A Soccer Shot with Lengthy Consequences—Case Report & Current Literature Review of Commotio Cordis
Abstract
1. Case Presentation
2. Patient Information (De-Identified)
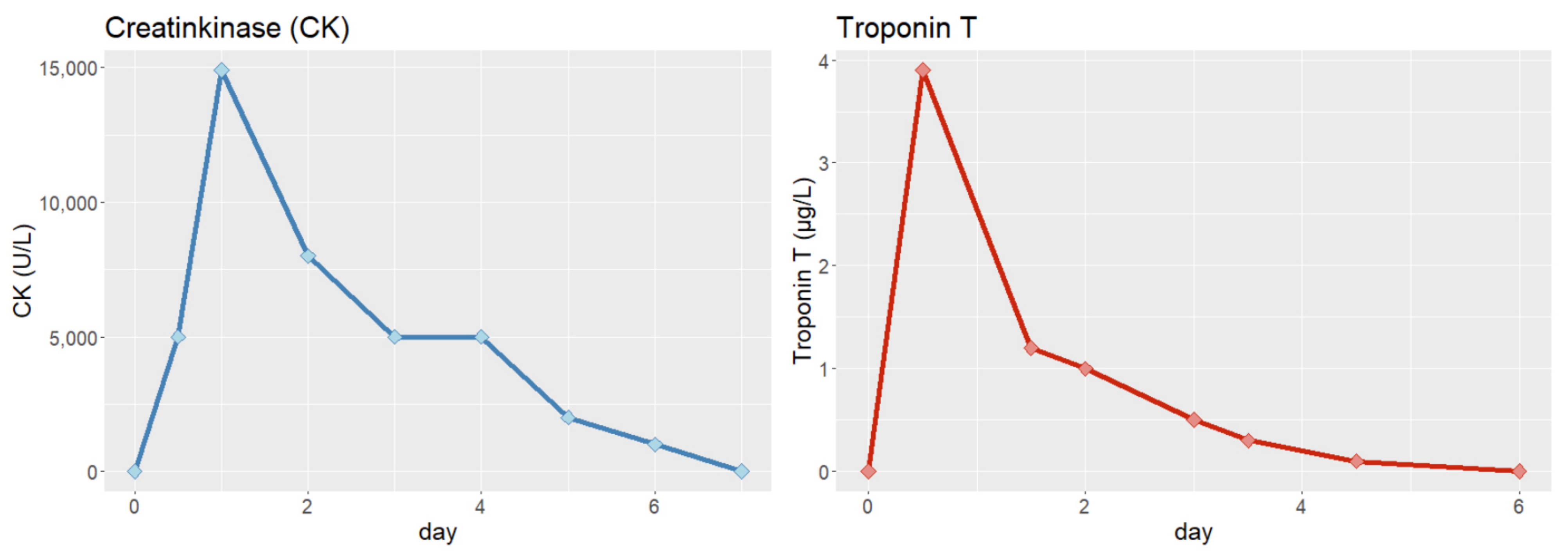
3. Physical Examination and Diagnostic Assessment
4. Differential Diagnosis
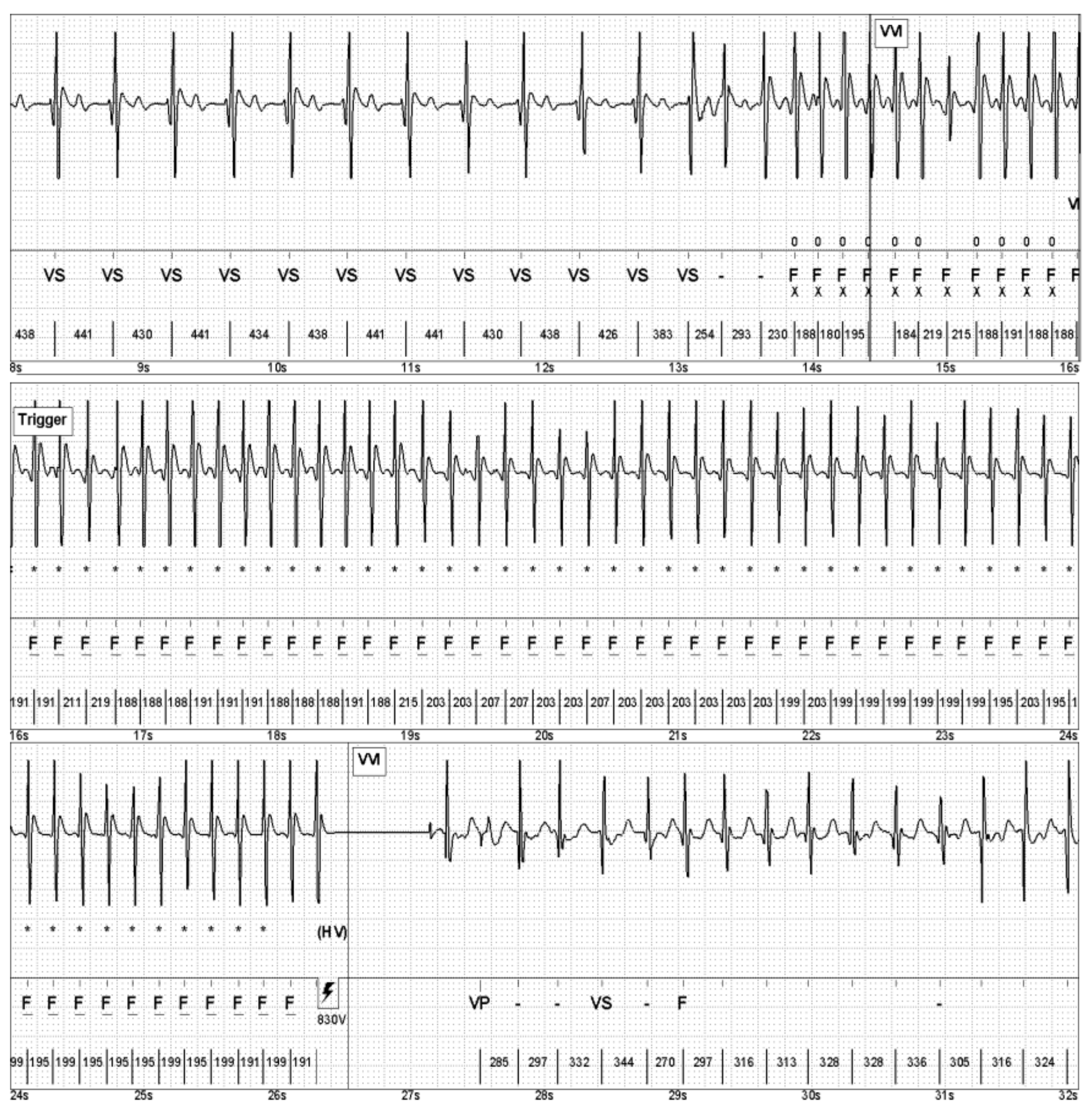
5. Interventions
6. Follow-Up
7. Outcomes
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zipes, D.P.; Ackerman, M.J.; Estes, N.A., 3rd; Grant, A.O.; Myerburg, R.J.; Van Hare, G. Task Force 7: Arrhythmias. J. Am. Coll. Cardiol. 2005, 45, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Kosicki, M.; Allen, F.; Steward, F.; Tomberg, K.; Pan, Y.; Bradley, A. Transvenous extraction of implantable cardioverter-defibrillator leads under advisory—A comparison of Riata, Sprint Fidelis, and non-recalled implantable cardioverter-defibrillator leads. Heart Rhythm 2013, 10, 1444–1450. [Google Scholar]
- El-Chami, M.F.; Merchant, F.M.; Levy, M.; Alam, M.B.; Rattan, R.; Hoskins, M.H.; Langberg, J.J.; Delurgio, D.; Lloyd, M.S.; Leon, A.R.; et al. Outcomes of Sprint Fidelis and Riata lead extraction: Data from 2 high-volume centers. Heart Rhythm 2015, 12, 1216–1220. [Google Scholar] [CrossRef]
- Chandra, N.; Bastiaenen, R.; Papadakis, M.; Sharma, S. Sudden cardiac death in young athletes: Practical challenges and diagnostic dilemmas. J. Am. Coll. Cardiol. 2013, 61, 1027–1040. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Stampe, N.K.; Jespersen, C.H.B.; Tfelt-Hansen, J.; Winkel, B.G. Exercise Testing Using Sprint Protocol vs Bruce Protocol in Catecholaminergic Polymorphic Ventricular Tachycardia. JACC Case Rep. 2022, 4, 996–1000. [Google Scholar] [CrossRef]
- Mascia, G.; Arbelo, E.; Hernandez-Ojeda, J.; Solimene, F.; Brugada, R.; Brugada, J. Brugada Syndrome and Exercise Practice: Current Knowledge, Shortcomings and Open Questions. Int. J. Sports Med. 2017, 38, 573–581. [Google Scholar]
- Corrado, D.; Drezner, J.A.; D’Ascenzi, F.; Zorzi, A. How to evaluate premature ventricular beats in the athlete: Critical review and proposal of a diagnostic algorithm. Br. J. Sports Med. 2020, 54, 1142–1148. [Google Scholar] [CrossRef]
- Link, M.S. Pathophysiology, prevention, and treatment of commotio cordis. Curr. Cardiol. Rep. 2014, 16, 495. [Google Scholar] [CrossRef]
- Menezes, R.G.; Fatima, H.; Hussain, S.A.; Ahmed, S.; Singh, P.K.; Kharoshah, M.A.; Madadin, M.; Ram, P.; Pant, S.; Luis, S.A. Commotio cordis: A review. Med. Sci. Law 2017, 57, 146–1451. [Google Scholar] [CrossRef]
- Link, M.S.; Estes, N.A.M., 3rd; Maron, B.J. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 13: Commotio Cordis: A Scientific Statement From the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2015, 66, 2439–2443. [Google Scholar] [CrossRef]
- Maron, B.J.; Gohman, T.E.; Kyle, S.B.; Estes, N.A., 3rd; Link, M.S. Clinical profile and spectrum of commotio cordis. JAMA 2002, 287, 1142–1146. [Google Scholar] [CrossRef]
- Link, M.S.; Wang, P.J.; Pandian, N.G.; Bharati, S.; Udelson, J.E.; Lee, M.Y.; Vecchiotti, M.A.; VanderBrink, B.A.; Mirra, G.; Maron, B.J.; et al. An experimental model of sudden death due to low-energy chest-wall impact (commotio cordis). N. Engl. J. Med. 1998, 338, 1805–1811. [Google Scholar] [CrossRef]
- Kohl, P.; Nesbitt, A.D.; Cooper, P.J.; Lei, M. Sudden cardiac death by Commotio cordis: Role of mechano-electric feedback. Cardiovasc. Res. 2001, 50, 280–289. [Google Scholar] [CrossRef]
- Alsheikh-Ali, A.A.; Madias, C.; Supran, S.; Link, M.S. Marked variability in susceptibility to ventricular fibrillation in an experimental commotio cordis model. Circulation 2010, 122, 2499–2504. [Google Scholar] [CrossRef] [PubMed]
- Bortnik, M.; Occhetta, E.; Ruggeri, C.; Marino, P. Transient trifascicular block complicating myocardial contusion after blunt chest trauma: A case report. J. Cardiovasc. Med. 2008, 9, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Haas, T.S.; Ahluwalia, A.; Garberich, R.F.; Estes, N.A., 3rd; Link, M.S. Increasing survival rate from commotio cordis. Heart Rhythm 2013, 10, 219–223. [Google Scholar] [CrossRef]
- Maron, B.J.; Estes, N.A., 3rd. Commotio cordis. N. Engl. J. Med. 2010, 362, 917–927. [Google Scholar] [CrossRef]
- Schaer, B.; Osswald, S.; Sticherling, C.; Tartini, R.; Pfisterer, M. Ventricular tachycardia in an ice-hockey player after a blunt chest trauma. Int. J. Cardiol. 2007, 114, 378–379. [Google Scholar] [CrossRef]
- Kalin, J.; Madias, C.; Alsheikh-Ali, A.A.; Link, M.S. Reduced diameter spheres increases the risk of chest blow-induced ventricular fibrillation (commotio cordis). Heart Rhythm 2011, 8, 1578–1581. [Google Scholar] [CrossRef]
- Link, M.S.; Maron, B.J.; Wang, P.J.; VanderBrink, B.A.; Zhu, W.; Estes, N.A., 3rd. Upper and lower limits of vulnerability to sudden arrhythmic death with chest-wall impact (commotio cordis). J. Am. Coll. Cardiol. 2003, 41, 99–104. [Google Scholar] [CrossRef]
- Michowitz, Y.; Tung, R.; Athill, C.; Shivkumar, K. Ventricular tachycardia from remote blunt chest trauma: Combined epicardial-endocardial right ventricular substrate characterization. Pacing Clin. Electrophysiol. 2012, 35, e127–e130. [Google Scholar] [CrossRef] [PubMed]
- Wolbrom, D.H.; Rahman, A.; Tschabrunn, C.M. Mechanisms and Clinical Management of Ventricular Arrhythmias following Blunt Chest Trauma. Cardiol. Res. Pract. 2016, 2016, 7270247. [Google Scholar] [CrossRef] [PubMed]



| Time zero, aged 32 years | Commotio cordis while playing soccer with survived cardiac death (ventricular fibrillation) |
| First month after CC | Recovery and implantation of VVI-ICD |
| Two years later | Inappropriate ICD shocks due to Riata® electrode malfunction; implantation of a new ICD lead without removal of the old one |
| Six years later | First appropriate ICD shock due to fast VT, repeated during playing soccer (performed against our advice) |
| Thirteen years later | Unsuccessful endocardial VT ablation attempt |
| Sixteen years later | Primarily successful VT epicardial ablation, complicated by iatrogenic palsy of the left phrenic nerve |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spitaler, P.; Stühlinger, M.; Adukauskaite, A.; Bauer, A.; Dichtl, W. A Soccer Shot with Lengthy Consequences—Case Report & Current Literature Review of Commotio Cordis. J. Clin. Med. 2023, 12, 2323. https://doi.org/10.3390/jcm12062323
Spitaler P, Stühlinger M, Adukauskaite A, Bauer A, Dichtl W. A Soccer Shot with Lengthy Consequences—Case Report & Current Literature Review of Commotio Cordis. Journal of Clinical Medicine. 2023; 12(6):2323. https://doi.org/10.3390/jcm12062323
Chicago/Turabian StyleSpitaler, Philipp, Markus Stühlinger, Agne Adukauskaite, Axel Bauer, and Wolfgang Dichtl. 2023. "A Soccer Shot with Lengthy Consequences—Case Report & Current Literature Review of Commotio Cordis" Journal of Clinical Medicine 12, no. 6: 2323. https://doi.org/10.3390/jcm12062323
APA StyleSpitaler, P., Stühlinger, M., Adukauskaite, A., Bauer, A., & Dichtl, W. (2023). A Soccer Shot with Lengthy Consequences—Case Report & Current Literature Review of Commotio Cordis. Journal of Clinical Medicine, 12(6), 2323. https://doi.org/10.3390/jcm12062323









