A., Montarello, N. J., Willemen, Y., Bække, P. S., Jørgensen, T. H., De Backer, O., & Sondergaard, L. (2023). Commissural Alignment and Coronary Access after Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine, 12(6), 2136. https://doi.org/10.3390/jcm12062136






