Surgical Risk Factors for Delayed Oral Feeding Autonomy in Patients with Left-Sided Congenital Diaphragmatic Hernia
Abstract
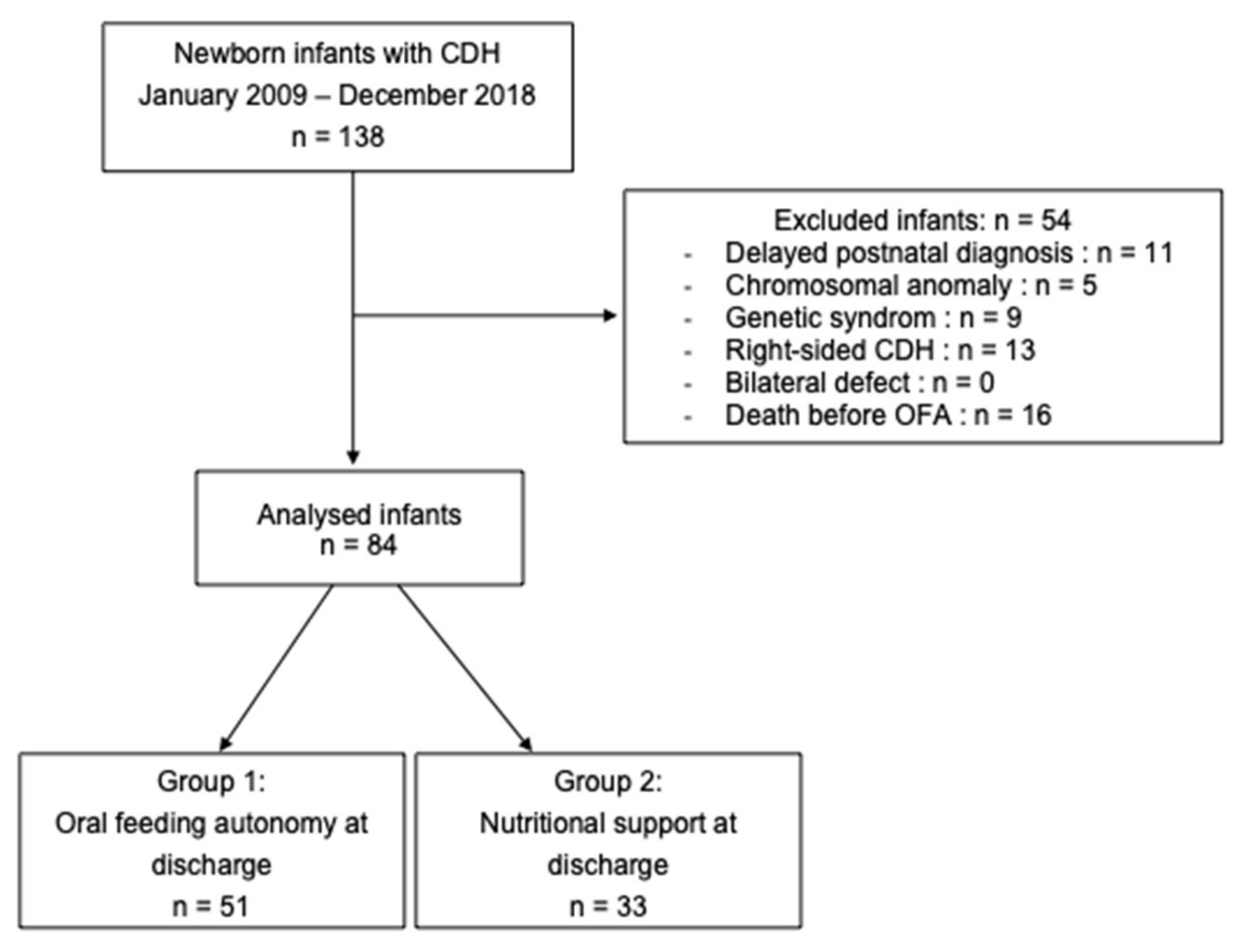
:1. Introduction
2. Materials and Methods
2.1. Population
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
- -
- Infants with a chromosomal anomaly or a genetic syndrome;
- -
- Delayed diagnosis of postnatal CDH (>24 h after birth);
- -
- Right-sided or bilateral defect;
- -
- Morgagni hernia;
- -
- Death occurring before discharge and before oral feeding autonomy (OFA) was acquired.
2.2. Treatment and Surgical Management
2.3. Data Collection
2.4. Method
2.4.1. Demographic Data
2.4.2. Hospitalization Data
2.4.3. Antenatal Data
2.4.4. Surgical Data
2.4.5. Postoperative Data
2.5. Adjustment on Patch Repair
- -
- -
- The need for a patch repair is one of the two main neonatal factors, with the requirement of ECMO almost constantly reported as associated with a failure to thrive and/or the need for nutritional support [19,20,21,22]. Because of a very small number of patients requiring ECMO in our population (n = 5), patch repair was the best adjustment variable in our study;
- -
2.6. Statistical Analysis
2.7. Ethical Agreement
3. Results
3.1. Population Characteristics
3.2. Factors Associated with Delayed Oral Feeding Autonomy
4. Discussion
4.1. Limitations of the Study
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harting, M.T.; Lally, K.P. The Congenital Diaphragmatic Hernia Study Group registry update. Semin. Fetal Neonatal Med. 2014, 19, 370–375. [Google Scholar] [CrossRef]
- Van Ginderdeuren, E.; Allegaert, K.; Decaluwe, H.; Deprest, J.; Debeer, A.; Proesmans, M. Clinical Outcome for Congenital Diaphragmatic Hernia at the Age of 1 Year in the Era of Fetal Intervention. Neonatology 2017, 112, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Nobuhara, K.K.; Lund, D.P.; Mitchell, J.; Kharasch, V.; Wilson, J.M. Long-term Outlook for Survivors of Congenital Diaphragmatic Hernia. Clin. Perinatol. 1996, 23, 873–887. [Google Scholar] [CrossRef]
- Pennaforte, T.; Rakza, T.; Fily, A.; Mur, S.; Diouta, L.; Sfeir, R.; Storme, L. Hernie de coupole diaphragmatique, devenir à long terme. Arch. Pédiatr. 2013, 20, S11–S18. [Google Scholar] [CrossRef]
- Jaillard, S.M.; Pierrat, V.; Dubois, A.; Truffert, P.; Lequien, P.; Wurtz, A.J.; Storme, L. Outcome at 2 years of infants with congenital diaphragmatic hernia, a population-based study. Ann. Thorac. Surg. 2003, 75, 250–256. [Google Scholar] [CrossRef]
- Muratore, C.S.; Utter, S.; Jaksic, T.; Lund, D.P.; Wilson, J.M. Nutritional morbidity in survivors of congenital diaphragmatic hernia. J. Pediatr. Surg. 2001, 36, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Wilken, M.; Bartmann, P.; Dovey, T.M.; Bagci, S. Characteristics of feeding tube dependency with respect to food aversive behaviour and growth. Appetite 2018, 123, 1–6. [Google Scholar] [CrossRef]
- Goday, P.S.; Huh, S.Y.; Silverman, A.; Lukens, C.T.; Dodrill, P.; Cohen, S.S.; Delaney, A.L.; Feuling, M.B.; Noel, J.R.; Gisel, E.; et al. Pediatric Feeding Disorder, Consensus Definition and Conceptual Framework. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Heiwegen, K.; van Rooij, I.A.; Scharbatke, H.; Roukema, J.; de Blaauw, I.; Botden, S.M. Factors related to long-term surgical morbidity in congenital diaphragmatic hernia survivors. J. Pediatr. Surg. 2018, 53, 508–512. [Google Scholar] [CrossRef]
- Jancelewicz, T.; Vu, L.T.; Keller, R.L.; Bratton, B.; Lee, H.; Farmer, D.; Harrison, M.; Miniati, D.; Mackenzie, T.; Hirose, S.; et al. Long-term surgical outcomes in congenital diaphragmatic hernia, observations from a single institution. J. Pediatr. Surg. 2010, 45, 155–160. [Google Scholar] [CrossRef]
- Yokota, K.; Uchida, H.; Kaneko, K.; Ono, Y.; Murase, N.; Makita, S.; Hayakawa, M. Surgical complications, especially gastroesophageal reflux disease, intestinal adhesion obstruction, and diaphragmatic hernia recurrence, are major sequelae in survivors of congenital diaphragmatic hernia. Pediatr. Surg. Int. 2014, 30, 895–899. [Google Scholar] [CrossRef]
- St Peter, S.D.; Valusek, P.A.; Tsao, K.; Holcomb, G.W., III; Ostlie, D.J.; Snyder, C.L. Abdominal Complications Related to Type of Repair for Congenital Diaphragmatic Hernia. J. Surg. Res. 2007, 140, 234–236. [Google Scholar] [CrossRef]
- Snoek, K.G.; Reiss, I.K.M.; Greenough, A.; Capolupo, I.; Urlesberger, B.; Wessel, L.; Storme, L.; Deprest, J.; van Heijst, A.; Tibboel, D. Standardized Postnatal Management of Infants with Congenital Diaphragmatic Hernia in Europe, The CDH EURO Consortium Consensus—2015 Update. Neonatology 2016, 110, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Storme, L.; Pennaforte, T.; Rakza, T.; Fily, A.; Sfeir, R.; Aubry, E.; Fayoux, P.; Deruelle, P.; Houfflin-Debarge, V.; Vaast, P.; et al. Per and post-natal medical management of congenital diaphragmatic hernia. Arch. Pediatr. Organe Off. Soc. Fr. Pediatr. 2010, 17 (Suppl. S3), S85–S92. [Google Scholar] [CrossRef]
- Storme, L.; Boubnova, J.; Mur, S.; Pognon, L.; Sharma, D.; Aubry, E.; Sfeir, R.; Vaast, P.; Rakza, T.; Benachi, A.; et al. Review shows that implementing a nationwide protocol for congenital diaphragmatic hernia was a key factor in reducing mortality and morbidity. Acta Paediatr. 2018, 107, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Heude, B.; Scherdel, P.; Werner, A.; Le Guern, M.; Gelbert, N.; Walther, D.; Arnould, M.; Bellaïche, M.; Chevallier, B.; Cheymol, J.; et al. A big-data approach to producing descriptive anthropometric references, a feasibility and validation study of paediatric growth charts. Lancet Digit. Health 2019, 1, e413–e423. [Google Scholar] [CrossRef] [Green Version]
- Haliburton, B.; Mouzaki, M.; Chiang, M.; Scaini, V.; Marcon, M.; Moraes, T.J.; Chiu, P.P. Long-term nutritional morbidity for congenital diaphragmatic hernia survivors, Failure to thrive extends well into childhood and adolescence. J. Pediatr. Surg. 2015, 50, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M.; Harting, M.T.; Calvo, R.Y.; Carroll, J.M.; Sykes, A.G.; Ignacio, R.C.; Ebanks, A.H.; Lazar, D.A. Identifying risk factors for enteral access procedures in neonates with congenital diaphragmatic hernia, A novel risk-assessment score. J. Pediatr. Surg. 2021, 56, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Pierog, A.; Aspelund, G.; Farkouh-Karoleski, C.; Wu, M.; Kriger, J.; Wynn, J.; Krishnan, U.; Mencin, A. Predictors of Low Weight and Tube Feedings in Children With Congenital Diaphragmatic Hernia at 1 Year of Age. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Fleming, H.; Dempsey, A.G.; Palmer, C.; Dempsey, J.; Friedman, S.; Galan, H.L.; Gien, J. Primary contributors to gastrostomy tube placement in infants with Congenital Diaphragmatic Hernia. J. Pediatr. Surg. 2021, 56, 1949–1956. [Google Scholar] [CrossRef]
- Verla, M.A.; Style, C.C.; Mehollin-Ray, A.R.; Fallon, S.C.; Vogel, A.M.; Fernandes, C.J.; Ikedionwu, C.A.; Lee, T.C.; Keswani, S.G.; Olutoye, O.O. Prenatal Imaging Features and Postnatal Factors Associated with Gastrointestinal Morbidity in Congenital Diaphragmatic Hernia. Fetal Diagn. Ther. 2020, 47, 252–260. [Google Scholar] [CrossRef]
- Rudra, S.; Adibe, O.O.; Malcolm, W.F.; Smith, P.B.; Cotten, C.M.; Greenberg, R.G. Gastrostomy tube placement in infants with congenital diaphragmatic hernia, Frequency, predictors, and growth outcomes. Early Hum. Dev. 2016, 103, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Brindle, M.E.; Brar, M.; Skarsgard, E.D.; Canadian Pediatric Surgery Network (CAPSNet). Patch repair is an independent predictor of morbidity and mortality in congenital diaphragmatic hernia. Pediatr. Surg. Int. 2011, 27, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Lally, K.P.; Lasky, R.E.; A Lally, P.; Bagolan, P.; Davis, C.F.; Frenckner, B.P.; Hirschl, R.M.; Langham, M.R.; Buchmiller, T.L.; Usui, N.; et al. Standardized reporting for congenital diaphragmatic hernia—An international consensus. J. Pediatr. Surg. 2013, 48, 2408–2415. [Google Scholar] [CrossRef]
- Congenital Diaphragmatic Hernia Study Group; Lally, K.P. Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics 2007, 120, e651–e657. [Google Scholar]
- Su, W.; Berry, M.; Puligandla, P.S.; Aspirot, A.; Flageole, H.; Laberge, J.-M. Predictors of gastroesophageal reflux in neonates with congenital diaphragmatic hernia. J. Pediatr. Surg. 2007, 42, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Diamond, I.R.; Mah, K.; Kim, P.C.; Bohn, D.; Gerstle, J.T.; Wales, P.W. Predicting the need for fundoplication at the time of congenital diaphragmatic hernia repair. J. Pediatr. Surg. 2007, 42, 1066–1070. [Google Scholar] [CrossRef]
- Dariel, A.; Rozé, J.-C.; Piloquet, H.; Podevin, G. Impact of prophylactic fundoplication on survival without growth disorder in left congenital diaphragmatic hernia requiring a patch repair. J. Pediatr. 2010, 157, 688–690.e1. [Google Scholar] [CrossRef] [Green Version]
- Maier, S.; Zahn, K.; Wessel, L.M.; Schaible, T.; Brade, J.; Reinshagen, K. Preventive antireflux surgery in neonates with congenital diaphragmatic hernia, a single-blinded prospective study. J. Pediatr. Surg. 2011, 46, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Chamond, C.; Morineau, M.; Gouizi, G.; Bargy, F.; Beaudoin, S. Preventive antireflux surgery in patients with congenital diaphragmatic hernia. World J. Surg. 2008, 32, 2454–2458. [Google Scholar] [CrossRef]
- Montalva, L.; Carricaburu, E.; Sfeir, R.; Fouquet, V.; Khen-Dunlop, N.; Hameury, F.; Panait, N.; Arnaud, A.; Lardy, H.; Schmitt, F.; et al. Anti-reflux surgery in children with congenital diaphragmatic hernia, A prospective cohort study on a controversial practice. J. Pediatr. Surg. 2022, 57, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Guner, Y.S.; Elliott, S.; Marr, C.C.; Greenholz, S.K. Anterior fundoplication at the time of congenital diaphragmatic hernia repair. Pediatr. Surg. Int. 2009, 25, 715–718. [Google Scholar] [CrossRef] [Green Version]
- Franken, J.; Mauritz, F.A.; Stellato, R.K.; Van der Zee, D.C.; Van Herwaarden-Lindeboom, M.Y.A. The Effect of Gastrostomy Placement on Gastric Function in Children, a Prospective Cohort Study. J. Gastrointest. Surg. 2017, 21, 1105–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khattak, I.; Kimber, C.; Kiely, E.; Spitz, L. Percutaneous endoscopic gastrostomy in paediatric practice, complications and outcome. J. Pediatr. Surg. 1998, 33, 67–72. [Google Scholar] [CrossRef]
- Mattei, P.; Rombeau, J.L. Review of the Pathophysiology and Management of Postoperative Ileus. World J. Surg. 2006, 30, 1382–1391. [Google Scholar] [CrossRef]
- Zahn, K.B.; Franz, A.-M.; Schaible, T.; Rafat, N.; Büttner, S.; Boettcher, M.; Wessel, L.M. Small Bowel Obstruction After Neonatal Repair of Congenital Diaphragmatic Hernia-Incidence and Risk-Factors Identified in a Large Longitudinal Cohort-Study. Front. Pediatr. 2022, 10, 846630. [Google Scholar] [CrossRef]
- Le Duc, K.; Mur, S.; Sharma, D.; Aubry, E.; Recher, M.; Rakza, T.; Storme, L. Prostaglandin E1 in infants with congenital diaphragmatic hernia (CDH) and life-threatening pulmonary hypertension. J. Pediatr. Surg. 2020, 55, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Grethel, E.J.; Cortes, R.A.; Wagner, A.J.; Clifton, M.S.; Lee, H.; Farmer, D.; Harrison, M.R.; Keller, R.L.; Nobuhara, K.K. Prosthetic patches for congenital diaphragmatic hernia repair, Surgisis vs Gore-Tex. J. Pediatr. Surg. 2006, 41, 29–33. [Google Scholar] [CrossRef]
| Characteristics | Total n = 84 | Group 1 n = 51 | Group 2 n = 33 | p-Value |
|---|---|---|---|---|
| Demographic data | ||||
| Gender-male | 45 (53.6) | 26 (51) | 19 (57.6) | 0.55 |
| Premature birth | 5 (6) | 3 (5.9) | 2 (6.1) | N/A |
| Gestational age | 39 (1.8) | 39.1 (1.4) | 38.8 (2.3) | 0.44 |
| Birth weight | 3250 (575) | 3287 (463) | 3193 (463) | 0.46 |
| Hospitalization data | ||||
| Age at NICU discharge (days) | 34.5 (16;60) | 22 (14;40) | 53 (38.90) | <0.001 |
| Age at hospital discharge (days) | 38 (20;69) | 23 (16;41) 1 | 68 (39;104) | <0.001 |
| Characteristic | Total n = 84 | Group 1 n = 51 | Group 2 n = 33 | ORcrude (95% CI) | p-Value | ORadjusted (95% CI) 1 | p-Value |
|---|---|---|---|---|---|---|---|
| Antenatal data | |||||||
| LHR | 51.5 (16.6) | 56.2 (17.0) 1 | 44.7 (13.5) 2 | 1.8 (1.1–2.8) 3 | 0.008 | 1.1 (0.7–1.9) 3 | 0.58 |
| Surgical data | |||||||
| ECMO support | 5 (6.0) | 2 (3.9) | 3 (9.1) | N/A | N/A | N/A | N/A |
| Laparotomy | 80 (95.2) | 47 (92.2) | 33 (100.0) | N/A | N/A | N/A | N/A |
| Thoracoscopy | 4 (4.8) | 4 (7.8) | 0 (0.0) | N/A | N/A | N/A | N/A |
| Liver up | 26 (32.1) | 10 (20.4) 4 | 16 (50.0) 5 | 3.9 (1.4–10.4) | 0.007 | 1.5 (0.4–5.2) | 0.53 |
| Stomach up | 49 (60.5) | 24 (49.0) 6 | 25 (78.1) 7 | 3.7 (1.3–10.2) | 0.011 | 2.2 (0.7–6.8) | 0.16 |
| Patch repair | 27 (32.1) | 8 (15.7) | 19 (57.6) | 7.3 (2.6–20.3) | <0.001 | N/A | N/A |
| Primary gastrostomy | 23 (27.4) | 3 (5.9) | 20 (60.6) | 24.6 (6.3–95.9) | <0.001 | 16.3 (3.5–74.4) | <0.001 |
| Operative time (minutes) | 57.5 (40; 65) | 50 (40; 60) 8 | 60 (50; 80) 9 | 1.5 (0.9–2.6) 10 | 0.086 | 1.1 (0.6–2.0) 10 | 0.64 |
| Postoperative events | |||||||
| Postoperative pleural effusion | 30 (35.7) | 11 (21.6) * | 19 (57.6) | 4.9 (1.8–12.9) | 0.001 | 2.8 (0.9–8.3) | 0.056 |
| Hernia recurrence | 3 (3.6) | 2 (3.9) | 1 (3.0) | N/A | N/A | N/A | N/A |
| Bowel obstruction | 11 (13.3) | 4 (7.8) | 7 (21.2) | 3.2 (0.8–11.9) | 0.087 | 3.7 (0.8–15.7) | 0.078 |
| Surgical reintervention | 11 (13.3) 11 | 3 (6.0) | 8 (24.2) | 5.0 (1.2–20.6) | 0.025 | 5.1 (1.1–23.7) | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourezma, M.; Mur, S.; Storme, L.; Cailliau, E.; Vaast, P.; Sfeir, R.; Lauriot Dit Prevost, A.; Aubry, E.; Le Duc, K.; Sharma, D., on behalf of French CDH Reference Center. Surgical Risk Factors for Delayed Oral Feeding Autonomy in Patients with Left-Sided Congenital Diaphragmatic Hernia. J. Clin. Med. 2023, 12, 2415. https://doi.org/10.3390/jcm12062415
Bourezma M, Mur S, Storme L, Cailliau E, Vaast P, Sfeir R, Lauriot Dit Prevost A, Aubry E, Le Duc K, Sharma D on behalf of French CDH Reference Center. Surgical Risk Factors for Delayed Oral Feeding Autonomy in Patients with Left-Sided Congenital Diaphragmatic Hernia. Journal of Clinical Medicine. 2023; 12(6):2415. https://doi.org/10.3390/jcm12062415
Chicago/Turabian StyleBourezma, Mélina, Sébastien Mur, Laurent Storme, Emeline Cailliau, Pascal Vaast, Rony Sfeir, Arthur Lauriot Dit Prevost, Estelle Aubry, Kévin Le Duc, and Dyuti Sharma on behalf of French CDH Reference Center. 2023. "Surgical Risk Factors for Delayed Oral Feeding Autonomy in Patients with Left-Sided Congenital Diaphragmatic Hernia" Journal of Clinical Medicine 12, no. 6: 2415. https://doi.org/10.3390/jcm12062415
APA StyleBourezma, M., Mur, S., Storme, L., Cailliau, E., Vaast, P., Sfeir, R., Lauriot Dit Prevost, A., Aubry, E., Le Duc, K., & Sharma, D., on behalf of French CDH Reference Center. (2023). Surgical Risk Factors for Delayed Oral Feeding Autonomy in Patients with Left-Sided Congenital Diaphragmatic Hernia. Journal of Clinical Medicine, 12(6), 2415. https://doi.org/10.3390/jcm12062415




