Nailfold Videocapillaroscopy for Non-Invasive Assessment of Microcirculation and Prognostic Correlation with Endothelial Dysfunction, Cardiovascular Risk Factors, and Non-HLA Antibodies in Heart Transplant Recipients: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Experimental Section
2.3. Statistical Methods
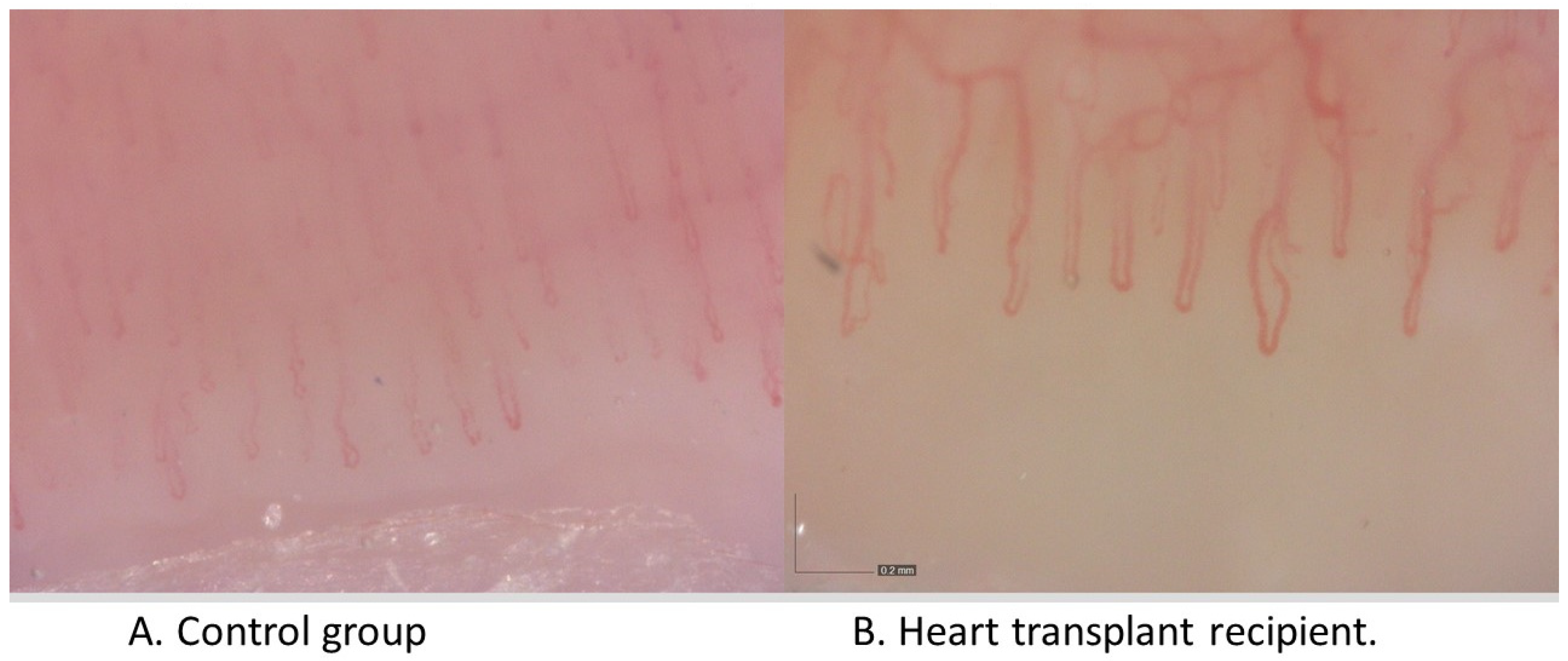
3. Results
3.1. Comparison of NCV Results between the Patient Groups (HTx vs. Healthy Controls)
3.2. Correlations within the HTx Group
3.3. Detailed Analysis of Individual HTx Recipients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moll, G.; Dai, Z.; Camara, N.O.S. Editorial: Advances in Heart Transplantation. Front. Immunol. 2022, 13, 960800. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Jaeger, B.R.; Kassab, G.S. Cardiac allograft vasculopathy: Microvascular arteriolar capillaries (“capioles”) and survival. Front. Biosci. (Elite Ed.) 2017, 9, 110–128. [Google Scholar] [CrossRef] [Green Version]
- Masha, L. Geographic Variation in US Heart Transplant Outcomes and Consequences for Health Care Equity. JAMA Netw. Open 2020, 3, e2028856. [Google Scholar] [CrossRef]
- Bakhtiyar, S.S.; Godfrey, E.L.; Ahmed, S.; Lamba, H.; Morgan, J.; Loor, G.; Civitello, A.; Cheema, F.H.; Etheridge, W.B.; Goss, J.; et al. Survival on the Heart Transplant Waiting List. JAMA Cardiol. 2020, 5, 1227–1235. [Google Scholar] [CrossRef]
- Smith, V.; Pizzorni, C.; Riccieri, V.; Decuman, S.; Brusselle, G.; De Pauw, M.; Deschepper, E.; Piette, Y.; Ruaro, B.; Sulli, A.; et al. Stabilization of Microcirculation in Patients with Early Systemic Sclerosis with Diffuse Skin Involvement following Rituximab Treatment: An Open-label Study. J. Rheumatol. 2016, 43, 995–996. [Google Scholar] [CrossRef] [Green Version]
- Sikorska, D.; Samborski, W.; Kaminska, D.; Kusztal, M.; Jablecki, J.; Nijakowski, K.; Oko, A.; Karczewski, M.; Korybalska, K.; Witowski, J. Abnormal Nailfold Capillaries in Patients after Hand Transplantation. J. Clin. Med. 2020, 9, 3422. [Google Scholar] [CrossRef]
- Bernero, E.; Sulli, A.; Ferrari, G.; Ravera, F.; Pizzorni, C.; Ruaro, B.; Zampogna, G.; Alessandri, E.; Cutolo, M. Prospective capillaroscopy-based study on transition from primary to secondary Raynaud’s phenomenon: Preliminary results. Reumatismo 2013, 65, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Catar, R.; Moll, G.; Kamhieh-Milz, J.; Luecht, C.; Chen, L.; Zhao, H.; Ernst, L.; Willy, K.; Girndt, M.; Fiedler, R.; et al. Expanded Hemodialysis Therapy Ameliorates Uremia-Induced Systemic Microinflammation and Endothelial Dysfunction by Modulating VEGF, TNF-α and AP-1 Signaling. Front. Immunol. 2021, 12, 774052. [Google Scholar] [CrossRef]
- Tona, F.; Marra, M.P.; Fedrigo, M.; Famoso, G.; Bellu, R.; Thiene, G.; Gerosa, G.; Angelini, A.; Iliceto, S. Recent developments on coronary microvasculopathy after heart transplantation: A new target in the therapy of cardiac allograft vasculopathy. Curr. Vasc. Pharmacol. 2012, 10, 206–215. [Google Scholar] [CrossRef]
- Heim, C.; Gocht, A.; Weyand, M.; Ensminger, S. New Targets for the Prevention of Chronic Rejection after Thoracic Organ Transplantation. Thorac. Cardiovasc. Surg. 2018, 66, 20–30. [Google Scholar] [CrossRef]
- Lee, M.S.; Finch, W.; Weisz, G.; Kirtane, A.J. Cardiac allograft vasculopathy. Rev. Cardiovasc. Med. 2011, 12, 143–152. [Google Scholar] [CrossRef]
- Sharples, L.D.; Jackson, C.H.; Parameshwar, J.; Wallwork, J.; Large, S.R. Diagnostic accuracy of coronary angiography and risk factors for post-heart-transplant cardiac allograft vasculopathy. Transplantation 2003, 76, 679–682. [Google Scholar] [CrossRef]
- Behrendt, D.; Ganz, P.; Fang, J.C. Cardiac allograft vasculopathy. Curr. Opin. Cardiol. 2000, 15, 422–429. [Google Scholar] [CrossRef]
- Taimeh, Z.; Loughran, J.; Birks, E.J.; Bolli, R. Vascular endothelial growth factor in heart failure. Nat. Rev. Cardiol. 2013, 10, 519–530. [Google Scholar] [CrossRef]
- Catar, R.A.; Bartosova, M.; Kawka, E.; Chen, L.; Marinovic, I.; Zhang, C.; Zhao, H.; Wu, D.; Zickler, D.; Stadnik, H.; et al. Angiogenic Role of Mesothelium-Derived Chemokine CXCL1 During Unfavorable Peritoneal Tissue Remodeling in Patients Receiving Peritoneal Dialysis as Renal Replacement Therapy. Front. Immunol. 2022, 13, 821681. [Google Scholar] [CrossRef]
- Anyfanti, P.; Angeloudi, E.; Dara, A.; Arvanitaki, A.; Bekiari, E.; Kitas, G.D.; Dimitroulas, T. Nailfold Videocapillaroscopy for the Evaluation of Peripheral Microangiopathy in Rheumatoid Arthritis. Life 2022, 12, 1167. [Google Scholar] [CrossRef]
- Sambataro, D.; Sambataro, G.; Libra, A.; Vignigni, G.; Pino, F.; Fagone, E.; Fruciano, M.; Gili, E.; Pignataro, F.; Del Papa, N.; et al. Nailfold Videocapillaroscopy is a Useful Tool to Recognize Definite Forms of Systemic Sclerosis and Idiopathic Inflammatory Myositis in Interstitial Lung Disease Patients. Diagnostics 2020, 10, 253. [Google Scholar] [CrossRef]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef]
- Minopoulou, I.; Theodorakopoulou, M.; Boutou, A.; Arvanitaki, A.; Pitsiou, G.; Doumas, M.; Sarafidis, P.; Dimitroulas, T. Nailfold Capillaroscopy in Systemic Sclerosis Patients with and without Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1528. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.E.; Ramirez-Lara, I.; Gomez-Delgado, F.; Yubero-Serrano, E.M.; Leon-Acuna, A.; Marin, C.; Alcala-Diaz, J.F.; Camargo, A.; Lopez-Moreno, J.; Perez-Martinez, P.; et al. Quantitative evaluation of capillaroscopic microvascular changes in patients with established coronary heart disease. Med. Clin. 2018, 150, 131–137. [Google Scholar] [CrossRef]
- Tibirica, E.; Souza, E.G.; De Lorenzo, A.; Oliveira, G.M. Reduced systemic microvascular density and reactivity in individuals with early onset coronary artery disease. Microvasc. Res. 2015, 97, 105–108. [Google Scholar] [CrossRef]
- Sobieszczańska-Małek, M.; Korewicki, J.; Komuda, K.; Karczmarz, M.; Szymańska, S.; Cicha-Mikołajczyk, A.; Bekta, P.; Parulski, A.; Pronicki, M.; Grajkowska, W.; et al. Heart Transplantation and Risk of Cardiac Vasculopathy Development: What Factors Are Important? Ann. Transplant. 2017, 22, 682–688. [Google Scholar] [CrossRef]
- Valantine, H. Cardiac allograft vasculopathy after heart transplantation: Risk factors and management. J. Heart Lung. Transplant. 2004, 23, S187–S193. [Google Scholar] [CrossRef]
- Van Keer, J.M.; Van Aelst, L.N.L.; Rega, F.; Droogne, W.; Voros, G.; Meyns, B.; Vanhaecke, J.; Emonds, M.P.; Janssens, S.; Naesens, M.; et al. Long-term outcome of cardiac allograft vasculopathy: Importance of the International Society for Heart and Lung Transplantation angiographic grading scale. J. Heart Lung Transplant. 2019, 38, 1189–1196. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Dragun, D.; Müller, D.N.; Bräsen, J.H.; Fritsche, L.; Nieminen-Kelhä, M.; Dechend, R.; Kintscher, U.; Rudolph, B.; Hoebeke, J.; Eckert, D.; et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N. Engl. J. Med. 2005, 352, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Cabral-Marques, O.; Moll, G.; Catar, R. Autoantibodies targeting G protein-coupled receptors: An evolving history in autoimmunity. Report of the 4th International Symposium. Autoimmun. Rev. 2023, 22, 103310. [Google Scholar] [CrossRef]
- Dragun, D.; Hegner, B. Non-HLA antibodies post-transplantation: Clinical relevance and treatment in solid organ transplantation. Contrib. Nephrol. 2009, 162, 129–139. [Google Scholar] [CrossRef]
- Dragun, D.; Catar, R.; Philippe, A. Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity. Kidney Int. 2016, 90, 280–288. [Google Scholar] [CrossRef]
- Catar, R.A.; Wischnewski, O.; Chen, L.; Heidecke, H.; Rutz, C.; Schülein, R.; Dragun, D.; Philippe, A.; Kusch, A. Non-HLA antibodies targeting angiotensin II Type 1 receptor and endothelin-1 Type A receptors induce endothelial injury via β2-arrestin link to mTOR pathway. Kidney Int. 2022, 101, 498–509. [Google Scholar] [CrossRef]
- Sikorska, D.; Kamińska, D.; Catar, R.; Banasik, M.; Heidecke, H.; Schulze-Forster, K.; Korybalska, K.; Rutkowski, R.; Łuczak, J.; Jabłecki, J.; et al. Non-HLA Antibodies in Hand Transplant Recipients Are Connected to Multiple Acute Rejection Episodes and Endothelial Activation. J. Clin. Med. 2022, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Banasik, M.; Boratynska, M.; Koscielska-Kasprzak, K.; Krajewska, M.; Mazanowska, O.; Kaminska, D.; Bartoszek, D.; Zabinska, M.; Myszka, M.; Nowakowska, B.; et al. The impact of non-HLA antibodies directed against endothelin-1 type A receptors (ETAR) on early renal transplant outcomes. Transpl. Immunol. 2014, 30, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banasik, M.; Boratynska, M.; Koscielska-Kasprzak, K.; Kaminska, D.; Bartoszek, D.; Zabinska, M.; Myszka, M.; Zmonarski, S.; Protasiewicz, M.; Nowakowska, B.; et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl. Int. 2014, 27, 1029–1038. [Google Scholar] [CrossRef]
- Dragun, D.; Philippe, A.; Catar, R.; Hegner, B. Autoimmune mediated G-protein receptor activation in cardiovascular and renal pathologies. Thromb. Haemost. 2009, 101, 643–648. [Google Scholar]
- Cabral-Marques, O.; Riemekasten, G. Vascular hypothesis revisited: Role of stimulating antibodies against angiotensin and endothelin receptors in the pathogenesis of systemic sclerosis. Autoimmun. Rev. 2016, 15, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Marques, O.; Riemekasten, G. Functional autoantibodies targeting G protein-coupled receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 648–656. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Marques, A.; Giil, L.M.; De Vito, R.; Rademacher, J.; Günther, J.; Lange, T.; Humrich, J.Y.; Klapa, S.; Schinke, S.; et al. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat. Commun. 2018, 9, 5224. [Google Scholar] [CrossRef] [Green Version]
- Cabral-Marques, O.; Halpert, G.; Schimke, L.F.; Ostrinski, Y.; Vojdani, A.; Baiocchi, G.C.; Freire, P.P.; Filgueiras, I.S.; Zyskind, I.; Lattin, M.T.; et al. Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity. Nat. Commun. 2022, 13, 1220. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Catar, R.; Schoon, J.; Qazi, T.H.; Sass, F.A.; Jacobi, D.; Blankenstein, A.; Reinke, S.; Kruger, D.; Streitz, M.; et al. Multi-Parameter Analysis of Biobanked Human Bone Marrow Stromal Cells Shows Little Influence for Donor Age and Mild Comorbidities on Phenotypic and Functional Properties. Front. Immunol. 2019, 10, 2474. [Google Scholar] [CrossRef]
- Catar, R.; Moll, G.; Hosp, I.; Simon, M.; Luecht, C.; Zhao, H.; Wu, D.; Chen, L.; Kamhieh-Milz, J.; Korybalska, K.; et al. Transcriptional Regulation of Thrombin-Induced Endothelial VEGF Induction and Proangiogenic Response. Cells 2021, 10, 910. [Google Scholar] [CrossRef]
- Shikama, M.; Sonoda, N.; Morimoto, A.; Suga, S.; Tajima, T.; Kozawa, J.; Maeda, N.; Otsuki, M.; Matsuoka, T.A.; Shimomura, I.; et al. Association of abdominal obesity with crossing capillaries in the finger nailfold in type 2 diabetes mellitus. Diabetol. Int. 2021, 12, 260–267. [Google Scholar] [CrossRef]
- Catar, R.; Witowski, J.; Zhu, N.; Lucht, C.; Derrac Soria, A.; Uceda Fernandez, J.; Chen, L.; Jones, S.A.; Fielding, C.A.; Rudolf, A.; et al. IL-6 Trans-Signaling Links Inflammation with Angiogenesis in the Peritoneal Membrane. J. Am. Soc. Nephrol. JASN 2017, 28, 1188–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansueto, N.; Rotondo, C.; Corrado, A.; Cantatore, F.P. Nailfold capillaroscopy: A comprehensive review on common findings and clinical usefulness in non-rheumatic disease. J. Med. Investig. 2021, 68, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Ajasllari, N.; Mancarella, L.; Brusi, V.; Meliconi, R.; Ursini, F. Nailfold capillaroscopy in common non-rheumatic conditions: A systematic review and applications for clinical practice. Microvasc. Res. 2020, 131, 104036. [Google Scholar] [CrossRef] [PubMed]
- McCartney, S.L.; Patel, C.; Del Rio, J.M. Long-term outcomes and management of the heart transplant recipient. Best Pr. Res. Clin. Anaesthesiol. 2017, 31, 237–248. [Google Scholar] [CrossRef]
- Abdelmaksoud, A.A.; Daifallah, S.M.; Salah, N.Y.; Saber, A.S. Nail fold microangiopathy in adolescents with type 1 diabetes: Relation to diabetic vascular complications. Microcirculation 2022, 29, e12771. [Google Scholar] [CrossRef]
- Bakirci, S.; Celik, E.; Acikgoz, S.B.; Erturk, Z.; Tocoglu, A.G.; Imga, N.N.; Kaya, M.; Tamer, A. The evaluation of nailfold videocapillaroscopy findings in patients with type 2 diabetes with and without diabetic retinopathy. North. Clin. Istanb. 2019, 6, 146–150. [Google Scholar] [CrossRef]
- Lambova, S.N.; Müller-Ladner, U. The specificity of capillaroscopic pattern in connective autoimmune diseases. A comparison with microvascular changes in diseases of social importance: Arterial hypertension and diabetes mellitus. Mod. Rheumatol. 2009, 19, 600–605. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Anyfanti, P.; Triantafyllou, G.; Zabulis, X.; Aslanidis, S.; Douma, S. Impaired metabolic profile is a predictor of capillary rarefaction in a population of hypertensive and normotensive individuals. J. Am. Soc. Hypertens. 2016, 10, 640–646. [Google Scholar] [CrossRef]
- Nakajima, T.; Nakano, S.; Kikuchi, A.; Matsunaga, Y.T. Nailfold capillary patterns correlate with age, gender, lifestyle habits, and fingertip temperature. PLoS ONE 2022, 17, e0269661. [Google Scholar] [CrossRef]
- Kardol-Hoefnagel, T.; Otten, H.G. A Comprehensive Overview of the Clinical Relevance and Treatment Options for Antibody-mediated Rejection Associated With Non-HLA Antibodies. Transplantation 2021, 105, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Apaydin, T.; Yalcinkaya, Y.; Ilgin, C.; Gogas Yavuz, D. Capillary microarchitectural changes in Cushing’s syndrome. Microvasc. Res. 2022, 141, 104323. [Google Scholar] [CrossRef] [PubMed]
| HTx Patients (n = 10) | Healthy (n = 12) | p-Values (MW or χ2 Test) | |
|---|---|---|---|
| Age (years) | 38 ± 14 | 35 ± 5 | 0.495 |
| Sex (male/female) | 5/5 | 5/7 | 0.682 |
| BMI (kg/m2) | 24.2 ± 5.1 | 23.4 ± 4.1 | 0.862 |
| Chronic diseases and/or CVD risk factors (n) | 10 | 0 | <0.001 |
| Smoking (n) | 0 | 0 | 1.000 |
| HTx Patients (n = 10) | Healthy Controls (n = 12) | p-Values (Mann–Whitney) | |
|---|---|---|---|
| Avascular Areas | 0 | 0 | 1.000 |
| Capillary Disorganization | 0 | 0 | 1.000 |
| Capillary Density (per mm) | 8 (7–10) | 8 (8–9) | 0.604 |
| Hemorrhages | 1 | 0 | 0.305 |
| Tortuous Capillaries | 7 | 5 | 0.184 |
| Branching Capillaries | 0 | 0 | 1.000 |
| Ectatic Capillaries | 6 | 4 | 0.211 |
| Giant Capillaries | 0 | 0 | 1.000 |
| Average Apical Loop Diameter (µm) | 18 (14–20) | 12 (11–13) | 0.001 |
| HTx Patients (n = 10) | Healthy Controls (n = 10) | p-Values (Mann–Whitney) | |
|---|---|---|---|
| VEGF (pg/mL) | 70 (20–111) | 50 (17–90) | 0.410 |
| Total Length (AU) | 5908 (5393–6384) | 6152 (5330–6531) | 0.869 |
| Total Branching Length (AU) | 5819 (5300–6340) | 6118 (5323–6518) | 0.668 |
| Total Segment Length (AU) | 3821 (3516–4254) | 3910 (3713–4564) | 0.531 |
| Total Branch Length (AU) | 1748 (1533–2085) | 1697 (1408–2015) | 0.410 |
| HTx Patients (n = 10) | Healthy Controls (n = 10) | p-Values (Mann–Whitney) | |
|---|---|---|---|
| Anti-AT1R-Ab (U/mL) | 24 (12–72) | 10 (8–12) | 0.017 |
| Anti-ETAR-Ab (U/mL) | 18 (12–66) | 10 (9–13) | 0.025 |
| Anti-PAR1-Ab (U/mL) | 4 (2-8) | 2 (2–5) | 0.417 |
| Anti-VEGF-A-Ab (U/mL) | 17 (8062) | 4 (17–90) | 0.003 |
| Correlations | R-Value | p-Value |
|---|---|---|
| Total Length and anti-AT1R-Ab | 0.757576 | 0.011143 |
| Total Length and anti-ETAR-Ab | 0.745455 | 0.013330 |
| Total Length and anti-VEGF-A-Ab | 0.765961 | 0.009787 |
| Total Branching Length and anti-AT1R-Ab | 0.757576 | 0.011143 |
| Total Branching Length and anti-ETAR-Ab | 0.745455 | 0.013330 |
| Total Branching Length and anti-VEGF-A-Ab | 0.765961 | 0.009787 |
| Total Branches Length and anti-AT1R-Ab | 0.781818 | 0.007547 |
| Total Branches Length and anti-ETAR-Ab | 0.842424 | 0.002220 |
| Total Branches Length and anti-VEGF-A-Ab | 0.802435 | 0.005211 |
| Number of Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Time Post-HTx (years) | 1 | 1 | 24 | 22 | 21 | 2 | 5 | 10 | 5 | 1 |
| NYHA Class | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Troponin T (ng/mL) | 22.0 | 9.3 | 4.7 | 5.4 | 1.9 | 1.9 | 4.6 | 1.9 | 9.7 | 1.9 |
| NT-proBNP (pg/mL) | 876 | 336 | 382 | 2426 | 314 | 50 | 265 | 732 | 424 | 265 |
| CRP (mg/L) | 2.30 | 3.92 | 1.24 | 0.66 | 1.00 | 1.14 | 1.89 | 1.23 | 0.43 | 2.8 |
| Total Cholesterol (mg/dL) | 176 | 186 | 118 | 196 | 150 | 146 | 206 | 94 | 110 | 162 |
| HDL (mg/dL) | 73 | 37 | 41 | 52 | 57 | 37 | 68 | 54 | 32 | 59 |
| LDL (mg/dL) | 69 | 116 | 52 | 120 | 79 | 84 | 126 | 31 | 55 | 81 |
| TG (mg/dL) | 168 | 164 | 126 | 119 | 71 | 124 | 59 | 47 | 114 | 111 |
| WBC (G/L) | 13.20 | 9.42 | 3.40 | 4.90 | 5.00 | 5.00 | 7.65 | 8.11 | 7.90 | 6.90 |
| Neutrophil (G/L) | 11.00 | 6.00 | 3.18 | 3.50 | 2.70 | 3.40 | 2.62 | 5.27 | 3.40 | 4.80 |
| Lymphocyte (G/L) | 1.00 | 2.20 | 1.10 | 0.97 | 1.70 | 0.83 | 3.23 | 1.87 | 3.10 | 1.26 |
| Glucose (mg/dL) | 150 | 113 | 117 | 87 | 100 | 87 | 96 | 88 | 95 | 92 |
| Normal Echocardiography (Y/N) | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Changes in Coronary Angiography (Y/N) | N | N | Y | Y (NS) | Y (NS) | N | N | N | N | N |
| VEGF-A (pg/mL) | 30.48 | 153.2 | 99.57 | 83.09 | 11.88 | 20.1 | 57.99 | 195.7 | 111 | 17.07 |
| Total Length (AU) | 3452 | 5393 | 4797 | 5862 | 6808 | 6988 | 6384 | 5954 | 6046 | 5553 |
| Total Branching Length (AU) | 3244 | 5301 | 4766 | 5703 | 6776 | 6920 | 6340 | 5937 | 6046 | 5302 |
| Total Segment Length (AU) | 1760 | 3874 | 3162 | 3715 | 5009 | 4641 | 4255 | 4207 | 3516 | 3769 |
| Total Branch Length (AU) | 1484 | 1427 | 1604 | 1989 | 1767 | 2279 | 2086 | 1730 | 2530 | 1533 |
| Anti-AT1R-Ab (U/mL) | 7.86 | 10.11 | 11.57 | 14.65 | 24.95 | 53.47 | 71.64 | 79.52 | 80.51 | 23.94 |
| Anti-ETAR-Ab (U/mL) | 8.41 | 10.37 | 11.99 | 15.74 | 20.16 | 47.93 | 66.32 | 73.51 | 75.92 | 11.81 |
| Anti-PAR1-Ab (U/mL) | 4.47 | 7.67 | 3.93 | 15.31 | 8.56 | 1.42 | 2.71 | 2.04 | 8.06 | 1.42 |
| Anti-VEGF-A-Ab (U/mL) | 4.13 | 5.44 | 8.14 | 9.63 | 25.31 | 45.97 | 61.91 | 63.07 | 63.07 | 8.60 |
| Capillary Disorganization (Y/N) | N | N | N | N | N | N | N | N | N | N |
| Average Diameter of Capillaries (µm) | 21 | 26 | 18 | 20 | 12 | 18 | 17 | 14 | 16 | 13 |
| Capillary Density (per mm) | 8 | 10 | 10 | 7 | 7 | 7 | 8 | 10 | 9 | 10 |
| Avascular Areas (Y/N) | N | N | N | N | N | N | N | N | N | N |
| Ectatic Capillaries (Y/N) | Y | Y | N | Y | N | Y | Y | Y | N | N |
| Giant Capillaries (Y/N) | N | N | N | N | N | N | N | N | N | N |
| Tortuous Capillaries (Y/N) | Y | Y | Y | N | N | Y | Y | Y | N | Y |
| Branching Capillaries (Y/N) | N | N | N | N | N | N | N | N | N | N |
| Hemorrhages (Y/N) | N | Y | N | N | N | N | N | N | N | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorska, D.; Kamińska, D.; Catar, R.; Wu, D.; Zhao, H.; Wang, P.; Kamhieh-Milz, J.; Banasik, M.; Kusztal, M.; Cielecka, M.; et al. Nailfold Videocapillaroscopy for Non-Invasive Assessment of Microcirculation and Prognostic Correlation with Endothelial Dysfunction, Cardiovascular Risk Factors, and Non-HLA Antibodies in Heart Transplant Recipients: A Pilot Study. J. Clin. Med. 2023, 12, 2302. https://doi.org/10.3390/jcm12062302
Sikorska D, Kamińska D, Catar R, Wu D, Zhao H, Wang P, Kamhieh-Milz J, Banasik M, Kusztal M, Cielecka M, et al. Nailfold Videocapillaroscopy for Non-Invasive Assessment of Microcirculation and Prognostic Correlation with Endothelial Dysfunction, Cardiovascular Risk Factors, and Non-HLA Antibodies in Heart Transplant Recipients: A Pilot Study. Journal of Clinical Medicine. 2023; 12(6):2302. https://doi.org/10.3390/jcm12062302
Chicago/Turabian StyleSikorska, Dorota, Dorota Kamińska, Rusan Catar, Dashan Wu, Hongfan Zhao, Pinchao Wang, Julian Kamhieh-Milz, Mirosław Banasik, Mariusz Kusztal, Magdalena Cielecka, and et al. 2023. "Nailfold Videocapillaroscopy for Non-Invasive Assessment of Microcirculation and Prognostic Correlation with Endothelial Dysfunction, Cardiovascular Risk Factors, and Non-HLA Antibodies in Heart Transplant Recipients: A Pilot Study" Journal of Clinical Medicine 12, no. 6: 2302. https://doi.org/10.3390/jcm12062302






