The Optimal Management of Inflammatory Bowel Disease in Patients with Cancer
Abstract
:1. Introduction
2. Risk of Developing Cancer in Patients with IBD
3. Inflammation-Related Cancer in Patients with IBD
3.1. Colorectal Carcinoma
3.2. Anal and Rectal Cancer
3.3. Small Bowel Cancer
3.4. Cholangiocarcinoma
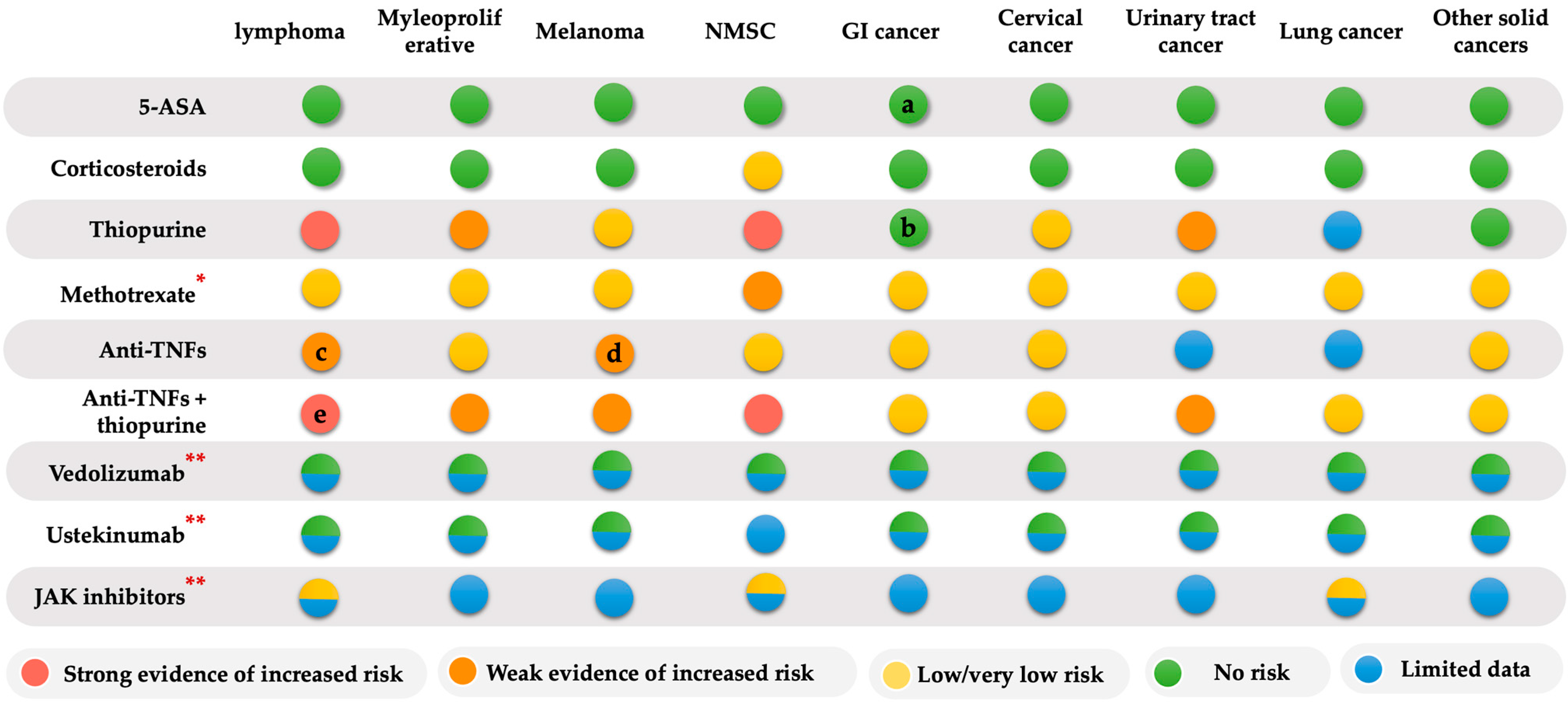
4. Risk of IBD Therapy-Related Cancer
4.1. Thiopurine and Cancer Risk
4.2. Methotrexate and Cancer Risk
4.3. Anti-Tumor Necrosis Factors (Anti-TNFs) and Cancer Risk
4.4. Combined Anti-TNF and Thiopurine Therapy and Cancer Risk
4.5. Vedolizumab and Cancer Risk
4.6. Ustekinumab and Cancer Risk
4.7. Small Molecules Therapy (JAK Inhibitors) and Cancer Risk
5. Management of IBD Therapy in Patients with a History of Previous Cancer
6. Management of IBD Therapy in Patients with Current or Active Cancer
6.1. Management of IBD Therapies in Patients with Active Cancer
6.2. Management of Chemotherapy and Radiation Therapy in IBD Patients
6.3. Management of Immune Checkpoint-Inhibitor (ICIs) Associated with IBD
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s Disease. Nat. Rev. Dis. Prim. 2020, 6, 22. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Kappelman, M.D.; Farkas, D.K.; Long, M.D.; Erichsen, R.; Sandler, R.S.; Sørensen, H.T.; Baron, J.A. Risk of Cancer in Patients with Inflammatory Bowel Diseases: A Nationwide Population-Based Cohort Study with 30 Years of Follow-up Evaluation. Clin. Gastroenterol. Hepatol. 2014, 12, 265–273.e1. [Google Scholar] [CrossRef] [Green Version]
- Beaugerie, L.; Itzkowitz, S.H. Cancers Complicating Inflammatory Bowel Disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Vavricka, S.; König, A.O.; Beaugerie, L.; Scharl, M.; Swiss IBDnet, An Official Working Group of the Swiss Society of Gastroenterology. Malignancies in Inflammatory Bowel Disease. Digestion 2020, 101, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The Four Epidemiological Stages in the Global Evolution of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.Y.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-First Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e4. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Berg, D.R.; Colombel, J.-F.; Ungaro, R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1896–1905. [Google Scholar] [CrossRef]
- Lo, B.; Zhao, M.; Vind, I.; Burisch, J. The Risk of Extraintestinal Cancer in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Population-Based Cohort Studies. Clin. Gastroenterol. Hepatol. 2021, 19, 1117–1138.e19. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Loftus, E.V. Risk of Cancer in Inflammatory Bowel Disease: Going up, Going down, or Still the Same? Curr. Opin. Gastroenterol. 2016, 32, 274–281. [Google Scholar] [CrossRef]
- Soularue, E.; Lepage, P.; Colombel, J.F.; Coutzac, C.; Faleck, D.; Marthey, L.; Collins, M.; Chaput, N.; Robert, C.; Carbonnel, F. Enterocolitis Due to Immune Checkpoint Inhibitors: A Systematic Review. Gut 2018, 67, 2056–2067. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.H.; Tatsioni, A.; Pedersen, N.; Shuhaibar, M.; Ramirez, V.H.; Politi, P.; Rombrechts, E.; Pierik, M.; Clofent, J.; Beltrami, M.; et al. Cancer in Inflammatory Bowel Disease 15years after Diagnosis in a Population-Based European Collaborative Follow-up Study. J. Crohn’s Colitis 2011, 5, 430–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, N.; Duricova, D.; Elkjaer, M.; Gamborg, M.; Munkholm, P.; Jess, T. Risk of Extra-Intestinal Cancer in Inflammatory Bowel Disease: Meta-Analysis of Population-Based Cohort Studies. Am. J. Gastroenterol. 2010, 105, 1480–1487. [Google Scholar] [CrossRef]
- Beaugerie, L. Inflammatory Bowel Disease Therapies and Cancer Risk: Where Are We and Where Are We Going? Gut 2012, 61, 476–483. [Google Scholar] [CrossRef]
- Gutierrez-Dalmau, A.; Campistol, J.M. Immunosuppressive Therapy and Malignancy in Organ Transplant Recipients: A Systematic Review. Drugs 2007, 67, 1167–1198. [Google Scholar] [CrossRef]
- Eaden, J.A. The Risk of Colorectal Cancer in Ulcerative Colitis: A Meta-Analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [Green Version]
- Jess, T.; Rungoe, C.; Peyrin–Biroulet, L. Risk of Colorectal Cancer in Patients with Ulcerative Colitis: A Meta-Analysis of Population-Based Cohort Studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef]
- Castaño-Milla, C.; Chaparro, M.; Gisbert, J.P. Systematic Review with Meta-Analysis: The Declining Risk of Colorectal Cancer in Ulcerative Colitis. Aliment. Pharmacol. Ther. 2014, 39, 645–659. [Google Scholar] [CrossRef]
- Beaugerie, L.; Svrcek, M.; Seksik, P.; Bouvier, A.; Simon, T.; Allez, M.; Brixi, H.; Gornet, J.; Altwegg, R.; Beau, P.; et al. Risk of Colorectal High-Grade Dysplasia and Cancer in a Prospective Observational Cohort of Patients with Inflammatory Bowel Disease. Gastroenterology 2013, 145, 166–175.e8. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Sulais, E.A.; Axelrad, J.E.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2022, jjac187. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Crothers, H.; Mytton, J.; Bosch, S.; Iqbal, T.; Ferguson, J.; Hirschfield, G.M. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People with Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology 2020, 159, 915–928. [Google Scholar] [CrossRef]
- Wijnands, A.M.; de Jong, M.E.; Lutgens, M.W.M.D.; Hoentjen, F.; Elias, S.G.; Oldenburg, B. Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Gastroenterology 2021, 160, 1584–1598. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Lytras, T.; Nikolopoulos, G.; Peyrin-Biroulet, L.; Danese, S. Systematic Review with Meta-Analysis: Use of 5-Aminosalicylates and Risk of Colorectal Neoplasia in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2017, 45, 1179–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Mei, Z.; Guo, Y.; Wang, G.; Wu, T.; Cui, X.; Huang, Z.; Zhu, Y.; Wen, D.; Song, J.; et al. Reduced Risk of Inflammatory Bowel Disease-Associated Colorectal Neoplasia with Use of Thiopurines: A Systematic Review and Meta-Analysis. J. Crohn’s Colitis 2018, 12, 546–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.J.; Qiu, X.Y.; Mao, X.Q.; Li, X.T.; Zhang, H.J. Systematic Review with Meta-Analysis: Thiopurines Decrease the Risk of Colorectal Neoplasia in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2018, 47, 318–331. [Google Scholar] [CrossRef] [Green Version]
- Laukoetter, M.G.; Mennigen, R.; Hannig, C.M.; Osada, N.; Rijcken, E.; Vowinkel, T.; Krieglstein, C.F.; Senninger, N.; Anthoni, C.; Bruewer, M. Intestinal Cancer Risk in Crohn’s Disease: A Meta-Analysis. J. Gastrointest. Surg. 2011, 15, 576–583. [Google Scholar] [CrossRef]
- Beaugerie, L.; Carrat, F.; Nahon, S.; Zeitoun, J.-D.; Sabaté, J.-M.; Peyrin-Biroulet, L.; Colombel, J.-F.; Allez, M.; Fléjou, J.-F.; Kirchgesner, J.; et al. High Risk of Anal and Rectal Cancer in Patients with Anal and/or Perianal Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 892–899.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baars, J.E.; Kuipers, E.J.; Dijkstra, G.; Hommes, D.W.; de Jong, D.J.; Stokkers, P.C.F.; Oldenburg, B.; Pierik, M.; Wahab, P.J.; van Bodegraven, A.A.; et al. Malignant Transformation of Perianal and Enterocutaneous Fistulas Is Rare: Results of 17 Years of Follow-up from the Netherlands. Scand. J. Gastroenterol. 2011, 46, 319–325. [Google Scholar] [CrossRef]
- Wan, Q.; Zhao, R.; Xia, L.; Wu, Y.; Zhou, Y.; Wang, Y.; Cui, Y.; Shen, X.; Wu, X.-T. Inflammatory Bowel Disease and Risk of Gastric, Small Bowel and Colorectal Cancer: A Meta-Analysis of 26 Observational Studies. J. Cancer Res. Clin. Oncol. 2021, 147, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Olén, O.; Sachs, M.C.; Erichsen, R.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Inflammatory Bowel Disease and Risk of Small Bowel Cancer: A Binational Population-Based Cohort Study from Denmark and Sweden. Gut 2020, 70, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.; Bennett, C.L.; Talamonti, M.S. Small Bowel Cancer in the United States: Changes in Epidemiology, Treatment, and Survival over the Last 20 Years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bojesen, R.D.; Riis, L.B.; Høgdall, E.; Nielsen, O.H.; Jess, T. Inflammatory Bowel Disease and Small Bowel Cancer Risk, Clinical Characteristics, and Histopathology: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2017, 15, 1900–1907.e2. [Google Scholar] [CrossRef]
- Biancone, L.; Armuzzi, A.; Scribano, M.L.; Castiglione, F.; D’incà, R.; Orlando, A.; Papi, C.; Daperno, M.; Vecchi, M.; Riegler, G.; et al. Cancer Risk in Inflammatory Bowel Disease: A 6-Year Prospective Multicenter Nested Case–Control IG-IBD Study. Inflamm. Bowel Dis. 2019, 26, 450–459. [Google Scholar] [CrossRef]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-Based Epidemiology, Malignancy Risk, and Outcome of Primary Sclerosing Cholangitis: Boonstra et Al. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef]
- Weismüller, T.J.; Trivedi, P.J.; Bergquist, A.; Imam, M.; Lenzen, H.; Ponsioen, C.Y.; Holm, K.; Gotthardt, D.; Färkkilä, M.A.; Marschall, H.-U.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate with Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984.e8. [Google Scholar] [CrossRef] [Green Version]
- Bowlus, C.L.; Lim, J.K.; Lindor, K.D. AGA Clinical Practice Update on Surveillance for Hepatobiliary Cancers in Patients with Primary Sclerosing Cholangitis: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 2416–2422. [Google Scholar] [CrossRef] [Green Version]
- Burak, K.; Angulo, P.; Pasha, T.M.; Egan, K.; Petz, J.; Lindor, K.D. Incidence and Risk Factors for Cholangiocarcinoma in Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2004, 99, 523–526. [Google Scholar] [CrossRef]
- Beaugerie, L.; Brousse, N.; Bouvier, A.M.; Colombel, J.F.; Lémann, M.; Cosnes, J.; Hébuterne, X.; Cortot, A.; Bouhnik, Y.; Gendre, J.P.; et al. Lymphoproliferative Disorders in Patients Receiving Thiopurines for Inflammatory Bowel Disease: A Prospective Observational Cohort Study. Lancet 2009, 374, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Kandiel, A. Increased Risk of Lymphoma among Inflammatory Bowel Disease Patients Treated with Azathioprine and 6-Mercaptopurine. Gut 2005, 54, 1121–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotlyar, D.S.; Lewis, J.D.; Beaugerie, L.; Tierney, A.; Brensinger, C.M.; Gisbert, J.P.; Loftus, E.V.; Peyrin-Biroulet, L.; Blonski, W.C.; Van Domselaar, M.; et al. Risk of Lymphoma in Patients with Inflammatory Bowel Disease Treated with Azathioprine and 6-Mercaptopurine: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 847–858.e4. [Google Scholar] [CrossRef]
- Peyrin–Biroulet, L.; Khosrotehrani, K.; Carrat, F.; Bouvier, A.; Chevaux, J.; Simon, T.; Carbonnel, F.; Colombel, J.; Dupas, J.; Godeberge, P.; et al. Increased Risk for Nonmelanoma Skin Cancers in Patients Who Receive Thiopurines for Inflammatory Bowel Disease. Gastroenterology 2011, 141, 1621–1628.e5. [Google Scholar] [CrossRef]
- Beaugerie, L.; Carrat, F.; Colombel, J.-F.; Bouvier, A.-M.; Sokol, H.; Babouri, A.; Carbonnel, F.; Laharie, D.; Faucheron, J.-L.; Simon, T.; et al. Risk of New or Recurrent Cancer under Immunosuppressive Therapy in Patients with IBD and Previous Cancer. Gut 2014, 63, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, B.; Svanström, H.; Schmiegelow, K.; Jess, T.; Hviid, A. Use of Azathioprine and the Risk of Cancer in Inflammatory Bowel Disease. Am. J. Epidemiol. 2013, 177, 1296–1305. [Google Scholar] [CrossRef]
- Algaba, A.; Guerra, I.; Marín-Jiménez, I.; Quintanilla, E.; López-Serrano, P.; García-Sánchez, M.C.; Casis, B.; Taxonera, C.; Moral, I.; Chaparro, M.; et al. Incidence, Management, and Course of Cancer in Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2015, 9, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Chaparro, M.; Ramas, M.; Benítez, J.M.; López-García, A.; Juan, A.; Guardiola, J.; Mínguez, M.; Calvet, X.; Márquez, L.; Salazar, L.I.F.; et al. Extracolonic Cancer in Inflammatory Bowel Disease: Data from the GETECCU Eneida Registry. Am. J. Gastroenterol. 2017, 112, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Levhar, N.; Ungar, B.; Kopylov, U.; Fudim, E.; Yavzori, M.; Picard, O.; Amariglio, N.; Chowers, Y.; Shemer-Avni, Y.; Mao, R.; et al. Propagation of EBV-Driven Lymphomatous Transformation of Peripheral Blood B Cells by Immunomodulators and Biologics Used in the Treatment of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1330–1339. [Google Scholar] [CrossRef]
- Huang, S.-Z.; Liu, Z.-C.; Liao, W.-X.; Wei, J.-X.; Huang, X.-W.; Yang, C.; Xia, Y.-H.; Li, L.; Ye, C.; Dai, S.-X. Risk of Skin Cancers in Thiopurines-Treated and Thiopurines-Untreated Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis: Meta-Analysis of Thiopurines Use. J. Gastroenterol. Hepatol. 2019, 34, 507–516. [Google Scholar] [CrossRef]
- Wheat, C.L.; Clark-Snustad, K.; Devine, B.; Grembowski, D.; Thornton, T.A.; Ko, C.W. Worldwide Incidence of Colorectal Cancer, Leukemia, and Lymphoma in Inflammatory Bowel Disease: An Updated Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1632439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magro, F.; Peyrin-Biroulet, L.; Sokol, H.; Aldeger, X.; Costa, A.; Higgins, P.D.; Joyce, J.C.; Katsanos, K.H.; Lopez, A.; de Xaxars, T.M.; et al. Extra-Intestinal Malignancies in Inflammatory Bowel Disease: Results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J. Crohn’s Colitis 2014, 8, 31–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazenberg, H.M.J.L.; de Boer, N.K.H.; Mulder, C.J.J.; Mom, S.H.; van Bodegraven, A.A.; Tack, G.J. Neoplasia and Precursor Lesions of the Female Genital Tract in IBD: Epidemiology, Role of Immunosuppressants, and Clinical Implications. Inflamm. Bowel Dis. 2018, 24, 510–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourrier, A.; Carrat, F.; Colombel, J.-F.; Bouvier, A.-M.; Abitbol, V.; Marteau, P.; Cosnes, J.; Simon, T.; Peyrin-Biroulet, L.; Beaugerie, L.; et al. Excess Risk of Urinary Tract Cancers in Patients Receiving Thiopurines for Inflammatory Bowel Disease: A Prospective Observational Cohort Study. Aliment. Pharmacol. Ther. 2016, 43, 252–261. [Google Scholar] [CrossRef]
- Algaba, A.; Guerra, I.; Castaño, A.; de la Poza, G.; Castellano, V.M.; López, M.; Bermejo, F. Risk of Cancer, with Special Reference to Extra-Intestinal Malignancies, in Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2013, 19, 9359–9365. [Google Scholar] [CrossRef]
- Derikx, L.A.A.P.; Nissen, L.H.C.; Drenth, J.P.H.; van Herpen, C.M.; Kievit, W.; Verhoeven, R.H.A.; Mulders, P.F.A.; Hulsbergen-van de Kaa, C.A.; Boers-Sonderen, M.J.; van den Heuvel, T.R.A.; et al. Better Survival of Renal Cell Carcinoma in Patients with Inflammatory Bowel Disease. Oncotarget 2015, 6, 38336–38347. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.H.C.; Assendorp, E.L.; van der Post, R.S.; Derikx, L.A.A.P.; de Jong, D.J.; Kievit, W.; Pierik, M.; van den Heuvel, T.; Verhoeven, R.; Overbeek, L.I.H.; et al. Impaired Gastric Cancer Survival in Patients with Inflammatory Bowel Disease. J. Gastrointest. Liver Dis. 2016, 25, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Mamtani, R.; Clark, A.S.; Scott, F.I.; Brensinger, C.M.; Boursi, B.; Chen, L.; Xie, F.; Yun, H.; Osterman, M.T.; Curtis, J.R.; et al. Association Between Breast Cancer Recurrence and Immunosuppression in Rheumatoid Arthritis and Inflammatory Bowel Disease: A Cohort Study. Arthritis Rheumatol. 2016, 68, 2403–2411. [Google Scholar] [CrossRef]
- Zenouzi, R.; Weismüller, T.J.; Jørgensen, K.K.; Bubenheim, M.; Lenzen, H.; Hübener, P.; Schulze, K.; Weiler-Normann, C.; Sebode, M.; Ehlken, H.; et al. No Evidence That Azathioprine Increases Risk of Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2016, 14, 1806–1812. [Google Scholar] [CrossRef]
- Kopylov, U.; Vutcovici, M.; Kezouh, A.; Seidman, E.; Bitton, A.; Afif, W. Risk of Lymphoma, Colorectal and Skin Cancer in Patients with IBD Treated with Immunomodulators and Biologics: A Quebec Claims Database Study. Inflamm. Bowel Dis. 2015, 21, 1847–1853. [Google Scholar] [CrossRef]
- Vanni, K.M.M.; Berliner, N.; Paynter, N.P.; Glynn, R.J.; MacFadyen, J.; Colls, J.; Lu, F.; Xu, C.; Ridker, P.M.; Solomon, D.H. Adverse Effects of Low-Dose Methotrexate in a Randomized Double-Blind Placebo-Controlled Trial: Adjudicated Hematologic and Skin Cancer Outcomes in the Cardiovascular Inflammation Reduction Trial. ACR Open Rheumatol. 2020, 2, 697–704. [Google Scholar] [CrossRef]
- Sepriano, A.; Kerschbaumer, A.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; de Wit, M.; et al. Safety of Synthetic and Biological DMARDs: A Systematic Literature Review Informing the 2019 Update of the EULAR Recommendations for the Management of Rheumatoid Arthritis. Ann. Rheum. Dis. 2020, 79, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Long, M.D.; Herfarth, H.H.; Pipkin, C.A.; Porter, C.Q.; Sandler, R.S.; Kappelman, M.D. Increased Risk for Non-Melanoma Skin Cancer in Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2010, 8, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Nugent, Z.; Demers, A.A.; Bernstein, C.N. Increased Risk of Nonmelanoma Skin Cancers among Individuals with Inflammatory Bowel Disease. Gastroenterology 2011, 141, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.H.C.; Pierik, M.; Derikx, L.A.A.P.; de Jong, E.; Kievit, W.; van den Heuvel, T.R.A.; van Rosendael, A.R.; Plasmeijer, E.I.; Dewint, P.; Verhoeven, R.H.A.; et al. Risk Factors and Clinical Outcomes in Patients with IBD with Melanoma. Inflamm. Bowel Dis. 2017, 23, 2018–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugué, P.-A.; Rebolj, M.; Hallas, J.; Garred, P.; Lynge, E. Risk of Cervical Cancer in Women with Autoimmune Diseases, in Relation with Their Use of Immunosuppressants and Screening: Population-Based Cohort Study. Int. J. Cancer 2015, 136, E711–E719. [Google Scholar] [CrossRef]
- Sebastian, S.; Neilaj, S. Practical Guidance for the Management of Inflammatory Bowel Disease in Patients with Cancer. Which Treatment? Ther. Adv. Gastroenterol. 2019, 12, 175628481881729. [Google Scholar] [CrossRef]
- Muller, M.; D’Amico, F.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. TNF Inhibitors and Risk of Malignancy in Patients with Inflammatory Bowel Diseases: A Systematic Review. J. Crohn’s Colitis 2021, 15, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.N.; Pasternak, B.; Basit, S.; Andersson, M.; Svanström, H.; Caspersen, S.; Munkholm, P.; Hviid, A.; Jess, T. Association Between Tumor Necrosis Factor-α Antagonists and Risk of Cancer in Patients with Inflammatory Bowel Disease. JAMA 2014, 311, 2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Bonovas, S. Systematic Review with Meta-Analysis: Biologics and Risk of Infection or Cancer in Elderly Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2020, 51, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Borren, N.Z.; Ananthakrishnan, A.N. Safety of Biologic Therapy in Older Patients with Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1736–1743.e4. [Google Scholar] [CrossRef] [Green Version]
- Osterman, M.T.; Sandborn, W.J.; Colombel, J.-F.; Robinson, A.M.; Lau, W.; Huang, B.; Pollack, P.F.; Thakkar, R.B.; Lewis, J.D. Increased Risk of Malignancy with Adalimumab Combination Therapy, Compared with Monotherapy, for Crohn’s Disease. Gastroenterology 2014, 146, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Biancone, L.; Orlando, A.; Kohn, A.; Colombo, E.; Sostegni, R.; Angelucci, E.; Rizzello, F.; Castiglione, F.; Benazzato, L.; Papi, C.; et al. Infliximab and Newly Diagnosed Neoplasia in Crohn’s Disease: A Multicentre Matched Pair Study. Gut 2006, 55, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Scharl, S.; Barthel, C.; Rossel, J.-B.; Biedermann, L.; Misselwitz, B.; Schoepfer, A.M.; Straumann, A.; Vavricka, S.R.; Rogler, G.; Scharl, M.; et al. Malignancies in Inflammatory Bowel Disease: Frequency, Incidence and Risk Factors—Results from the Swiss IBD Cohort Study. Am. J. Gastroenterol. 2019, 114, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Chupin, A.; Perduca, V.; Meyer, A.; Bellanger, C.; Carbonnel, F.; Dong, C. Systematic Review with Meta-Analysis: Comparative Risk of Lymphoma with Anti-Tumour Necrosis Factor Agents and/or Thiopurines in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2020, 52, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.A.; Marden, S.M.; Persing, S.M.; Larson, R.J.; Sands, B.E. Risk of Lymphoma Associated with Combination Anti–Tumor Necrosis Factor and Immunomodulator Therapy for the Treatment of Crohn’s Disease: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2009, 7, 874–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaitre, M.; Kirchgesner, J.; Rudnichi, A.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Association Between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients with Inflammatory Bowel Disease. JAMA 2017, 318, 1679. [Google Scholar] [CrossRef] [PubMed]
- Askling, J.; Fahrbach, K.; Nordstrom, B.; Ross, S.; Schmid, C.H.; Symmons, D. Cancer Risk with Tumor Necrosis Factor Alpha (TNF) Inhibitors: Meta-Analysis of Randomized Controlled Trials of Adalimumab, Etanercept, and Infliximab Using Patient Level Data: Cancer risk in trials of anti-tnf. Pharmacoepidem. Drug Saf. 2011, 20, 119–130. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Sandborn, W.J.; Ghosh, S.; Wolf, D.C.; Panaccione, R.; Feagan, B.; Reinisch, W.; Robinson, A.M.; Lazar, A.; Kron, M.; et al. Four-Year Maintenance Treatment with Adalimumab in Patients with Moderately to Severely Active Ulcerative Colitis: Data from ULTRA 1, 2, and 3. Am. J. Gastroenterol. 2014, 109, 1771–1780. [Google Scholar] [CrossRef] [Green Version]
- D’Haens, G.; Reinisch, W.; Panaccione, R.; Satsangi, J.; Petersson, J.; Bereswill, M.; Arikan, D.; Perotti, E.; Robinson, A.M.; Kalabic, J.; et al. Open: Lymphoma Risk and Overall Safety Profile of Adalimumab in Patients with Crohn’s Disease with up to 6 Years of Follow-up in the PYRAMID Registry. Am. J. Gastroenterol. 2018, 113, 872–882. [Google Scholar] [CrossRef]
- D’Haens, G.; Reinisch, W.; Colombel, J.-F.; Panes, J.; Ghosh, S.; Prantera, C.; Lindgren, S.; Hommes, D.W.; Huang, Z.; Boice, J.; et al. Five-Year Safety Data From ENCORE, a European Observational Safety Registry for Adults with Crohn’s Disease Treated with Infliximab [Remicade ®] or Conventional Therapy. ECCOJC 2016, 11, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Long, M.D.; Martin, C.F.; Pipkin, C.A.; Herfarth, H.H.; Sandler, R.S.; Kappelman, M.D. Risk of Melanoma and Nonmelanoma Skin Cancer Among Patients with Inflammatory Bowel Disease. Gastroenterology 2012, 143, 390–399.e1. [Google Scholar] [CrossRef] [Green Version]
- Esse, S.; Mason, K.J.; Green, A.C.; Warren, R.B. Melanoma Risk in Patients Treated with Biologic Therapy for Common Inflammatory Diseases: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2020, 156, 787. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Diamond, R.H.; Langholff, W.; Londhe, A.; Sandborn, W.J. Drug Therapies and the Risk of Malignancy in Crohn’s Disease: Results from the TREATTM Registry. Am. J. Gastroenterol. 2014, 109, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Kotlyar, D.S.; Osterman, M.T.; Diamond, R.H.; Porter, D.; Blonski, W.C.; Wasik, M.; Sampat, S.; Mendizabal, M.; Lin, M.V.; Lichtenstein, G.R. A Systematic Review of Factors That Contribute to Hepatosplenic T-Cell Lymphoma in Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2011, 9, 36–41.e1. [Google Scholar] [CrossRef]
- Shah, E.D.; Coburn, E.S.; Nayyar, A.; Lee, K.J.; Koliani-Pace, J.L.; Siegel, C.A. Systematic Review: Hepatosplenic T-Cell Lymphoma on Biologic Therapy for Inflammatory Bowel Disease, Including Data from the Food and Drug Administration Adverse Event Reporting System. Aliment. Pharmacol. Ther. 2020, 51, 527–533. [Google Scholar] [CrossRef]
- Cohen, R.D.; Bhayat, F.; Blake, A.; Travis, S. The Safety Profile of Vedolizumab in Ulcerative Colitis and Crohn’s Disease: 4 Years of Global Post-Marketing Data. J. Crohn’s Colitis 2020, 14, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Card, T.; Ungaro, R.; Bhayat, F.; Blake, A.; Hantsbarger, G.; Travis, S. Vedolizumab Use Is Not Associated with Increased Malignancy Incidence: GEMINI LTS Study Results and Post-Marketing Data. Aliment. Pharmacol. Ther. 2020, 51, 149–157. [Google Scholar] [CrossRef]
- Loftus, E.V.; Feagan, B.G.; Panaccione, R.; Colombel, J.-F.; Sandborn, W.J.; Sands, B.E.; Danese, S.; D’Haens, G.; Rubin, D.T.; Shafran, I.; et al. Long-Term Safety of Vedolizumab for Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2020, 52, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 578–590.e4. [Google Scholar] [CrossRef]
- Abreu, M.T.; Rowbotham, D.S.; Danese, S.; Sandborn, W.J.; Miao, Y.; Zhang, H.; Tikhonov, I.; Panaccione, R.; Hisamatsu, T.; Scherl, E.J.; et al. Efficacy and Safety of Maintenance Ustekinumab for Ulcerative Colitis Through 3 Years: UNIFI Long-Term Extension. J. Crohn’s Colitis 2022, 16, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Garre, A.; Iborra, M.; Sierra-Ausín, M.; Barreiro-de Acosta, M.; Fernández-Clotet, A.; de Castro, L.; Boscá-Watts, M.; Casanova, M.J.; López-García, A.; et al. Effectiveness and Safety of Ustekinumab in Ulcerative Colitis: Real-World Evidence from the ENEIDA Registry. J. Crohn’s Colitis 2021, 15, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Baston-Rey, I.; Fernández-Salgado, E.; González García, J.; Ramos, L.; Diz-Lois Palomares, M.T.; Argüelles-Arias, F.; Iglesias Flores, E.; Cabello, M.; Rubio Iturria, S.; et al. Long-Term Real-World Effectiveness and Safety of Ustekinumab in Crohn’s Disease Patients: The SUSTAIN Study. Inflamm. Bowel Dis. 2022, 28, 1725–1736. [Google Scholar] [CrossRef]
- Kopylov, U.; Hanzel, J.; Liefferinckx, C.; De Marco, D.; Imperatore, N.; Plevris, N.; Baston-Rey, I.; Harris, R.J.; Truyens, M.; Domislovic, V.; et al. Effectiveness of Ustekinumab Dose Escalation in Crohn’s Disease Patients with Insufficient Response to Standard-Dose Subcutaneous Maintenance Therapy. Aliment. Pharmacol. Ther. 2020, 52, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Papp, K.A.; Gooderham, M.; Pariser, D.M.; Augustin, M.; Kerdel, F.A.; Fakharzadeh, S.; Goyal, K.; Calabro, S.; Langholff, W.; et al. Drug Survival of Biologic Therapy in a Large, Disease-based Registry of Patients with Psoriasis: Results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1148–1158. [Google Scholar] [CrossRef] [Green Version]
- Olivera, P.A.; Lasa, J.S.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Safety of Janus Kinase Inhibitors in Patients with Inflammatory Bowel Diseases or Other Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1554–1573.e12. [Google Scholar] [CrossRef]
- Curtis, J.R.; Lee, E.B.; Kaplan, I.V.; Kwok, K.; Geier, J.; Benda, B.; Soma, K.; Wang, L.; Riese, R. Tofacitinib, an Oral Janus Kinase Inhibitor: Analysis of Malignancies across the Rheumatoid Arthritis Clinical Development Programme. Ann. Rheum. Dis. 2016, 75, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- OCEBM Levels of Evidence—Centre for Evidence-Based Medicine (CEBM), University of Oxford. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 6 February 2023).
- Acuna, S.A.; Huang, J.W.; Dossa, F.; Shah, P.S.; Kim, S.J.; Baxter, N.N. Cancer Recurrence after Solid Organ Transplantation: A Systematic Review and Meta-Analysis. Transplant. Rev. 2017, 31, 240–248. [Google Scholar] [CrossRef]
- Bernheim, O.; Colombel, J.-F.; Ullman, T.A.; Laharie, D.; Beaugerie, L.; Itzkowitz, S.H. The Management of Immunosuppression in Patients with Inflammatory Bowel Disease and Cancer. Gut 2013, 62, 1523–1528. [Google Scholar] [CrossRef]
- Shelton, E.; Laharie, D.; Scott, F.I.; Mamtani, R.; Lewis, J.D.; Colombel, J.-F.; Ananthakrishnan, A.N. Cancer Recurrence Following Immune-Suppressive Therapies in Patients with Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2016, 151, 97–109.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micic, D.; Komaki, Y.; Alavanja, A.; Rubin, D.T.; Sakuraba, A. Risk of Cancer Recurrence Among Individuals Exposed to Antitumor Necrosis Factor Therapy: A Systematic Review and Meta-Analysis of Observational Studies. J. Clin. Gastroenterol. 2019, 53, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Poullenot, F.; Amiot, A.; Nachury, M.; Viennot, S.; Altwegg, R.; Bouhnik, Y.; Abitbol, V.; Nancey, S.; Vuitton, L.; Peyrin-Biroulet, L.; et al. Comparative Risk of Incident Cancer in Patients with Inflammatory Bowel Disease with Prior Non-Digestive Malignancy According to Immunomodulator: A Multicentre Cohort Study. J. Crohn’s Colitis 2022, 16, 1523–1530. [Google Scholar] [CrossRef]
- Vedamurthy, A.; Gangasani, N.; Ananthakrishnan, A.N. Vedolizumab or Tumor Necrosis Factor Antagonist Use and Risk of New or Recurrent Cancer in Patients with Inflammatory Bowel Disease with Prior Malignancy: A Retrospective Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, 88–95. [Google Scholar] [CrossRef]
- Annese, V.; Beaugerie, L.; Egan, L.; Biancone, L.; Bolling, C.; Brandts, C.; Dierickx, D.; Dummer, R.; Fiorino, G.; Gornet, J.M.; et al. European Evidence-Based Consensus: Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2015, 9, 945–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [Green Version]
- Axelrad, J.E.; Fowler, S.A.; Friedman, S.; Ananthakrishnan, A.N.; Yajnik, V. Effects of Cancer Treatment on Inflammatory Bowel Disease Remission and Reactivation. Clin. Gastroenterol. Hepatol. 2012, 10, 1021–1027.e1. [Google Scholar] [CrossRef]
- Rajca, S.; Seksik, P.; Bourrier, A.; Sokol, H.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J. Impact of the Diagnosis and Treatment of Cancer on the Course of Inflammatory Bowel Disease. J. Crohn’s Colitis 2014, 8, 819–824. [Google Scholar] [CrossRef] [Green Version]
- de Boer, N.K.H.; Peyrin-Biroulet, L.; Jharap, B.; Sanderson, J.D.; Meijer, B.; Atreya, I.; Barclay, M.L.; Colombel, J.-F.; Lopez, A.; Beaugerie, L.; et al. Thiopurines in Inflammatory Bowel Disease: New Findings and Perspectives. J. Crohn’s Colitis 2018, 12, 610–620. [Google Scholar] [CrossRef] [Green Version]
- Sultan, K.; Korelitz, B.I.; Present, D.; Katz, S.; Sunday, S.; Shapira, I. Prognosis of Lymphoma in Patients Following Treatment with 6-Mercaptopurine/Azathioprine for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 1855–1858. [Google Scholar] [CrossRef]
- Raaschou, P.; Simard, J.F.; Neovius, M.; Askling, J.; Anti-Rheumatic Therapy in Sweden Study Group. Does Cancer That Occurs during or after Anti-Tumor Necrosis Factor Therapy Have a Worse Prognosis? A National Assessment of Overall and Site-Specific Cancer Survival in Rheumatoid Arthritis Patients Treated with Biologic Agents. Arthritis Rheum. 2011, 63, 1812–1822. [Google Scholar] [CrossRef] [Green Version]
- Koc, Ö.M.; van Kampen, R.J.W.; van Bodegraven, A.A. Cancer-Associated Chemotherapy Induces Less IBD Exacerbations and a Reduction of IBD Medication Afterwards. Inflamm. Bowel Dis. 2018, 24, 1606–1611. [Google Scholar] [CrossRef]
- Grimsdottir, S.; Attauabi, M.; Dahl, E.K.; Burisch, J.; Seidelin, J.B. Systematic Review with Meta-Analysis: The Impact of Cancer Treatments on the Disease Activity of Inflammatory Bowel Diseases. J. Crohn’s Colitis 2023, jjad010. [Google Scholar] [CrossRef]
- Bodofsky, S.; Freeman, R.H.; Hong, S.S.; Chundury, A.; Hathout, L.; Deek, M.P.; Jabbour, S.K. Inflammatory Bowel Disease-Associated Malignancies and Considerations for Radiation Impacting Bowel: A Scoping Review. J. Gastrointest. Oncol. 2022, 13, 2565–2582. [Google Scholar] [CrossRef]
- Feagins, L.A.; Kim, J.; Chandrakumaran, A.; Gandle, C.; Naik, K.H.; Cipher, D.J.; Hou, J.K.; Yao, M.D.; Gaidos, J.K.J. Rates of Adverse IBD-Related Outcomes for Patients with IBD and Concomitant Prostate Cancer Treated with Radiation Therapy. Inflamm. Bowel Dis. 2020, 26, 728–733. [Google Scholar] [CrossRef]
- Green, S.; Stock, R.G.; Greenstein, A.J. Rectal cancer and inflammatory bowel disease: Natural history and implications for radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Ruan, A.B.; Srivoleti, P.; Giobbie-Hurder, A.; Braschi-Amirfarzan, M.; Srivastava, A.; Buchbinder, E.I.; Ott, P.A.; Kehl, K.L.; Awad, M.M.; et al. Safety of Immune Checkpoint Inhibitors in Patients with Pre-Existing Inflammatory Bowel Disease and Microscopic Colitis. JCO Oncol. Pract. 2020, 16, e933–e942. [Google Scholar] [CrossRef]
- Marthey, L.; Mateus, C.; Mussini, C.; Nachury, M.; Nancey, S.; Grange, F.; Zallot, C.; Peyrin-Biroulet, L.; Rahier, J.F.; Bourdier de Beauregard, M.; et al. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. ECCOJC 2016, 10, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; De Felice, K.M.; Loftus, E.V.; Khanna, S. Systematic Review: Colitis Associated with Anti-CTLA-4 Therapy. Aliment. Pharmacol. Ther. 2015, 42, 406–417. [Google Scholar] [CrossRef]
- Johnston, R.L.; Lutzky, J.; Chodhry, A.; Barkin, J.S. Cytotoxic T-Lymphocyte-Associated Antigen 4 Antibody-Induced Colitis and Its Management with Infliximab. Dig. Dis. Sci. 2009, 54, 2538–2540. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, V.; Hertervig, E.; Gedeon, P.; Kopljar, M.; Griph, H.; Kinhult, S.; Carneiro, A.; Marsal, J. Vedolizumab Treatment for Immune Checkpoint Inhibitor-Induced Enterocolitis. Cancer Immunol. Immunother. 2017, 66, 581–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishu, S.; Melia, J.; Sharfman, W.; Lao, C.D.; Fecher, L.A.; Higgins, P.D.R. Efficacy and Outcome of Tofacitinib in Immune Checkpoint Inhibitor Colitis. Gastroenterology 2021, 160, 932–934.e3. [Google Scholar] [CrossRef] [PubMed]




| Inflammation-Related Cancer | IBD Therapy-Related Cancer |
|---|---|
| Colorectal cancer | Melanoma |
| Small intestinal cancer | Non-melanoma skin cancer |
| Intestinal lymphoma | Lymphoproliferative, hematological malignancy |
| Anal carcinoma | Cervical cancer |
| Cholangiocarcinoma | Urinary tract cancer |
| Low Risk (<10%) | Intermediate Risk (11–25%) | High Risk (>25%) |
|---|---|---|
| Lymphoma (HL and NHL) | Uterine body | Myeloma |
| Thyroid | Gastrointestinal cancer, colon | Skin cancer (Melanoma and NMSC) |
| Uterine and cervix | Prostate | Symptomatic renal carcinoma |
| Testicle | Breast | Bladder |
| Incidental asymptomatic renal tumor | Lung | Sarcoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wetwittayakhlang, P.; Tselekouni, P.; Al-Jabri, R.; Bessissow, T.; Lakatos, P.L. The Optimal Management of Inflammatory Bowel Disease in Patients with Cancer. J. Clin. Med. 2023, 12, 2432. https://doi.org/10.3390/jcm12062432
Wetwittayakhlang P, Tselekouni P, Al-Jabri R, Bessissow T, Lakatos PL. The Optimal Management of Inflammatory Bowel Disease in Patients with Cancer. Journal of Clinical Medicine. 2023; 12(6):2432. https://doi.org/10.3390/jcm12062432
Chicago/Turabian StyleWetwittayakhlang, Panu, Paraskevi Tselekouni, Reem Al-Jabri, Talat Bessissow, and Peter L. Lakatos. 2023. "The Optimal Management of Inflammatory Bowel Disease in Patients with Cancer" Journal of Clinical Medicine 12, no. 6: 2432. https://doi.org/10.3390/jcm12062432
APA StyleWetwittayakhlang, P., Tselekouni, P., Al-Jabri, R., Bessissow, T., & Lakatos, P. L. (2023). The Optimal Management of Inflammatory Bowel Disease in Patients with Cancer. Journal of Clinical Medicine, 12(6), 2432. https://doi.org/10.3390/jcm12062432






