Effect of Frozen-Thawed Embryo Transfer on the Metabolism of Children in Early Childhood
Abstract
:1. Introduction
2. Method
2.1. Study Design and Population
2.2. Medical History
2.3. IVF Procedures
2.4. Biochemical Analysis
2.5. Metabolomic Analysis
2.5.1. Sample Preparation
2.5.2. Data Analysis and Processing
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Biochemical Profile
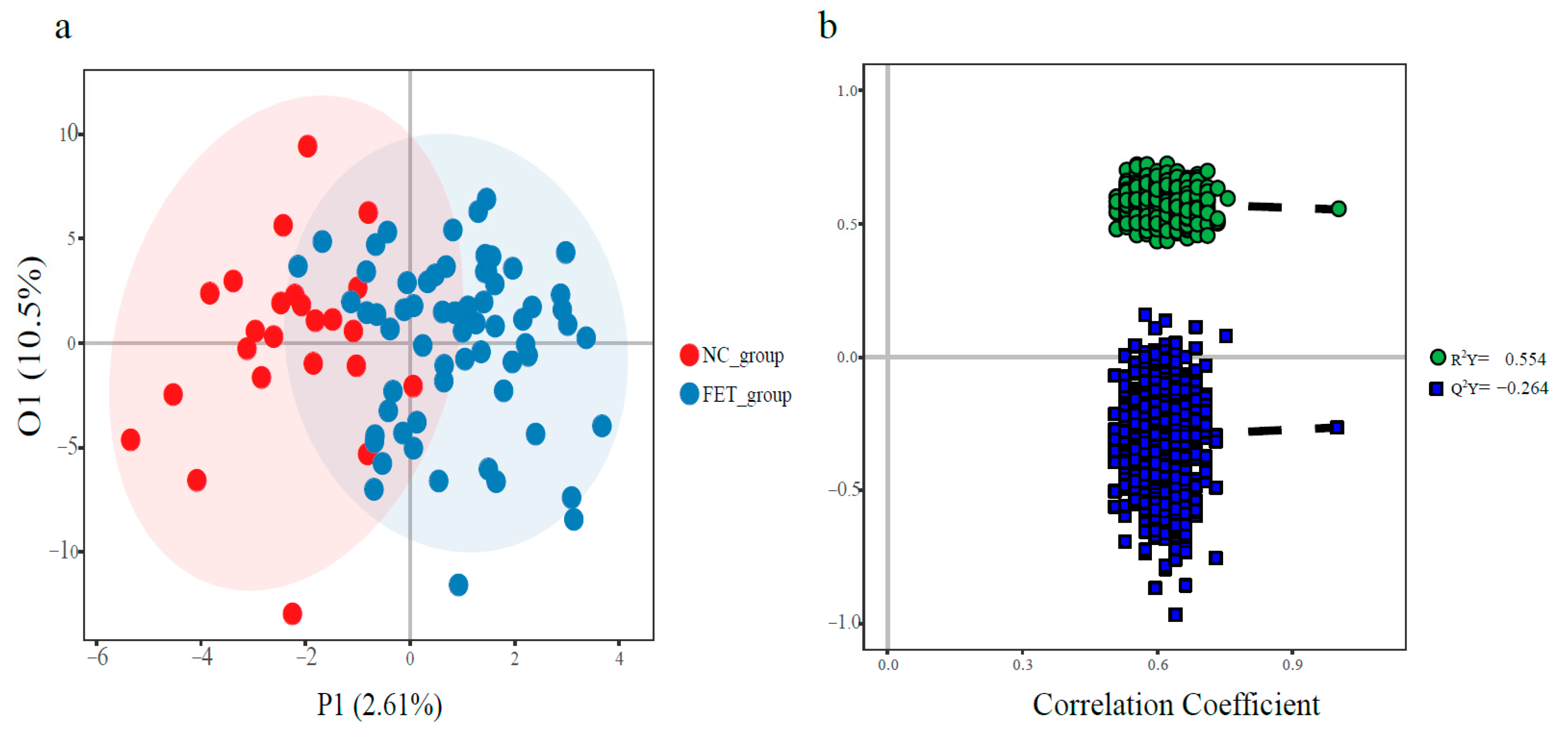
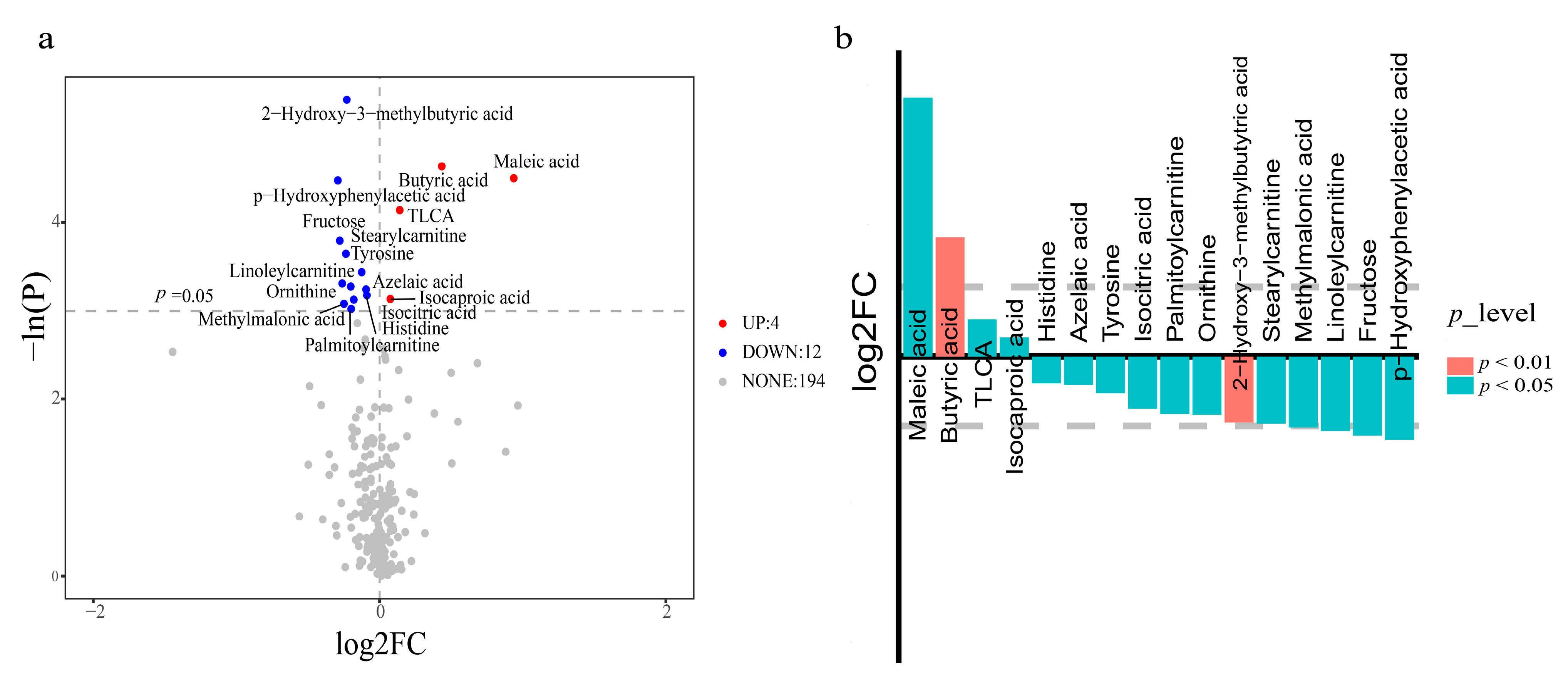
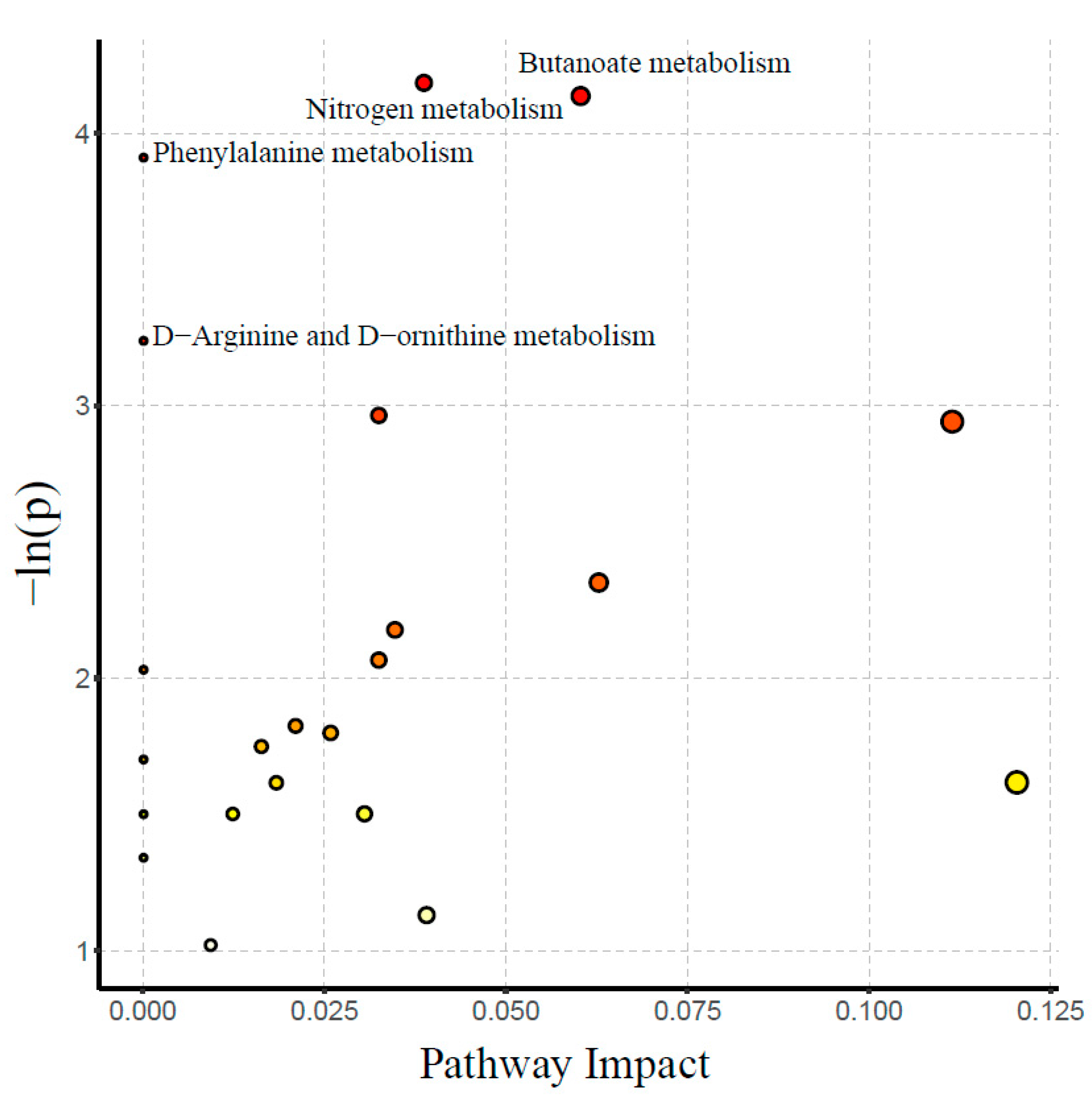
3.3. Metabolomic Profile
4. Discussion
4.1. Glycolipid Metabolism
4.2. Amino Acid Metabolism
4.3. Bile Acids (BAs) and Short-Chain Fatty Acids (SFACs) Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wennerholm, U.-B.; Bergh, C. Perinatal outcome in children born after assisted reproductive technologies. Ups. J. Med. Sci. 2020, 125, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trounson, A.; Mohr, L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature 1983, 305, 707–709. [Google Scholar] [CrossRef]
- Belva, F.; Henriet, S.; Van den Abbeel, E.; Camus, M.; Devroey, P.; Van der Elst, J.; Liebaers, I.; Haentjens, P.; Bonduelle, M. Neonatal outcome of 937 children born after transfer of cryopreserved embryos obtained by ICSI and IVF and comparison with outcome data of fresh ICSI and IVF cycles. Hum. Reprod. 2008, 23, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, S.; Pinborg, A. Large for gestational age and macrosomia in singletons born after frozen/thawed embryo transfer (FET) in assisted reproductive technology (ART). Birth Defects Res. 2018, 110, 630–643. [Google Scholar] [CrossRef]
- Sha, T.; Yin, X.; Cheng, W.; Massey, I.Y. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: A meta-analysis. Fertil. Steril. 2018, 109, 330–342. [Google Scholar] [CrossRef] [Green Version]
- Roque, M.; Lattes, K.; Serra, S.; Solà, I.; Geber, S.; Carreras, R.; Checa, M.A. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: A systematic review and meta-analysis. Fertil. Steril. 2013, 99, 156–162. [Google Scholar] [CrossRef]
- Wei, D.; Liu, J.-Y.; Sun, Y.; Shi, Y.; Zhang, B.; Liu, J.-Q.; Tan, J.; Liang, X.; Cao, Y.; Wang, Z.; et al. Frozen versus fresh single blastocyst transfer in ovulatory women: A multicentre, randomised controlled trial. Lancet 2019, 393, 1310–1318. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, 290–296. [Google Scholar] [CrossRef] [Green Version]
- Hermann, G.M.; Dallas, L.M.; Haskell, S.E.; Roghair, R.D. Neonatal macrosomia is an independent risk factor for adult metabolic syndrome. Neonatology 2010, 98, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Calle, A.; Miranda, A.; Fernandez-Gonzalez, R.; Pericuesta, E.; Laguna, R.; Gutierrez-Adan, A. Male mice produced by in vitro culture have reduced fertility and transmit organomegaly and glucose intolerance to their male offspring. Biol. Reprod. 2012, 87, 34. [Google Scholar] [CrossRef]
- Chen, M.; Wu, L.; Wu, F.; Wittert, G.A.; Norman, R.J.; Robker, R.L.; Heilbronn, L.K. Impaired glucose metabolism in response to high fat diet in female mice conceived by in vitro fertilization (IVF) or ovarian stimulation alone. PLoS ONE 2014, 9, e113155. [Google Scholar] [CrossRef] [Green Version]
- Cerny, D.; Sartori, C.; Rimoldi, S.F.; Meister, T.; Soria, R.; Bouillet, E.; Scherrer, U.; Rexhaj, E. Assisted Reproductive Technologies Predispose to Insulin Resistance and Obesity in Male Mice Challenged With a High-Fat Diet. Endocrinology 2017, 158, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Vrooman, L.A.; Bartolomei, M.S. Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod. Toxicol. 2017, 68, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Qin, N.; Zhou, Z.; Zhao, W.; Zou, K.; Shi, W.; Yu, C.; Liu, X.; Dong, Z.; Mao, Y.; Liu, X.; et al. Abnormal Glucose Metabolism in Male Mice Offspring Conceived by Fertilization and Frozen-Thawed Embryo Transfer. Front. Cell Dev. Biol. 2021, 9, 637781. [Google Scholar] [CrossRef]
- Ceelen, M.; van Weissenbruch, M.M.; Roos, J.C.; Vermeiden, J.P.W.; van Leeuwen, F.E.; Delemarre-van de Waal, H.A. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J. Clin. Endocrinol. Metab. 2007, 92, 3417–3423. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Zhou, W.; Xi, B.; Ma, J.; Hu, J.; Fang, M.; Hu, K.; Qin, Y.; You, L.; Cao, Y.; et al. Increased risk of metabolic dysfunction in children conceived by assisted reproductive technology. Diabetologia 2020, 63, 2150–2157. [Google Scholar] [CrossRef]
- Green, M.P.; Mouat, F.; Miles, H.L.; Hopkins, S.A.; Derraik, J.G.B.; Hofman, P.L.; Peek, J.C.; Cutfield, W.S. Phenotypic differences in children conceived from fresh and thawed embryos in in vitro fertilization compared with naturally conceived children. Fertil.Steril. 2013, 99, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, M.; Källén, B. Combined use of selective serotonin reuptake inhibitors and sedatives/hypnotics during pregnancy: Risk of relatively severe congenital malformations or cardiac defects. A register study. BMJ Open 2013, 3, e002166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yang, Y.; Peng, W.; Chang, J.; Mei, Y.; Yan, L.; Chen, Y.; Wei, X.; Liu, Y.; Wang, Y.; et al. A 7-Year Report of Spectrum of Inborn Errors of Metabolism on Full-Term and Premature Infants in a Chinese Neonatal Intensive Care Unit. Front. Genet. 2019, 10, 1302. [Google Scholar] [CrossRef] [PubMed]
- Chatillon-Boissier, K.; Genod, A.; Denis-Belicard, E.; Felloni, B.; Chene, G.; Seffert, P.; Chauleur, C. Prospective randomised study of long versus short agonist protocol with poor responder patients during in vitro fertilization. Gynecol Obs. Fertil. 2012, 40, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, A.; Selman, H.; Valeri, C.; Napoletano, S.; Sbracia, M.; Antonini, G.; Biagiotti, G.; Pacchiarotti, A. Ovarian Stimulation Protocol in IVF: An Up-to-Date Review of the Literature. Curr. Pharm. Biotechnol. 2016, 17, 303–315. [Google Scholar] [CrossRef] [PubMed]
- La Marca, A.; Sunkara, S.K. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: From theory to practice. Hum. Reprod. Update 2014, 20, 124–140. [Google Scholar] [CrossRef] [Green Version]
- Nargund, G.; Datta, A.K.; Fauser, B. Mild stimulation for in vitro fertilization. Fertil. Steril. 2017, 108, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Yin, X.; Zhang, S.; Jiang, H.; Tan, K.; Li, J.; Xiong, B.; Gong, F.; Zhang, C.; Pan, X.; et al. Clinical outcome of preimplantation genetic diagnosis and screening using next generation sequencing. Gigascience 2014, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Y.; Chen, Q.; Fu, Y.; Wang, Y.; Hong, Q.; Lyu, Q.; Ai, A.; Shoham, Z. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil. Steril. 2015, 104, 62–70.e63. [Google Scholar] [CrossRef]
- Xue, Y.; Tong, X.; Jiang, L.; Zhu, H.; Yang, L.; Zhang, S. Effect of vitrification versus slow freezing of human day 3 embryos on β-hCG levels. J. Assist. Reprod. Genet. 2014, 31, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Cao, B.; Guo, X.; Li, W.; Li, S.; Chen, J.; Zhou, W.; Zheng, C.; Wei, Y. Apolipoprotein B-100 peptide 210 antibody inhibits atherosclerosis by regulation of macrophages that phagocytize oxidized lipid. Am. J. Transl. Res. 2018, 10, 1817–1828. [Google Scholar] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Jiao, Y.; Gao, Y.; Zhang, P.; Huang, W.; Liu, Y.; Huang, Y.; Tian, Y.; Wan, J.B.; Zhang, Z.; et al. An extendable all-in-one injection twin derivatization LC-MS/MS strategy for the absolute quantification of multiple chemical-group-based submetabolomes. Anal. Chim. Acta 2019, 1063, 99–109. [Google Scholar] [CrossRef]
- Bian, X.; Li, N.; Tan, B.; Sun, B.; Guo, M.Q.; Huang, G.; Fu, L.; Hsiao, W.L.W.; Liu, L.; Wu, J.L. Polarity-Tuning Derivatization-LC-MS Approach for Probing Global Carboxyl-Containing Metabolites in Colorectal Cancer. Anal. Chem. 2018, 90, 11210–11215. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-Y.; Liu, X.-M.; Jin, L.; Wang, T.-T.; Ullah, K.; Sheng, J.-Z.; Huang, H.-F. Cardiovascular and metabolic profiles of offspring conceived by assisted reproductive technologies: A systematic review and meta-analysis. Fertil. Steril. 2017, 107, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Bruls, Y.M.; de Ligt, M.; Lindeboom, L.; Phielix, E.; Havekes, B.; Schaart, G.; Kornips, E.; Wildberger, J.E.; Hesselink, M.K.; Muoio, D.; et al. Carnitine supplementation improves metabolic flexibility and skeletal muscle acetylcarnitine formation in volunteers with impaired glucose tolerance: A randomised controlled trial. EBioMedicine 2019, 49, 318–330. [Google Scholar] [CrossRef] [Green Version]
- Sjöblom, C.; Roberts, C.T.; Wikland, M.; Robertson, S.A. Granulocyte-macrophage colony-stimulating factor alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology 2005, 146, 2142–2153. [Google Scholar] [CrossRef]
- Ceelen, M.; van Weissenbruch, M.M.; Prein, J.; Smit, J.J.; Vermeiden, J.P.W.; Spreeuwenberg, M.; van Leeuwen, F.E.; Delemarre-van de Waal, H.A. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8-18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum. Reprod. 2009, 24, 2788–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, L.D.; Koulman, A.; Griffin, J.L. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: Progress from the metabolome. Lancet Diabetes Endocrinol. 2014, 2, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Feng, R.; Guo, F.; Li, Y.; Jiao, J.; Sun, C. Targeted metabolomic analysis reveals the association between the postprandial change in palmitic acid, branched-chain amino acids and insulin resistance in young obese subjects. Diabetes Res. Clin. Pract. 2015, 108, 84–93. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kido, J.; Matsumoto, S.; Shimizu, K.; Nakamura, K. Associations among amino acid, lipid, and glucose metabolic profiles in childhood obesity. BMC Pediatr. 2019, 19, 273. [Google Scholar] [CrossRef]
- Perng, W.; Gillman, M.W.; Fleisch, A.F.; Michalek, R.D.; Watkins, S.M.; Isganaitis, E.; Patti, M.E.; Oken, E. Metabolomic profiles and childhood obesity. Obesity 2014, 22, 2570–2578. [Google Scholar] [CrossRef] [Green Version]
- Moran-Ramos, S.; Ocampo-Medina, E.; Gutierrez-Aguilar, R.; Macías-Kauffer, L.; Villamil-Ramírez, H.; López-Contreras, B.E.; León-Mimila, P.; Vega-Badillo, J.; Gutierrez-Vidal, R.; Villarruel-Vazquez, R.; et al. An Amino Acid Signature Associated with Obesity Predicts 2-Year Risk of Hypertriglyceridemia in School-Age Children. Sci. Rep. 2017, 7, 5607. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Suhre, K.; Meisinger, C.; Döring, A.; Altmaier, E.; Belcredi, P.; Gieger, C.; Chang, D.; Milburn, M.V.; Gall, W.E.; Weinberger, K.M.; et al. Metabolic footprint of diabetes: A multiplatform metabolomics study in an epidemiological setting. PLoS ONE 2010, 5, e13953. [Google Scholar] [CrossRef] [Green Version]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goffredo, M.; Mass, K.; Parks, E.J.; Wagner, D.A.; McClure, E.A.; Graf, J.; Savoye, M.; Pierpont, B.; Cline, G.; Santoro, N. Role of Gut Microbiota and Short Chain Fatty Acids in Modulating Energy Harvest and Fat Partitioning in Youth. J. Clin. Endocrinol. Metab. 2016, 101, 4367–4376. [Google Scholar] [CrossRef] [PubMed]
- Rahat-Rozenbloom, S.; Fernandes, J.; Gloor, G.B.; Wolever, T.M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. 2014, 38, 1525–1531. [Google Scholar] [CrossRef] [Green Version]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef] [Green Version]
| Children Factors | NC Group (n = 24) | FET Group (n = 65) | p Value |
|---|---|---|---|
| Age at follow-up (months) | 37.20 (32.05–41.30) | 33.50 (26.30–35.30) | 0.002 ** |
| Gender | 0.651 | ||
| Boy | 12 (50.00%) | 29 (44.60%) | |
| Girl | 12 (50.00%) | 36 (55.40%) | |
| BMI (kg/m2) | 15.40 (14.83–16.15) | 15.90 (15.10–16.70) | 0.377 |
| Gestational week | 38.85 (38.20–39.55) | 39.00 (38.00–39.00) | 0.303 |
| PTB (<37 weeks) | 0 (0.0%) | 3 (4.6%) | 0.683 |
| Birthweight (g) | 3395.00 (3200.00–3586.25) | 3460.00 (3000.00–3710.00) | 0.757 |
| LBW (<2500 g) | 6 (7.2%) | 0 (0.0%) | |
| Macrosomia (>4000 g) | 1 (4.2%) | 7 (10.8%) | 0.583 |
| SGA | 0 (0.0%) | 6 (9.2%) | 0.287 |
| LGA | 1 (4.2%) | 10 (15.4%) | 0.287 |
| NICU | 0 (0.0%) | 0 (0.0%) | 0.379 |
| Breastfeedingtotal duration (months) | 6.00 (6.00–11.50) | 8.00 (6.00–12.00) | 0.703 |
| Maternal factors | |||
| Ovum age (years) | 29.50 (27.00–34.00) | 32.00 (28.00–35.00) | 0.256 |
| Pregnant age (years) | 29.50 (27.00–34.00) | 32.00 (28.00–35.00) | 0.179 |
| BMI (kg/m2) | 19.95 (18.40–23.35) | 19.90 (19.90–21.00) | 0.875 |
| Type of delivery | |||
| Cesarean section | 7 (29.20%) | 21 (32.30%) | 0.777 |
| Vaginal | 17 (70.80%) | 44 (67.70%) | |
| Smoking | 0 (0.0%) | 2 (3.1%) | 0.949 |
| Passive smoking | 10 (41.7%) | 29 (45.3%) | 0.759 |
| Drinking | 8 (33.33%) | 9 (13.8%) | 0.076 |
| GDM | 11 (13.30%) | 1 (3.40%) | |
| Prenatal factors | |||
| Sperm age | 31.00 (28.00–33.00) | 33.00 (30.00–36.50) | 0.077 |
| BMI (kg/m2) | 24.20 (21.80–26.55) | 23.90 (21.95–24.85) | 0.694 |
| Smoking | 5 (20.8%) | 20 (30.8%) | 0.355 |
| Biochemical Markers | NC Group | FET Group | p Value |
|---|---|---|---|
| Fasting blood glucose | 5.00 (4.80–5.20) | 5.20 (4.90–5.40) | 0.049 * |
| TC (mmol/L) | 4.42 (3.88–5.01) | 4.39 (3.83–4.98) | 0.805 |
| TG (mmol/L) | 0.67 (0.54–0.85) | 0.70 (0.57–0.87) | 0.718 |
| HDL (mmol/L) | 1.35 (1.12–1.47) | 1.15 (1.36–1.58) | 0.372 |
| LDL (mmol/L) | 2.25 (1.91–2.99) | 1.99 (2.26–2.94) | 0.894 |
| APOE (g/L) | 179.80 (118.88–235.66) | 263.93 (180.95–387.52) | 0.007 ** |
| Insulin (IU/L) | 4.02 (3.50–6.34) | 3.32 (3.00–4.02) | 0.002 ** |
| HOMA-IR | 0.90 (0.77–1.59) | 0.78 (0.68–0.94) | 0.018 * |
| Leptin (pg/mL) | 207.58 (108.44–257.65) | 229.20 (129.13–322.98) | 0.305 |
| FT3 (pmol/L) | 4.44 (4.02–6.45) | 4.88 (4.27–6.65) | 0.240 |
| FT4 (pmol/L) | 15.78 (15.17–16.62) | 15.61 (14.30–18.24) | 0.631 |
| TSH (mIU/L) | 3.49 (4.33–6.45) | 4.34 (3.65–4.99) | 1.000 |
| CRP (ng/mL) | 1017.01 (568.47–2263.48) | 1039.35 (455.94.47–4467.87) | 0.901 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.-H.; Wu, T.; Zhang, C.; Su, K.-Z.; Wu, Y.-T.; Huang, H.-F. Effect of Frozen-Thawed Embryo Transfer on the Metabolism of Children in Early Childhood. J. Clin. Med. 2023, 12, 2322. https://doi.org/10.3390/jcm12062322
Dong Z-H, Wu T, Zhang C, Su K-Z, Wu Y-T, Huang H-F. Effect of Frozen-Thawed Embryo Transfer on the Metabolism of Children in Early Childhood. Journal of Clinical Medicine. 2023; 12(6):2322. https://doi.org/10.3390/jcm12062322
Chicago/Turabian StyleDong, Ze-Han, Ting Wu, Chen Zhang, Kai-Zhen Su, Yan-Ting Wu, and He-Feng Huang. 2023. "Effect of Frozen-Thawed Embryo Transfer on the Metabolism of Children in Early Childhood" Journal of Clinical Medicine 12, no. 6: 2322. https://doi.org/10.3390/jcm12062322
APA StyleDong, Z.-H., Wu, T., Zhang, C., Su, K.-Z., Wu, Y.-T., & Huang, H.-F. (2023). Effect of Frozen-Thawed Embryo Transfer on the Metabolism of Children in Early Childhood. Journal of Clinical Medicine, 12(6), 2322. https://doi.org/10.3390/jcm12062322








