The Role and Clinical Relevance of Osteopontin in Allergic Airway Diseases
Abstract
1. Introduction
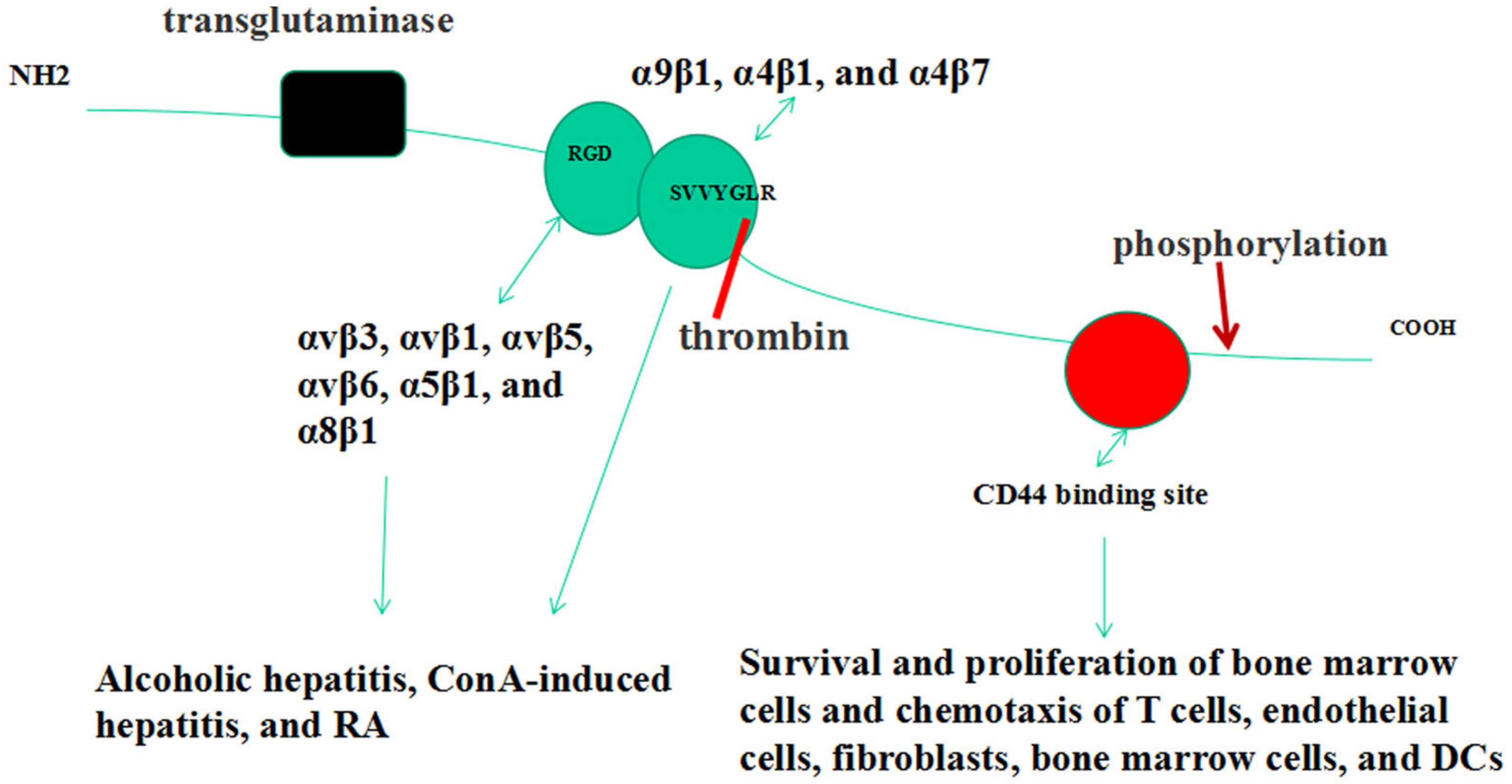
2. OPN Gene & Structure
3. Forms of OPN
4. OPN Receptors
5. OPN Post-Translational Modifications
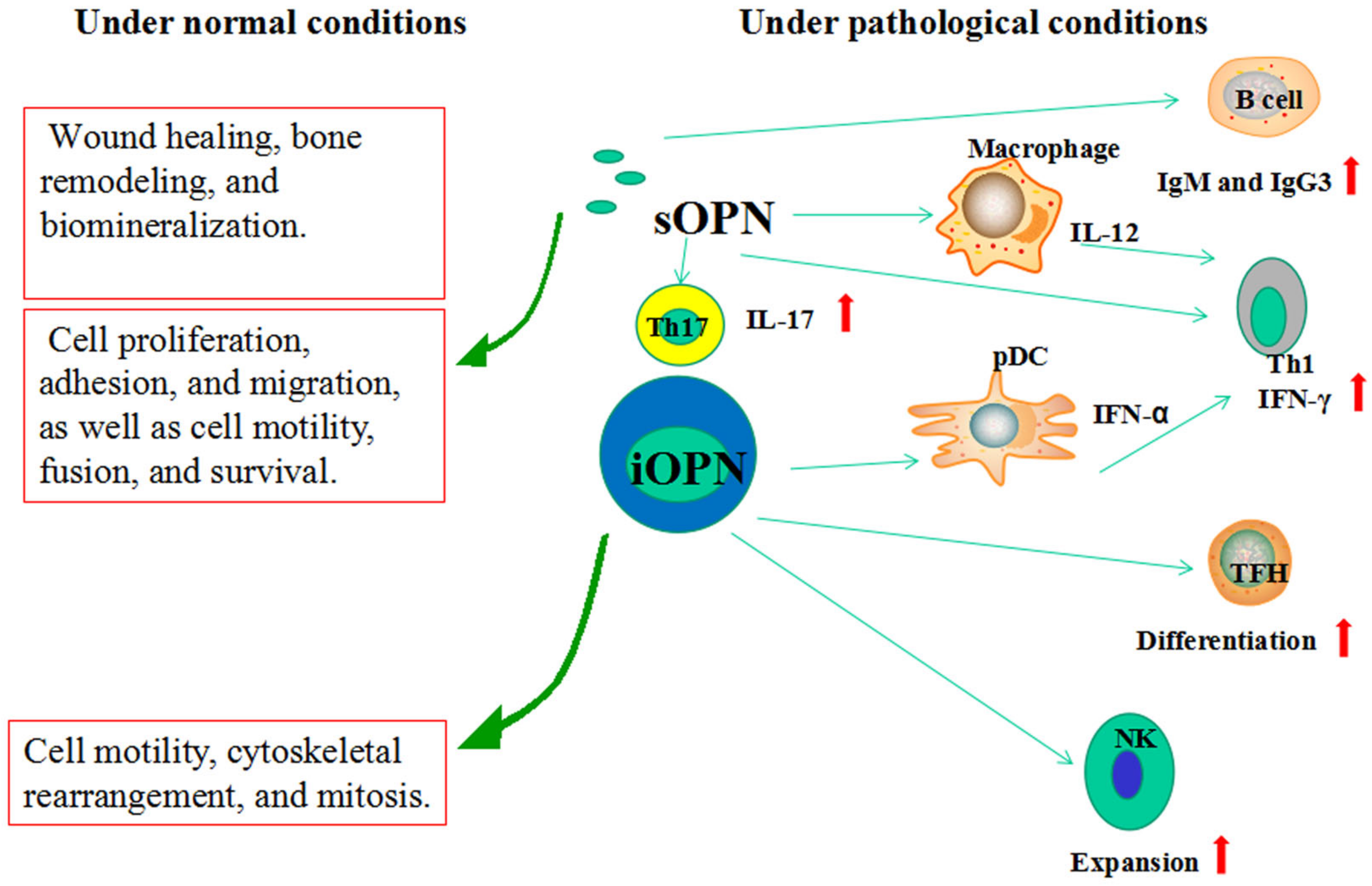
6. Functions of OPN
6.1. sOPN
6.2. iOPN
7. The Role and Regulation of OPN in CRSwNP
8. The Role and Regulation of OPN in AR
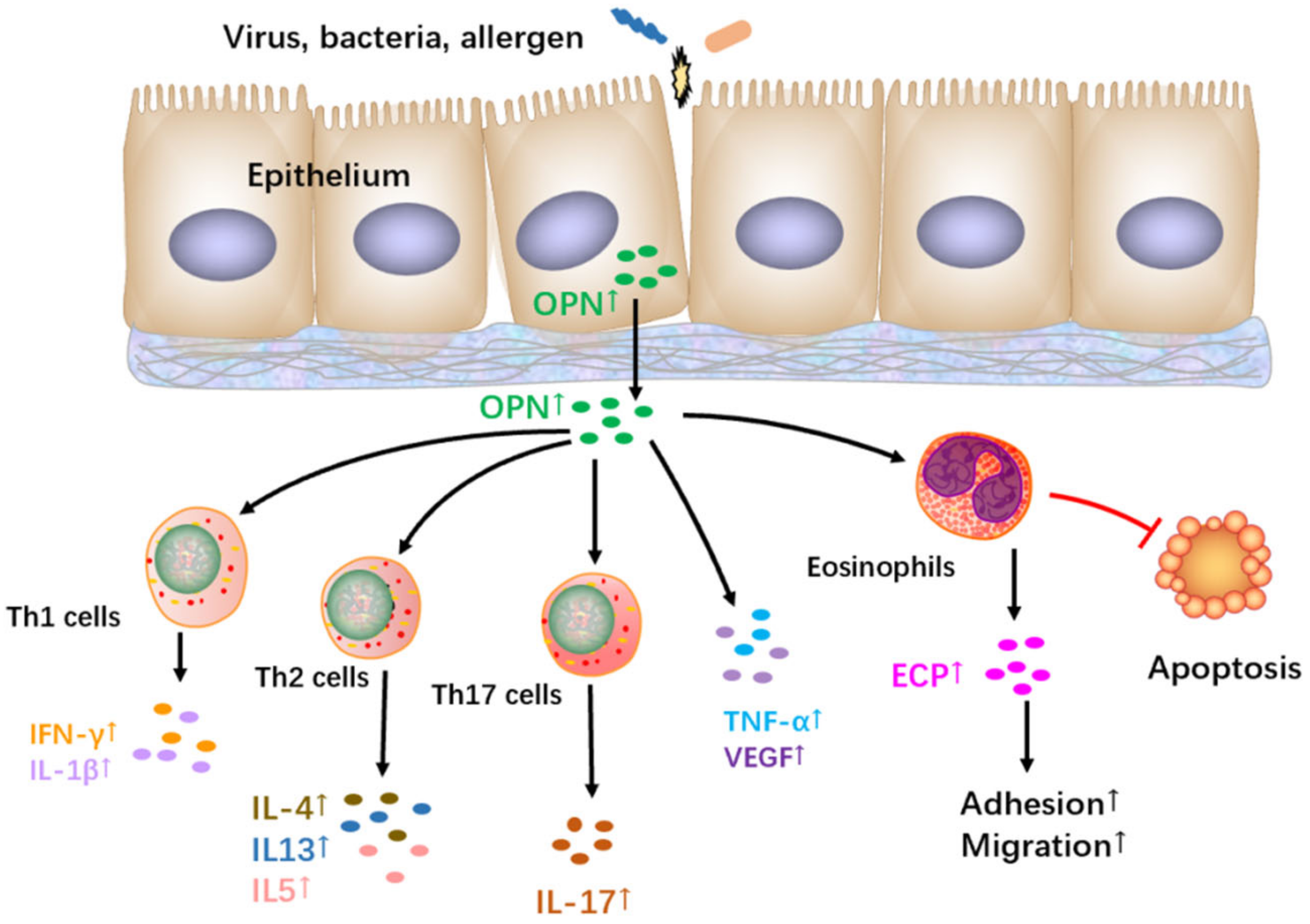
9. The Role and Regulation of OPN in Allergic Asthma
| Asthma Model | Modalities | Findings | References |
|---|---|---|---|
| An acute model | Anti-OPN antibodies at sensitization and challenge stage | Different DC subpopulation recruitment | [81] |
| An acute model | rOPN at challenge stage | Inhibited OVA-specific IgE production | [81] |
| A tolerance induction model | rOPN at sensitization stage | Induced accumulation of IFN-β-producing plasmacytoid dendritic cells and regulatory T cells in mediastinal lymph nodes | [123] |
| A chronic disease model | rOPN at challenge stage | Enhanced remodeling | [113] |
10. Clinical Relevance
11. Summary and Opinions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dykewicz, M.S.; Hamilos, D.L. Rhinitis and sinusitis. J. Allergy Clin. Immunol. 2010, 125, S103–S115. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.P. Endotyping asthma: New insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008, 372, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T.; Polosa, R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet 2006, 368, 780–793. [Google Scholar] [CrossRef]
- Hamilos, D.L. Drivers of chronic rhinosinusitis: Inflammation versus infection. J. Allergy Clin. Immunol. 2015, 136, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Nakayama, T.; Asaka, D.; Inoue, N.; Tsurumoto, T.; Takaishi, S.; Otori, N.; Kojima, H.; Matsuda, A.; Oboki, K.; et al. Distinct gene expression profiles and regulation networks of nasal polyps in eosinophilic and non-eosinophilic chronic rhinosinusitis. Int. Forum. Allergy Rhinol. 2018, 8, 592–604. [Google Scholar] [CrossRef]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef]
- Kim, D.W.; Cho, S.H. Emerging Endotypes of Chronic Rhinosinusitis and Its Application to Precision Medicine. Allergy Asthma. Immunol. Res. 2017, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kato, A. Group 2 Innate Lymphoid Cells in Airway Diseases. Chest 2019, 156, 141–149. [Google Scholar] [CrossRef]
- Pawankar, R.; Hayashi, M.; Yamanishi, S.; Igarashi, T. The paradigm of cytokine networks in allergic airway inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 41–48. [Google Scholar] [CrossRef]
- Castillo, E.F.; Zheng, H.; Yang, X.O. Orchestration of epithelial-derived cytokines and innate immune cells in allergic airway inflammation. Cytokine. Growth. Factor. Rev. 2018, 39, 19–25. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Godar, M.; Blanchetot, C.; de Haard, H.; Lambrecht, B.N.; Brusselle, G. Personalized medicine with biologics for severe type 2 asthma: Current status and future prospects. MAbs 2018, 10, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.; Casale, T.; Wenzel, S.; Bousquet, J.; Deniz, Y.; Reisner, C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J. Allergy Clin. Immunol. 2005, 115, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Diver, S.; Khalfaoui, L.; Emson, C.; Wenzel, S.E.; Menzies-Gow, A.; Wechsler, M.E.; Johnston, J.; Molfino, N.; Parnes, J.R.; Megally, A.; et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. Respir. Med. 2021, 9, 1299–1312. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet. 2012, 380, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Hellings, P.W.; Mullol, J.; Naclerio, R.M.; Chao, J.; Amin, N.; Grabher, A.; Swanson, B.N.; Hamilton, J.D.; Guillonneau, S.; et al. Dupilumab improves patient-reported outcomes in patients with chronic rhinosinusitis with nasal polyps and comorbid asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 2447–2449.e2. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; O’Byrne, P.M.; Boulet, L.P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C.; et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Ruddy, M.K.; Pavord, I.D.; Israel, E.; Rabe, K.F.; Ford, L.B.; Maspero, J.F.; Abdulai, R.M.; Hu, C.C.; Martincova, R.; et al. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 1656–1668. [Google Scholar] [CrossRef]
- Corren, J.; Garcia Gil, E.; Parnes, J.; Pham, T.-H.; Griffiths, J. Tezepelumab treatment effect on annualized rate of exacerbations by baseline biomarkers in uncontrolled severe asthma patients: Phase 2b PATHWAY study. In B15. Immunotherapy for Lung Disease; American Thoracic Society: New York, NY, USA, 2019; p. A2621. [Google Scholar]
- Wen, S.R.; Liu, G.J.; Feng, R.N.; Gong, F.C.; Zhong, H.; Duan, S.R.; Bi, S. Increased levels of IL-23 and osteopontin in serum and cerebrospinal fluid of multiple sclerosis patients. J. Neuroimmunol. 2012, 244, 94–96. [Google Scholar] [CrossRef]
- Su, C.M.; Chiang, Y.C.; Huang, C.Y.; Hsu, C.J.; Fong, Y.C.; Tang, C.H. Osteopontin Promotes Oncostatin M Production in Human Osteoblasts: Implication of Rheumatoid Arthritis Therapy. J. Immunol. 2015, 195, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Komine-Aizawa, S.; Masuda, H.; Mazaki, T.; Shiono, M.; Hayakawa, S.; Takayama, T. Plasma osteopontin predicts inflammatory bowel disease activities. Int. Surg. 2015, 100, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Comabella, M.; Pericot, I.; Goertsches, R.; Nos, C.; Castillo, M.; Blas Navarro, J.; Río, J.; Montalban, X. Plasma osteopontin levels in multiple sclerosis. J. Neuroimmunol. 2005, 158, 231–239. [Google Scholar] [CrossRef]
- Metwally, R.M.; Hasan, A.S.; R, E.G. Association of Osteopontin gene single nucleotide polymorphism with lupus nephritis. Int. J. Rheum. Dis. 2022, 25, 571–575. [Google Scholar] [CrossRef]
- Yamamoto, N.; Nakashima, T.; Torikai, M.; Naruse, T.; Morimoto, J.; Kon, S.; Sakai, F.; Uede, T. Successful treatment of collagen-induced arthritis in non-human primates by chimeric anti-osteopontin antibody. Int. Immunopharmacol. 2007, 7, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Sakai, F.; Kon, S.; Morimoto, J.; Kimura, C.; Yamazaki, H.; Okazaki, I.; Seki, N.; Fujii, T.; Uede, T. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J. Clin. Investig. 2003, 112, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Park, J.H.; Jo, M.S.; Shin, J.M.; Kim, D.W.; Park, I.H. Eosinophil-Derived Osteopontin Induces the Expression of Pro-Inflammatory Mediators and Stimulates Extracellular Matrix Production in Nasal Fibroblasts: The Role of Osteopontin in Eosinophilic Chronic Rhinosinusitis. Front. Immunol. 2022, 13, 777928. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, X.; Yu, H.J.; Hua, X.Y.; Cui, Y.H.; Huang, S.K.; Liu, Z. The expression of osteopontin and its association with Clara cell 10 kDa protein in allergic rhinitis. Clin. Exp. Allergy 2010, 40, 1632–1641. [Google Scholar] [CrossRef]
- Zeng, Q.; Xi, L.; Zeng, Y.; Liu, W.; Zhou, L. Osteopontin mediated eosinophils activation by group II innate lymphoid cells. World Allergy Organ. J. 2022, 15, 100659. [Google Scholar] [CrossRef]
- Liu, W.L.; Zhang, H.; Zheng, Y.; Wang, H.T.; Chen, F.H.; Xu, L.; Wei, Y.; Sun, Y.Q.; Shi, J.B.; Li, H.B. Expression and regulation of osteopontin in chronic rhinosinusitis with nasal polyps. Clin. Exp. Allergy 2015, 45, 414–422. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.H.; Wang, H.; Long, X.B.; You, X.J.; Gao, Q.X.; Cui, Y.H.; Liu, Z. Expression of osteopontin in chronic rhinosinusitis with and without nasal polyps. Allergy 2009, 64, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Uede, T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol. Int. 2011, 61, 265–280. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Kaleta, B. The role of osteopontin in kidney diseases. Inflamm. Res. 2019, 68, 93–102. [Google Scholar] [CrossRef]
- Gimba, E.R.; Tilli, T.M. Human osteopontin splicing isoforms: Known roles, potential clinical applications and activated signaling pathways. Cancer Lett. 2013, 331, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Lamort, A.S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef] [PubMed]
- Rittling, S.R.; Singh, R. Osteopontin in Immune-mediated Diseases. J. Dent. Res. 2015, 94, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Subraman, V.; Thiyagarajan, M.; Malathi, N.; Rajan, S.T. OPN-Revisited. J. Clin. Diagn. Res. 2015, 9, ZE10–ZE13. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, J.; Kon, S.; Matsui, Y.; Uede, T. Osteopontin; as a target molecule for the treatment of inflammatory diseases. Curr. Drug. Targets. 2010, 11, 494–505. [Google Scholar] [CrossRef]
- Inoue, M.; Shinohara, M.L. Intracellular osteopontin (iOPN) and immunity. Immunol. Res. 2011, 49, 160–172. [Google Scholar] [CrossRef]
- Castello, L.M.; Raineri, D.; Salmi, L.; Clemente, N.; Vaschetto, R.; Quaglia, M.; Garzaro, M.; Gentilli, S.; Navalesi, P.; Cantaluppi, V.; et al. Osteopontin at the Crossroads of Inflammation and Tumor Progression. Mediators. Inflamm. 2017, 2017, 4049098. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.L.; Jansson, M.; Hwang, E.S.; Werneck, M.B.; Glimcher, L.H.; Cantor, H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc. Natl. Acad. Sci. USA 2005, 102, 17101–17106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, W.; Li, Y.C.; Zhang, G.J.; Yao, K.; Hu, R.; Wu, B. The OPN gene polymorphism confers the susceptibility and response to Ara-C based chemotherapy in Chinese AML patients. Cell. Physiol. Biochem. 2015, 35, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; He, C.; Jing, W.; Zhou, X.; Chen, R.; Cao, L.; Zhu, M.; Jia, R.; Wang, H.; Guo, Y.; et al. Intracellular Osteopontin inhibits toll-like receptor signaling and impedes liver carcinogenesis. Cancer Res. 2015, 75, 86–97. [Google Scholar] [CrossRef]
- Yokosaki, Y.; Matsuura, N.; Sasaki, T.; Murakami, I.; Schneider, H.; Higashiyama, S.; Saitoh, Y.; Yamakido, M.; Taooka, Y.; Sheppard, D. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J. Biol. Chem. 1999, 274, 36328–36334. [Google Scholar] [CrossRef]
- Bayless, K.J.; Davis, G.E. Identification of dual alpha 4beta1 integrin binding sites within a 38 amino acid domain in the N-terminal thrombin fragment of human osteopontin. J. Biol. Chem. 2001, 276, 13483–13489. [Google Scholar] [CrossRef]
- Christensen, B.; Schack, L.; Kläning, E.; Sørensen, E.S. Osteopontin is cleaved at multiple sites close to its integrin-binding motifs in milk and is a novel substrate for plasmin and cathepsin D. J. Biol. Chem. 2010, 285, 7929–7937. [Google Scholar] [CrossRef]
- Diao, H.; Kon, S.; Iwabuchi, K.; Kimura, C.; Morimoto, J.; Ito, D.; Segawa, T.; Maeda, M.; Hamuro, J.; Nakayama, T.; et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity 2004, 21, 539–550. [Google Scholar] [CrossRef]
- Marastoni, D.; Magliozzi, R.; Bolzan, A.; Pisani, A.I.; Rossi, S.; Crescenzo, F.; Montemezzi, S.; Pizzini, F.B.; Calabrese, M. CSF Levels of CXCL12 and Osteopontin as Early Markers of Primary Progressive Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1083. [Google Scholar] [CrossRef]
- Chabas, D.; Baranzini, S.E.; Mitchell, D.; Bernard, C.C.; Rittling, S.R.; Denhardt, D.T.; Sobel, R.A.; Lock, C.; Karpuj, M.; Pedotti, R.; et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 2001, 294, 1731–1735. [Google Scholar] [CrossRef]
- Murugaiyan, G.; Mittal, A.; Weiner, H.L. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J. Immunol. 2008, 181, 7480–7488. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, A.; Scutera, S.; Sozzani, S.; Musso, T. Role of osteopontin in dendritic cell shaping of immune responses. Cytokine. Growth. Factor. Rev. 2019, 50, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Huang, C.J.; Chao, J.R.; Chen, S.T.; Lee, S.F.; Yen, J.J.; Yang-Yen, H.F. Coupling of osteopontin and its cell surface receptor CD44 to the cell survival response elicited by interleukin-3 or granulocyte-macrophage colony-stimulating factor. Mol. Cell. Biol. 2000, 20, 2734–2742. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Ledbetter, S.R.; Claffey, K.P.; Papadopoulos-Sergiou, A.; Peruzzi, C.A.; Detmar, M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am. J. Pathol. 1996, 149, 293–305. [Google Scholar] [PubMed]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Zohar, R.; Suzuki, N.; Suzuki, K.; Arora, P.; Glogauer, M.; McCulloch, C.A.; Sodek, J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J. Cell. Physiol. 2000, 184, 118–130. [Google Scholar] [CrossRef]
- Spinelli, F.R.; Garufi, C.; Truglia, S.; Pacucci, V.A.; Morello, F.; Miranda, F.; Perricone, C.; Ceccarelli, F.; Valesini, G.; Conti, F. The role of osteopontin as a candidate biomarker of renal involvement in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2019, 37, 899–905. [Google Scholar]
- Boskey, A.L.; Christensen, B.; Taleb, H.; Sørensen, E.S. Post-translational modification of osteopontin: Effects on in vitro hydroxyapatite formation and growth. Biochem. Biophys. Res. Commun. 2012, 419, 333–338. [Google Scholar] [CrossRef]
- Christensen, B.; Nielsen, M.S.; Haselmann, K.F.; Petersen, T.E.; Sørensen, E.S. Post-translationally modified residues of native human osteopontin are located in clusters: Identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. Biochem. J. 2005, 390, 285–292. [Google Scholar] [CrossRef]
- Khan, S.A.; Cook, A.C.; Kappil, M.; Günthert, U.; Chambers, A.F.; Tuck, A.B.; Denhardt, D.T. Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: Novel post-transcriptional, post-translational regulation. Clin. Exp. Metastasis 2005, 22, 663–673. [Google Scholar] [CrossRef]
- Sørensen, E.S.; Højrup, P.; Petersen, T.E. Posttranslational modifications of bovine osteopontin: Identification of twenty-eight phosphorylation and three O-glycosylation sites. Protein. Sci. 1995, 4, 2040–2049. [Google Scholar] [CrossRef]
- Kaartinen, M.T.; Pirhonen, A.; Linnala-Kankkunen, A.; Mäenpää, P.H. Cross-linking of osteopontin by tissue transglutaminase increases its collagen binding properties. J. Biol. Chem. 1999, 274, 1729–1735. [Google Scholar] [CrossRef]
- Beninati, S.; Senger, D.R.; Cordella-Miele, E.; Mukherjee, A.B.; Chackalaparampil, I.; Shanmugam, V.; Singh, K.; Mukherjee, B.B. Osteopontin: Its transglutaminase-catalyzed posttranslational modifications and cross-linking to fibronectin. J. Biochem. 1994, 115, 675–682. [Google Scholar] [CrossRef]
- Clemente, N.; Raineri, D.; Cappellano, G.; Boggio, E.; Favero, F.; Soluri, M.F.; Dianzani, C.; Comi, C.; Dianzani, U.; Chiocchetti, A. Osteopontin Bridging Innate and Adaptive Immunity in Autoimmune Diseases. J. Immunol. Res 2016, 2016, 7675437. [Google Scholar] [CrossRef] [PubMed]
- Gerlic, M.; Croker, B.A. Myelopoiesis embraces its inner weakness. Nat. Immunol. 2017, 18, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Caputo, S.; Bellone, M. Osteopontin and the immune system: Another brick in the wall. Cell. Mol. Immunol. 2018, 15, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Nishimichi, N.; Hayashita-Kinoh, H.; Chen, C.; Matsuda, H.; Sheppard, D.; Yokosaki, Y. Osteopontin undergoes polymerization in vivo and gains chemotactic activity for neutrophils mediated by integrin alpha9beta1. J. Biol. Chem. 2011, 286, 11170–11178. [Google Scholar] [CrossRef]
- Kanayama, M.; Xu, S.; Danzaki, K.; Gibson, J.R.; Inoue, M.; Gregory, S.G.; Shinohara, M.L. Skewing of the population balance of lymphoid and myeloid cells by secreted and intracellular osteopontin. Nat. Immunol. 2017, 18, 973–984. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Buttari, F.; Gilio, L.; Iezzi, E.; Galifi, G.; Carbone, F.; Micillo, T.; Dolcetti, E.; Azzolini, F.; Bruno, A.; et al. Osteopontin Is Associated with Multiple Sclerosis Relapses. Biomedicines 2023, 11, 178. [Google Scholar] [CrossRef]
- Wong, C.K.; Lit, L.C.; Tam, L.S.; Li, E.K.; Lam, C.W. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology 2005, 44, 602–606. [Google Scholar] [CrossRef]
- Kivisäkk, P.; Healy, B.C.; Francois, K.; Gandhi, R.; Gholipour, T.; Egorova, S.; Sevdalinova, V.; Quintana, F.; Chitnis, T.; Weiner, H.L.; et al. Evaluation of circulating osteopontin levels in an unselected cohort of patients with multiple sclerosis: Relevance for biomarker development. Mult. Scler. 2014, 20, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wu, Y.; Liu, N. Osteopontin in autoimmune disorders: Current knowledge and future perspective. Inflammopharmacology 2022, 30, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Luo, W.; Li, Y.; Gao, S.; Lei, G. Role of osteopontin in rheumatoid arthritis. Rheumatol. Int. 2015, 35, 589–595. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Lu, L.; Bu, J.; Werneck, M.B.; Kobayashi, K.S.; Glimcher, L.H.; Cantor, H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat. Immunol. 2006, 7, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.L.; Kim, J.H.; Garcia, V.A.; Cantor, H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: Role of intracellular osteopontin. Immunity 2008, 29, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, J.; Katagiri, Y.; Tada, N.; Murakami, M.; Ikeda, T.; Sato, M.; Hirokawa, K.; Okada, S.; Hatano, M.; Tokuhisa, T.; et al. Introduction of an osteopontin gene confers the increase in B1 cell population and the production of anti-DNA autoantibodies. Lab. Investig. 1998, 78, 1523–1533. [Google Scholar]
- Chehimi, J.; Elder, M.; Greene, J.; Noroski, L.; Stiehm, E.R.; Winkelstein, J.A.; Sullivan, K.E. Cytokine and chemokine dysregulation in hyper-IgE syndrome. Clin. Immunol. 2001, 100, 49–56. [Google Scholar] [CrossRef]
- Seier, A.M.; Renkl, A.C.; Schulz, G.; Uebele, T.; Sindrilaru, A.; Iben, S.; Liaw, L.; Kon, S.; Uede, T.; Weiss, J.M. Antigen-specific induction of osteopontin contributes to the chronification of allergic contact dermatitis. Am. J. Pathol. 2010, 176, 246–258. [Google Scholar] [CrossRef]
- Weiss, J.M.; Renkl, A.C.; Maier, C.S.; Kimmig, M.; Liaw, L.; Ahrens, T.; Kon, S.; Maeda, M.; Hotta, H.; Uede, T.; et al. Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J. Exp. Med. 2001, 194, 1219–1229. [Google Scholar] [CrossRef]
- Xanthou, G.; Alissafi, T.; Semitekolou, M.; Simoes, D.C.; Economidou, E.; Gaga, M.; Lambrecht, B.N.; Lloyd, C.M.; Panoutsakopoulou, V. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat. Med. 2007, 13, 570–578. [Google Scholar] [CrossRef]
- Frenzel, D.F.; Weiss, J.M. Osteopontin and allergic disease: Pathophysiology and implications for diagnostics and therapy. Expert. Rev. Clin. Immunol. 2011, 7, 93–109. [Google Scholar] [CrossRef]
- Liu, W.; Xia, W.; Fan, Y.; Wang, H.; Zuo, K.; Lai, Y.; Li, H.; Liu, Z.; Shi, J.; Xu, G. Elevated serum osteopontin level is associated with blood eosinophilia and asthma comorbidity in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2012, 130, 1416–1418.e1416. [Google Scholar] [CrossRef] [PubMed]
- Konno, S.; Kurokawa, M.; Uede, T.; Nishimura, M.; Huang, S.K. Role of osteopontin, a multifunctional protein, in allergy and asthma. Clin. Exp. Allergy 2011, 41, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Mao, H.; Ouyang, H.; Xin, Y. Osteopontin induced vascular endothelial growth factor production in dispersed nasal polyp cells through the phosphatidylinositol 3-kinase-protein kinase B and the extracellular signal-regulated kinase 1/2 pathways. Am. J. Rhinol. Allergy 2017, 31, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zeng, Q.; Chen, Y.; Luo, R.Z. Role of Leptin/Osteopontin Axis in the Function of Eosinophils in Allergic Rhinitis with Obesity. Mediators. Inflamm. 2018, 2018, 9138904. [Google Scholar] [CrossRef]
- Zeng, Q.; Luo, X.; Han, M.; Liu, W.; Li, H. Leptin/Osteopontin Axis Regulated Type 2T Helper Cell Response in Allergic Rhinitis with Obesity. EBioMedicine 2018, 32, 43–49. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, Q.; Zhou, L.; Li, Y.; Chen, Y.; Luo, R. Leptin/osteopontin axis contributes to enhanced T helper 17 type responses in allergic rhinitis. Pediatr. Allergy Immunol. 2018, 29, 622–629. [Google Scholar] [CrossRef]
- O’Neil, S.E.; Malmhäll, C.; Samitas, K.; Pullerits, T.; Bossios, A.; Lötvall, J. Quantitative expression of osteopontin in nasal mucosa of patients with allergic rhinitis: Effects of pollen exposure and nasal glucocorticoid treatment. Allergy Asthma. Clin. Immunol. 2010, 6, 28. [Google Scholar] [CrossRef]
- Wang, C.; Wang, K.; Liu, S.; Qin, X.; Chen, K.; Zhang, T. Decreased level of osteopontin in children with allergic rhinitis during sublingual immunotherapy. Int. J. Pediatr. Otorhinolaryngol. 2016, 81, 15–20. [Google Scholar] [CrossRef]
- Konno, S.; Golden, D.B.; Schroeder, J.; Hamilton, R.G.; Lichtenstein, L.M.; Huang, S.K. Increased expression of osteopontin is associated with long-term bee venom immunotherapy. J. Allergy Clin. Immunol. 2005, 115, 1063–1067. [Google Scholar] [CrossRef]
- Chaker, A.M.; Shamji, M.H.; Dumitru, F.A.; Calderon, M.A.; Scadding, G.W.; Makatsori, M.; Jones, I.; He, Q.A.; Subramanian, K.K.; Arm, J.P.; et al. Short-term subcutaneous grass pollen immunotherapy under the umbrella of anti-IL-4: A randomized controlled trial. J. Allergy Clin. Immunol. 2016, 137, 452–461.e9. [Google Scholar] [CrossRef] [PubMed]
- Navas, A.; Ruiz-Leon, B.; Serrano, P.; Martí, M.; Espinazo, M.L.; Blanco, N.; Molina, J.; Alonso, C.; Jurado, A.; Moreno-Aguilar, C. Natural and Induced Tolerance to Hymenoptera Venom: A Single Mechanism? Toxins 2022, 14, 426. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, H.; Wang, Z.; Liu, Y. MicroRNA let-7a up-regulates OPN expression in a mouse model of allergic rhinitis. J. Laryngol. Otol. 2017, 131, 955–960. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Busse, W.W. Severe asthma: Lessons from the Severe Asthma Research Program. J. Allergy Clin. Immunol. 2007, 119, 14–21; quiz 22–13. [Google Scholar] [CrossRef]
- Forton, A.C.; Petri, M.A.; Goldman, D.; Sullivan, K.E. An osteopontin (SPP1) polymorphism is associated with systemic lupus erythematosus. Hum. Mutat. 2002, 19, 459. [Google Scholar] [CrossRef]
- Niino, M.; Kikuchi, S.; Fukazawa, T.; Yabe, I.; Tashiro, K. Genetic polymorphisms of osteopontin in association with multiple sclerosis in Japanese patients. J. Neuroimmunol. 2003, 136, 125–129. [Google Scholar] [CrossRef]
- Mochida, S.; Hashimoto, M.; Matsui, A.; Naito, M.; Inao, M.; Nagoshi, S.; Nagano, M.; Egashira, T.; Mishiro, S.; Fujiwara, K. Genetic polymorphims in promoter region of osteopontin gene may be a marker reflecting hepatitis activity in chronic hepatitis C patients. Biochem. Biophys. Res. Commun. 2004, 313, 1079–1085. [Google Scholar] [CrossRef]
- Tanino, Y.; Hizawa, N.; Konno, S.; Fukui, Y.; Takahashi, D.; Maeda, Y.; Huang, S.K.; Nishimura, M. Sequence variants of the secreted phosphoprotein 1 gene are associated with total serum immunoglobulin E levels in a Japanese population. Clin. Exp. Allergy 2006, 36, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Samitas, K.; Zervas, E.; Vittorakis, S.; Semitekolou, M.; Alissafi, T.; Bossios, A.; Gogos, H.; Economidou, E.; Lötvall, J.; Xanthou, G.; et al. Osteopontin expression and relation to disease severity in human asthma. Eur. Respir. J. 2011, 37, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Samitas, K.; Zervas, E.; Xanthou, G.; Panoutsakopoulou, V.; Gaga, M. Osteopontin is increased in the bronchoalveolar lavage fluid and bronchial tissue of smoking asthmatics. Cytokine 2013, 61, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lou, W.; Fu, F. Association between osteopontin expression and asthma: A meta-analysis. J. Int. Med. Res. 2019, 47, 3513–3521. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Yang, L.; Zhao, F.Q.; Shi, S.M.; Tan, P. Osteopontin levels are elevated in patients with asthma. J. Int. Med. Res. 2011, 39, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Akelma, A.Z.; Cizmeci, M.N.; Kanburoglu, M.K.; Bozkaya, D.; Catal, F.; Mete, E.; Kutukoglu, I.; Namuslu, M. Elevated level of serum osteopontin in school-age children with asthma. Allergol. Immunopathol. 2014, 42, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Hirahara, K.; Kiuchi, M.; Wada, T.; Ichikawa, T.; Kanno, T.; Okano, M.; Kokubo, K.; Onodera, A.; Sakurai, D.; et al. Amphiregulin-Producing Pathogenic Memory T Helper 2 Cells Instruct Eosinophils to Secrete Osteopontin and Facilitate Airway Fibrosis. Immunity 2018, 49, 134–150.e6. [Google Scholar] [CrossRef] [PubMed]
- Delimpoura, V.; Bakakos, P.; Tseliou, E.; Bessa, V.; Hillas, G.; Simoes, D.C.; Papiris, S.; Loukides, S. Increased levels of osteopontin in sputum supernatant in severe refractory asthma. Thorax 2010, 65, 782–786. [Google Scholar] [CrossRef]
- Hillas, G.; Loukides, S.; Kostikas, K.; Simoes, D.; Petta, V.; Konstantellou, E.; Emmanouil, P.; Papiris, S.; Koulouris, N.; Bakakos, P. Increased levels of osteopontin in sputum supernatant of smoking asthmatics. Cytokine 2013, 61, 251–255. [Google Scholar] [CrossRef]
- Vignola, A.M.; Kips, J.; Bousquet, J. Tissue remodeling as a feature of persistent asthma. J. Allergy Clin. Immunol. 2000, 105, 1041–1053. [Google Scholar] [CrossRef]
- Davies, D.E.; Wicks, J.; Powell, R.M.; Puddicombe, S.M.; Holgate, S.T. Airway remodeling in asthma: New insights. J. Allergy Clin. Immunol. 2003, 111, 215–225; quiz 226. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Ito, I.; Niimi, A.; Izuhara, K.; Ohta, S.; Ono, J.; Iwata, T.; Matsumoto, H.; Mishima, M. Osteopontin and periostin are associated with a 20-year decline of pulmonary function in patients with asthma. Am. J. Respir. Crit. Care. Med. 2014, 190, 472–474. [Google Scholar] [CrossRef]
- Kohan, M.; Bader, R.; Puxeddu, I.; Levi-Schaffer, F.; Breuer, R.; Berkman, N. Enhanced osteopontin expression in a murine model of allergen-induced airway remodelling. Clin. Exp. Allergy 2007, 37, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Simoes, D.C.; Xanthou, G.; Petrochilou, K.; Panoutsakopoulou, V.; Roussos, C.; Gratziou, C. Osteopontin deficiency protects against airway remodeling and hyperresponsiveness in chronic asthma. Am. J. Respir. Crit. Care. Med. 2009, 179, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Vetrone, S.A.; Montecino-Rodriguez, E.; Kudryashova, E.; Kramerova, I.; Hoffman, E.P.; Liu, S.D.; Miceli, M.C.; Spencer, M.J. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J. Clin. Investig. 2009, 119, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Kohan, M.; Breuer, R.; Berkman, N. Osteopontin induces airway remodeling and lung fibroblast activation in a murine model of asthma. Am. J. Respir. Cell. Mol. Biol. 2009, 41, 290–296. [Google Scholar] [CrossRef]
- Jeffery, P.K. Remodeling in asthma and chronic obstructive lung disease. Am. J. Respir. Crit. Care. Med. 2001, 164, S28–S38. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, H.H.; Robinson, D.S. The role of eosinophils in airway tissue remodelling in asthma. Curr. Opin. Immunol. 2007, 19, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Puxeddu, I.; Berkman, N.; Ribatti, D.; Bader, R.; Haitchi, H.M.; Davies, D.E.; Howarth, P.H.; Levi-Schaffer, F. Osteopontin is expressed and functional in human eosinophils. Allergy 2010, 65, 168–174. [Google Scholar] [CrossRef]
- Takahashi, A.; Kurokawa, M.; Konno, S.; Ito, K.; Kon, S.; Ashino, S.; Nishimura, T.; Uede, T.; Hizawa, N.; Huang, S.K.; et al. Osteopontin is involved in migration of eosinophils in asthma. Clin. Exp. Allergy 2009, 39, 1152–1159. [Google Scholar] [CrossRef]
- Nagasaka, A.; Matsue, H.; Matsushima, H.; Aoki, R.; Nakamura, Y.; Kambe, N.; Kon, S.; Uede, T.; Shimada, S. Osteopontin is produced by mast cells and affects IgE-mediated degranulation and migration of mast cells. Eur. J. Immunol. 2008, 38, 489–499. [Google Scholar] [CrossRef]
- Bulfone-Paus, S.; Paus, R. Osteopontin as a new player in mast cell biology. Eur. J. Immunol. 2008, 38, 338–341. [Google Scholar] [CrossRef]
- Jia, Q.; Huang, Z.; Wang, G.; Sun, X.; Wu, Y.; Yang, B.; Yang, T.; Liu, J.; Li, P.; Li, J. Osteopontin: An important protein in the formation of kidney stones. Front. Pharmacol. 2022, 13, 1036423. [Google Scholar] [CrossRef]
- De Heer, H.J.; Hammad, H.; Soullié, T.; Hijdra, D.; Vos, N.; Willart, M.A.; Hoogsteden, H.C.; Lambrecht, B.N. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 2004, 200, 89–98. [Google Scholar] [CrossRef]
- Alissafi, T.; Kourepini, E.; Simoes, D.C.M.; Paschalidis, N.; Aggelakopoulou, M.; Sparwasser, T.; Boon, L.; Hammad, H.; Lambrecht, B.N.; Panoutsakopoulou, V. Osteopontin Promotes Protective Antigenic Tolerance against Experimental Allergic Airway Disease. J. Immunol. 2018, 200, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Konno, S.; Matsukura, S.; Kawaguchi, M.; Ieki, K.; Suzuki, S.; Odaka, M.; Watanabe, S.; Homma, T.; Sato, M.; et al. Effects of corticosteroids on osteopontin expression in a murine model of allergic asthma. Int. Arch. Allergy Immunol. 2009, 149 (Suppl. S1), 7–13. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.; Howard-Thompson, A.; George, C.; Hoover, R.M.; Self, T.H. Smoking and asthma. J. Am. Board. Fam. Med. 2011, 24, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lanckacker, E.; Dentener, M.; Bracke, K.; Provoost, S.; De Grove, K.; Brusselle, G.; Wouters, E.; Maes, T.; Joos, G. Aggravation of Allergic Airway Inflammation by Cigarette Smoke in Mice Is CD44-Dependent. PLoS ONE 2016, 11, e0151113. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.K.T.; Nguyen, T.V.T.; Kim, S.H.; Cao, T.B.T.; Luu, Q.Q.; Kim, S.H.; Park, H.S. Osteopontin contributes to late-onset asthma phenotypes in adult asthma patients. Exp. Mol. Med. 2020, 52, 253–265. [Google Scholar] [CrossRef] [PubMed]
| Regulatory Factors | Effect on OPN |
|---|---|
| IL-1β, TNF-α, IFN-γ, IL-6, IL-17A, IL-13, TGF-β | Upregulation |
| Leptin | |
| MicroRNA let-7a | |
| Smoking | |
| Aging | |
| Viral infection | |
| IL-4 | Downregulation |
| Clara cell 10-kDa protein | |
| Corticosteroids |
| OPN Manipulation | Function | Application |
|---|---|---|
| Local or systemic OPN | A diagnostic and prognostic biomarker | Asthma [103], cancers [37], hepatitis [37] |
| Anti-OPN antibodies | Promoting or inhibiting inflammation in different setting of diseases | Asthma [37], cancers [37], hepatitis [37], collagen-induced arthritis [26] |
| OPN siRNA | Inhibiting Th1-related inflammation | Cancers [37], hepatitis [37] |
| Recombinant OPN | Enhancing remodeling in airways and inhibiting Th1/Th17-related inflammation | Asthma [113], collagn-induced arthritis [22] |
| ASK8007 | Blocking the function of OPN | Rheumatoid arthritis [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Fu, L.; Liu, Z. The Role and Clinical Relevance of Osteopontin in Allergic Airway Diseases. J. Clin. Med. 2023, 12, 2433. https://doi.org/10.3390/jcm12062433
Liu Y, Fu L, Liu Z. The Role and Clinical Relevance of Osteopontin in Allergic Airway Diseases. Journal of Clinical Medicine. 2023; 12(6):2433. https://doi.org/10.3390/jcm12062433
Chicago/Turabian StyleLiu, Yang, Li Fu, and Zheng Liu. 2023. "The Role and Clinical Relevance of Osteopontin in Allergic Airway Diseases" Journal of Clinical Medicine 12, no. 6: 2433. https://doi.org/10.3390/jcm12062433
APA StyleLiu, Y., Fu, L., & Liu, Z. (2023). The Role and Clinical Relevance of Osteopontin in Allergic Airway Diseases. Journal of Clinical Medicine, 12(6), 2433. https://doi.org/10.3390/jcm12062433







