Why Does Obesity as an Inflammatory Condition Predispose to Colorectal Cancer?
Abstract
1. Introduction
2. Colorectal Cancer
2.1. Epidemiology of Colorectal Cancer
2.2. Risk Factors of Colorectal Cancer
3. Obesity and Colorectal Cancer
3.1. Epidemiology of Obesity as a Risk Factor for Colorectal Cancer
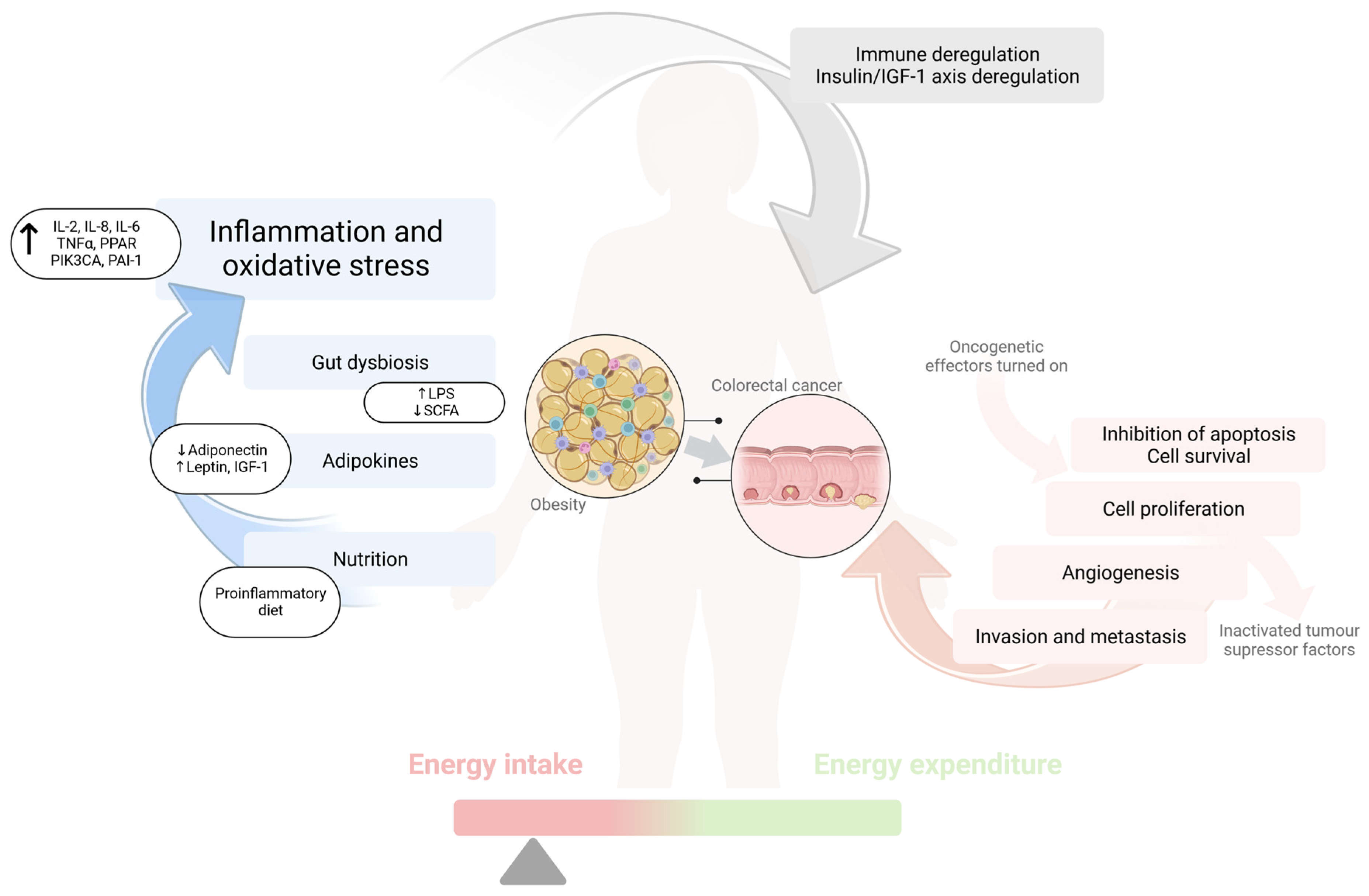
3.2. Pathogenesis of Colorectal Cancer in Obesity
4. Gut Microbiota and Colorectal Cancer
5. Diet and Colorectal Cancer
5.1. Diet Predisposing to Obesity, Inflammation, and Colorectal Cancer
5.2. Diet Protecting against the Development of Obesity, Inflammation, and Colorectal Cancer
6. Sedentary Lifestyle
7. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer |
| WHO | World Health Organization |
| IARC | International Agency for Research on Cancer |
| IBD | inflammatory bowel disease |
| IGF-1 | insulin-like growth factor (IGF)-1 |
| IGF-1R | IGF-1 receptor |
| IL | interleukin |
| TNF | tumor necrosis factor |
| WHR | waist-to-hip ratio |
| WC | waist circumference |
| HR | hazard ratio |
| RR | relative risk |
| LPS | lipopolysaccharide |
| BCAA | branched-chain amino acid |
| SCFAs | short-chain fatty acids |
| TLR-4 | toll-like receptor 4 |
References
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 12 January 2020).
- Giovannucci, E. Modifiable Risk Factors for Colon Cancer. Gastroenterol. Clin. N. Am. 2002, 31, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Stryjkowska-Góra, A.; Rudzki, S. Title: Risk Factors for the Diagnosis of Colorectal Cancer. Cancer Control. 2022, 29, 10732748211056692. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a Key Event in Cancer Development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Izano, M.; Wei, E.K.; Tai, C.; Swede, H.; Gregorich, S.; Harris, T.B.; Klepin, H.; Satterfield, S.; Murphy, R.; Newman, A.B.; et al. Chronic Inflammation and Risk of Colorectal and Other Obesity-Related Cancers: The Health, Aging and Body Composition Study. Int. J. Cancer 2016, 138, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Nimptsch, K.; Pischon, T. Obesity and Colorectal Cancer. Front. Biosci. (Elite Ed.) 2013, 5, 61–77. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Yio, X. Inflammation and Cancer IV. Colorectal Cancer in Inflammatory Bowel Disease: The Role of Inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef]
- Bou Malhab, L.J.; Abdel-Rahman, W.M. Obesity and Inflammation: Colorectal Cancer Engines. Curr. Mol. Pharmacol. 2022, 15, 620–646. [Google Scholar] [CrossRef]
- Vazzana, N.; Riondino, S.; Toto, V.; Guadagni, F.; Roselli, M.; Davi, G.; Ferroni, P. Obesity-Driven Inflammation and Colorectal Cancer. Curr. Med. Chem. 2012, 19, 5837–5853. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.-Y.; Osterreicher, C.H.; Hung, K.E.; et al. Adenoma-Linked Barrier Defects and Microbial Products Drive IL-23/IL-17-Mediated Tumour Growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef]
- Obesity-Linked Gut Microbiome Dysbiosis Associated with Derangements in Gut Permeability and Intestinal Cellular Homeostasis Independent of Diet—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30250849/ (accessed on 27 December 2022).
- GBD 2019 Diseases and Injuries Collaborators Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef] [PubMed]
- Cancer Statistics, 2021—Siegel—2021—CA: A Cancer Journal for Clinicians—Wiley Online Library. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21654 (accessed on 27 December 2022).
- Bailey, C.E.; Hu, C.-Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing Disparities in the Age-Related Incidences of Colon and Rectal Cancers in the United States, 1975-2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhao, Z.; Deng, Y.; Zheng, Z.; Huang, Y.; Huang, S.; Chi, P. The Global, Regional, and National Early-Onset Colorectal Cancer Burden and Trends from 1990 to 2019: Results from the Global Burden of Disease Study 2019. BMC Public Health 2022, 22, 1896. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global Patterns and Trends in Colorectal Cancer Incidence in Young Adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.-G.; Marsoni, S.; Bardelli, A.; Siena, S. Early-Onset Colorectal Cancer in Young Individuals. Mol. Oncol. 2019, 13, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, Y.; Wang, F.; Zhang, P.; Shi, C.; Zou, Y.; Qin, H. Obesity and Risk of Colorectal Cancer: A Systematic Review of Prospective Studies. PLoS ONE 2013, 8, e53916. [Google Scholar] [CrossRef]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and Colorectal Cancer. Gut 2013, 62, 933–947. [Google Scholar] [CrossRef]
- Soltani, G.; Poursheikhani, A.; Yassi, M.; Hayatbakhsh, A.; Kerachian, M.; Kerachian, M.A. Obesity, Diabetes and the Risk of Colorectal Adenoma and Cancer. BMC Endocr. Disord. 2019, 19, 113. [Google Scholar] [CrossRef]
- Harber, I.; Zeidan, D.; Aslam, M.N. Colorectal Cancer Screening: Impact of COVID-19 Pandemic and Possible Consequences. Life 2021, 11, 1297. [Google Scholar] [CrossRef]
- Abu-Freha, N.; Hizkiya, R.; Abu-Abed, M.; Michael, T.; Jacob, B.M.; Rouvinov, K.; Schwartz, D.; Reshef, A.; Netz, U.; Pinsk, I.; et al. The Impact of the COVID-19 Pandemic on Colorectal and Gastric Cancer Diagnosis, Disease Stage and Mortality. Front. Med. 2022, 9, 954878. [Google Scholar] [CrossRef]
- Grady, W.M. Genetic Testing for High-Risk Colon Cancer Patients. Gastroenterology 2003, 124, 1574–1594. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, A.S.; Higgins, J.P.T.; Pharoah, P. Relative and Absolute Risk of Colorectal Cancer for Individuals with a Family History: A Meta-Analysis. Eur. J. Cancer 2006, 42, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.E.; Sutherland, R.L.; Town, S.; Chow, K.; Fan, J.; Forbes, N.; Heitman, S.J.; Hilsden, R.J.; Brenner, D.R. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1229–1240.e5. [Google Scholar] [CrossRef] [PubMed]
- Kampman, E. A First-Degree Relative with Colorectal Cancer: What Are We Missing? Cancer Epidemiol. Biomark. Prevent. 2007, 16, 1–3. [Google Scholar] [CrossRef]
- Hampel, H.; Frankel, W.L.; Martin, E.; Arnold, M.; Khanduja, K.; Kuebler, P.; Clendenning, M.; Sotamaa, K.; Prior, T.; Westman, J.A.; et al. Feasibility of Screening for Lynch Syndrome Among Patients With Colorectal Cancer. J. Clin. Oncol. 2008, 26, 5783–5788. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Roy, H.K.; Lynch, H.T. Lynch Syndrome in the 21st Century: Clinical Perspectives. QJM 2016, 109, 151–158. [Google Scholar] [CrossRef]
- Vasen, H.F.A.; Tomlinson, I.; Castells, A. Clinical Management of Hereditary Colorectal Cancer Syndromes. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 88–97. [Google Scholar] [CrossRef]
- Leong, R.W.L.; Koo, J.H. Colorectal Cancer in Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2009, 24, 503–505. [Google Scholar] [CrossRef]
- Castaño-Milla, C.; Chaparro, M.; Gisbert, J.P. Systematic Review with Meta-Analysis: The Declining Risk of Colorectal Cancer in Ulcerative Colitis. Aliment Pharmacol. Ther. 2014, 39, 645–659. [Google Scholar] [CrossRef]
- Platz, E.A.; Willett, W.C.; Colditz, G.A.; Rimm, E.B.; Spiegelman, D.; Giovannucci, E. Proportion of Colon Cancer Risk That Might Be Preventable in a Cohort of Middle-Aged US Men. Cancer Causes Control 2000, 11, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Pischon, T.; Jenab, M.; Bueno-de-Mesquita, H.B.; Fedirko, V.; Norat, T.; Romaguera, D.; Knüppel, S.; Boutron-Ruault, M.-C.; Dossus, L.; et al. Combined Impact of Healthy Lifestyle Factors on Colorectal Cancer: A Large European Cohort Study. BMC Med. 2014, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Alcohol Drinking and Colorectal Cancer Risk: An Overall and Dose-Response Meta-Analysis of Published Studies—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21307158/ (accessed on 27 December 2022).
- Effects of Regular Aspirin on Long-Term Cancer Incidence and Metastasis: A Systematic Comparison of Evidence from Observational Studies versus Randomised Trials—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/22440112/ (accessed on 27 December 2022).
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary Fibre, Whole Grains, and Risk of Colorectal Cancer: Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Boughanem, H.; Canudas, S.; Becerra-Tomás, N.; Fernández de la Puente, M.; Babio, N.; Macias-Gonzalez, M.; Salas-Salvadó, J. Circulating Vitamin D Levels and Colorectal Cancer Risk: A Meta-Analysis and Systematic Review of Case-Control and Prospective Cohort Studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.; Bouras, E.; van den Brandt, P.A.; Muller, D.C.; Papadopoulou, A.; Heath, A.K.; Critselis, E.; Gunter, M.J.; Vineis, P.; Ferrari, P.; et al. A Prospective Diet-Wide Association Study for Risk of Colorectal Cancer in EPIC. Clin. Gastroenterol. Hepatol. 2022, 20, 864–873.e13. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Morris, J.S.; Bradbury, K.E.; Cross, A.J.; Gunter, M.J.; Murphy, N. Physical Activity, Sedentary Behaviour and Colorectal Cancer Risk in the UK Biobank. Br. J. Cancer 2018, 118, 920–929. [Google Scholar] [CrossRef]
- Andersen, V.; Vogel, U. Systematic Review: Interactions between Aspirin, and Other Nonsteroidal Anti-Inflammatory Drugs, and Polymorphisms in Relation to Colorectal Cancer. Aliment Pharmacol. Ther. 2014, 40, 147–159. [Google Scholar] [CrossRef]
- Nan, H.; Hutter, C.M.; Lin, Y.; Jacobs, E.J.; Ulrich, C.M.; White, E.; Baron, J.A.; Berndt, S.I.; Brenner, H.; Butterbach, K.; et al. Association of Aspirin and NSAID Use with Risk of Colorectal Cancer According to Genetic Variants. JAMA 2015, 313, 1133–1142. [Google Scholar] [CrossRef]
- Borges Canha, M. Role of Colonic Microbiota in Colorectal Carcinogenesis: A Systematic Review. Rev. Esp. Enferm. Dig. 2015, 107, 659–671. [Google Scholar] [CrossRef]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human Gut Microbiome and Risk for Colorectal Cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Chaplin, A.; Rodriguez, R.M.; Segura-Sampedro, J.J.; Ochogavía-Seguí, A.; Romaguera, D.; Barceló-Coblijn, G. Insights behind the Relationship between Colorectal Cancer and Obesity: Is Visceral Adipose Tissue the Missing Link? Int. J. Mol. Sci. 2022, 23, 13128. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.M.; Brown, K.A.; Iyengar, N.M. Targeting Obesity-Related Dysfunction in Hormonally Driven Cancers. Br. J. Cancer 2021, 125, 495–509. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Ironmonger, L.; Steele, R.J.C.; Ormiston-Smith, N.; Crawford, C.; Seims, A. A Review of Sex-Related Differences in Colorectal Cancer Incidence, Screening Uptake, Routes to Diagnosis, Cancer Stage and Survival in the UK. BMC Cancer 2018, 18, 906. [Google Scholar] [CrossRef]
- Goodarzi, G.; Mozaffari, H.; Raeisi, T.; Mehravar, F.; Razi, B.; Ghazi, M.L.; Garousi, N.; Alizadeh, S.; Janmohammadi, P. Metabolic Phenotypes and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Cohort Studies. BMC Cancer 2022, 22, 89. [Google Scholar] [CrossRef]
- Li, H.; Yang, G.; Xiang, Y.-B.; Gao, J.; Zhang, X.; Zheng, W.; Gao, Y.-T.; Shu, X.-O. Body Weight, Fat Distribution and Colorectal Cancer Risk: A Report from Cohort Studies of 134 255 Chinese Men and Women. Int. J. Obes. 2013, 37, 783–789. [Google Scholar] [CrossRef]
- MacInnis, R.J.; English, D.R.; Hopper, J.L.; Haydon, A.M.; Gertig, D.M.; Giles, G.G. Body Size and Composition and Colon Cancer Risk in Men. Cancer Epidemiol. Biomark. Prev. 2004, 13, 553–559. [Google Scholar] [CrossRef]
- Garcia, H.; Song, M. Early-Life Obesity and Adulthood Colorectal Cancer Risk: A Meta-Analysis. Rev. Panam. Salud Publica 2019, 43, e3. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Yang, C.-M.; Shi, B.-M. Body Fatness at an Early Age and Risk of Colorectal Cancer. Int. J. Cancer 2018, 142, 729–740. [Google Scholar] [CrossRef]
- Christakoudi, S.; Riboli, E.; Evangelou, E.; Tsilidis, K.K. Associations of Body Shape Phenotypes with Sex Steroids and Their Binding Proteins in the UK Biobank Cohort. Sci. Rep. 2022, 12, 10774. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Boakye, D.; Chen, X.; Jansen, L.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Associations of Body Mass Index at Different Ages With Early-Onset Colorectal Cancer. Gastroenterology 2022, 162, 1088–1097.e3. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Koh, W.-P.; Yu, M.C.; Yuan, J.-M. Body Mass Index and Risk of Colorectal Cancer in Chinese Singaporeans: The Singapore Chinese Health Study. Cancer 2011, 117, 3841–3849. [Google Scholar] [CrossRef]
- Otani, T.; Iwasaki, M.; Inoue, M. Shoichiro Tsugane for the Japan Public Health Center-based Prospective Study Group Body Mass Index, Body Height, and Subsequent Risk of Colorectal Cancer in Middle-Aged and Elderly Japanese Men and Women: Japan Public Health Center-Based Prospective Study. Cancer Causes Control 2005, 16, 839–850. [Google Scholar] [CrossRef]
- Kuriyama, S.; Tsubono, Y.; Hozawa, A.; Shimazu, T.; Suzuki, Y.; Koizumi, Y.; Suzuki, Y.; Ohmori, K.; Nishino, Y.; Tsuji, I. Obesity and Risk of Cancer in Japan. Int. J. Cancer 2005, 113, 148–157. [Google Scholar] [CrossRef]
- Adams, K.F.; Leitzmann, M.F.; Albanes, D.; Kipnis, V.; Mouw, T.; Hollenbeck, A.; Schatzkin, A. Body Mass and Colorectal Cancer Risk in the NIH-AARP Cohort. Am. J. Epidemiol. 2007, 166, 36–45. [Google Scholar] [CrossRef]
- Oxentenko, A.S.; Bardia, A.; Vierkant, R.A.; Wang, A.H.; Anderson, K.E.; Campbell, P.T.; Sellers, T.A.; Folsom, A.R.; Cerhan, J.R.; Limburg, P.J. Body Size and Incident Colorectal Cancer: A Prospective Study of Older Women. Cancer Prev. Res. 2010, 3, 1608–1620. [Google Scholar] [CrossRef]
- Kasprzak, A. Insulin-Like Growth Factor 1 (IGF-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.F.; Lewis, L.R.; Levine, A.E. Characterization of the Synergistic Effect of Insulin and Transferrin and the Regulation of Their Receptors on a Human Colon Carcinoma Cell Line. Int. J. Cancer 1990, 45, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like Growth Factor (IGF)-I, IGF Binding Protein-3, and Cancer Risk: Systematic Review and Meta-Regression Analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Rinaldi, S.; Cleveland, R.; Norat, T.; Biessy, C.; Rohrmann, S.; Linseisen, J.; Boeing, H.; Pischon, T.; Panico, S.; Agnoli, C.; et al. Serum Levels of IGF-I, IGFBP-3 and Colorectal Cancer Risk: Results from the EPIC Cohort, plus a Meta-Analysis of Prospective Studies. Int. J. Cancer 2010, 126, 1702–1715. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Painter, J.E.; Atkin, W.S.; Potten, C.S.; Shalet, S.M.; O’Dwyer, S.T. High-Risk Colorectal Adenomas and Serum Insulin-like Growth Factors. Br. J. Surg. 2001, 88, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.; Naffah de Souza, C.; Câmara, N.O.S.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2016, 6, 637. [Google Scholar] [CrossRef]
- Suganami, T.; Ogawa, Y. Adipose Tissue Macrophages: Their Role in Adipose Tissue Remodeling. J. Leukoc. Biol. 2010, 88, 33–39. [Google Scholar] [CrossRef]
- Hao, N.-B.; Lü, M.-H.; Fan, Y.-H.; Cao, Y.-L.; Zhang, Z.-R.; Yang, S.-M. Macrophages in Tumor Microenvironments and the Progression of Tumors. Clin. Dev. Immunol. 2012, 2012, 948098. [Google Scholar] [CrossRef]
- Springer, N.L.; Iyengar, N.M.; Bareja, R.; Verma, A.; Jochelson, M.S.; Giri, D.D.; Zhou, X.K.; Elemento, O.; Dannenberg, A.J.; Fischbach, C. Obesity-Associated Extracellular Matrix Remodeling Promotes a Macrophage Phenotype Similar to Tumor-Associated Macrophages. Am. J. Pathol. 2019, 189, 2019–2035. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, T.; Schwartz, B. Leptin Promotes Motility and Invasiveness in Human Colon Cancer Cells by Activating Multiple Signal-Transduction Pathways. Int. J. Cancer 2008, 123, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Hosono, K.; Uchiyama, T.; Sakai, E.; Sugiyama, M.; Takahashi, H.; Nakajima, N.; Wada, K.; Takeda, K.; Nakagama, H.; et al. Leptin Acts as a Growth Factor for Colorectal Tumours at Stages Subsequent to Tumour Initiation in Murine Colon Carcinogenesis. Gut 2011, 60, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Kotelevets, L.; Tsocas, A.; Laigneau, J.-P.; Sobhani, I.; Chastre, E.; Lehy, T. Leptin Stimulates the Proliferation of Human Colon Cancer Cells in Vitro but Does Not Promote the Growth of Colon Cancer Xenografts in Nude Mice or Intestinal Tumorigenesis in Apc(Min/+) Mice. Gut 2005, 54, 1136–1145. [Google Scholar] [CrossRef]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Rittig, K.; Balletshofer, B.; Machann, J.; Schick, F.; Porubska, K.; Fritsche, A.; Häring, H.-U.; Stefan, N. The Relationships of Plasma Adiponectin with a Favorable Lipid Profile, Decreased Inflammation, and Less Ectopic Fat Accumulation Depend on Adiposity. Clin. Chem. 2006, 52, 1934–1942. [Google Scholar] [CrossRef]
- Fenton, J.I.; Birmingham, J.M.; Hursting, S.D.; Hord, N.G. Adiponectin Blocks Multiple Signaling Cascades Associated with Leptin-Induced Cell Proliferation in ApcMin/+ Colon Epithelial Cells. Int. J. Cancer 2008, 122, 2437–2445. [Google Scholar] [CrossRef]
- Danielsen, S.A.; Eide, P.W.; Nesbakken, A.; Guren, T.; Leithe, E.; Lothe, R.A. Portrait of the PI3K/AKT Pathway in Colorectal Cancer. Biochim. Biophys. Acta 2015, 1855, 104–121. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Duong, H.-Q. The Molecular Characteristics of Colorectal Cancer: Implications for Diagnosis and Therapy. Oncol. Lett. 2018, 16, 9–18. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Neal, M.D.; Leaphart, C.; Levy, R.; Prince, J.; Billiar, T.R.; Watkins, S.; Li, J.; Cetin, S.; Ford, H.; Schreiber, A.; et al. Enterocyte TLR4 Mediates Phagocytosis and Translocation of Bacteria Across the Intestinal Barrier. J. Immunol. 2006, 176, 3070–3079. [Google Scholar] [CrossRef] [PubMed]
- Kuugbee, E.D.; Shang, X.; Gamallat, Y.; Bamba, D.; Awadasseid, A.; Suliman, M.A.; Zang, S.; Ma, Y.; Chiwala, G.; Xin, Y.; et al. Structural Change in Microbiota by a Probiotic Cocktail Enhances the Gut Barrier and Reduces Cancer via TLR2 Signaling in a Rat Model of Colon Cancer. Digest. Dis. Sci. 2016, 61, 2908–2920. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Edmundson, P.; Araujo-Perez, F.; McCoy, A.N.; Galanko, J.; Keku, T.O. Association of Plasma Endotoxin, Inflammatory Cytokines and Risk of Colorectal Adenomas. BMC Cancer 2013, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Serino, M.; Luche, E.; Waget, A.; Pardo, G.; Salvador, J.; Ricart, W.; Frühbeck, G.; Burcelin, R.; et al. Circulating Lipopolysaccharide-Binding Protein (LBP) as a Marker of Obesity-Related Insulin Resistance. Int. J. Obes. 2012, 36, 1442–1449. [Google Scholar] [CrossRef]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, S.A.; da Conceição, L.L.; Siqueira, N.P.; Rosa, D.D.; da Silva, L.L.; Peluzio, M. Review of the Mechanisms of Probiotic Actions in the Prevention of Colorectal Cancer. Nutr. Res. 2017, 37, 1–19. [Google Scholar] [CrossRef]
- Czajkowska, A.; Szponar, B. Short chain fatty acids (SCFA), the products of gut bacteria metabolism and their role in the host. Postepy Hig. Med. Dosw. 2018, 72, 131–142. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef]
- Zheng, D.-W.; Li, R.-Q.; An, J.-X.; Xie, T.-Q.; Han, Z.-Y.; Xu, R.; Fang, Y.; Zhang, X.-Z. Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer. Adv. Mater. 2020, 32, 2004529. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, W.-C.; Kong, M.-S.; Shi, H.N.; Walker, W.A.; Lin, C.-Y.; Huang, C.-T.; Lin, Y.-C.; Jung, S.-M.; Lin, T.-Y. Oral Inoculation of Probiotics Lactobacillus Acidophilus NCFM Suppresses Tumour Growth Both in Segmental Orthotopic Colon Cancer and Extra-Intestinal Tissue. Br. J. Nutr. 2012, 107, 1623–1634. [Google Scholar] [CrossRef]
- Gagnière, J. Gut Microbiota Imbalance and Colorectal Cancer. World J. Gastroenterol. 2016, 22, 501. [Google Scholar] [CrossRef]
- Greenblum, S.; Turnbaugh, P.J.; Borenstein, E. Metagenomic Systems Biology of the Human Gut Microbiome Reveals Topological Shifts Associated with Obesity and Inflammatory Bowel Disease. Proc. Natl. Acad. Sci. USA 2012, 109, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Carter, J.; Harari, S.; Pei, Z. The Interrelationships of the Gut Microbiome and Inflammation in Colorectal Carcinogenesis. Clin. Lab. Med. 2014, 34, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal Relationships among the Gut Microbiome, Short-Chain Fatty Acids and Metabolic Diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Salmerón, M.; Lucena, S.R.; Chocarro-Calvo, A.; García-Martínez, J.M.; Martín Orozco, R.M.; García-Jiménez, C. Metabolic and Hormonal Remodeling of Colorectal Cancer Cell Signalling by Diabetes. Endocr. Rel. Cancer 2021, 28, R191–R206. [Google Scholar] [CrossRef] [PubMed]
- Pauli, J.R.; Ropelle, E.R.; Cintra, D.E.; Carvalho-Filho, M.A.; Moraes, J.C.; De Souza, C.T.; Velloso, L.A.; Carvalheira, J.B.C.; Saad, M.J.A. Acute Physical Exercise Reverses S -Nitrosation of the Insulin Receptor, Insulin Receptor Substrate 1 and Protein Kinase B/Akt in Diet-Induced Obese Wistar Rats: Acute Exercise/Insulin Resistance. J. Physiol. 2008, 586, 659–671. [Google Scholar] [CrossRef]
- Tremblay, F.; Krebs, M.; Dombrowski, L.; Brehm, A.; Bernroider, E.; Roth, E.; Nowotny, P.; Waldhäusl, W.; Marette, A.; Roden, M. Overactivation of S6 Kinase 1 as a Cause of Human Insulin Resistance During Increased Amino Acid Availability. Diabetes 2005, 54, 2674–2684. [Google Scholar] [CrossRef]
- Neis, E.P.J.G.; Dejong, C.H.C.; Rensen, S.S. The Role of Microbial Amino Acid Metabolism in Host Metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef]
- Sze Marc, A.; Schloss Patrick, D. Leveraging Existing 16S RRNA Gene Surveys To Identify Reproducible Biomarkers in Individuals with Colorectal Tumors. mBio 2018, 9, e00630-18. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium Nucleatum Infection Is Prevalent in Human Colorectal Carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Yu, T.; Fang, X.; Lou, L.; Xin, S.; Ji, L.; Jiang, F.; Lou, Y. Fusobacterium Nucleatum Promotes the Progression of Colorectal Cancer Through Cdk5-Activated Wnt/β-Catenin Signaling. Front. Microbiol. 2021, 11, 545251. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hong, X.; Wang, J.; Sun, T.; Yu, T.; Yu, Y.; Fang, J.; Xiong, H. Metformin Elicits Antitumour Effect by Modulation of the Gut Microbiota and Rescues Fusobacterium Nucleatum-Induced Colorectal Tumourigenesis. EBioMedicine 2020, 61, 103037. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; DeSantis, T.Z.; Weinmaier, T.; McMurdie, P.J.; Cope, J.L.; Altrichter, A.; Yamal, J.-M.; Hollister, E.B. Leveraging Sequence-Based Faecal Microbial Community Survey Data to Identify a Composite Biomarker for Colorectal Cancer. Gut 2018, 67, 882. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.M.; Campbell, B.J.; Hart, C.A.; Mpofu, C.; Nayar, M.; Singh, R.; Englyst, H.; Williams, H.F.; Rhodes, J.M. Enhanced Escherichia Coli Adherence and Invasion in Crohn’s Disease and Colon Cancer 1. Gastroenterology 2004, 127, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Buc, E.; Sauvanet, P.; Darcha, C.; Dubois, D.; Pereira, B.; Déchelotte, P.; Bonnet, R.; Pezet, D.; Darfeuille-Michaud, A. Colonization of the Human Gut by E. coli and Colorectal Cancer Risk. Clin. Cancer Res. 2014, 20, 859–867. [Google Scholar] [CrossRef]
- Goldin, B.R.; Gorbach, S.L. The Relationship Between Diet and Rat Fecal Bacterial Enzymes Implicated in Colon Cancer23. J. Natl. Cancer Inst. 1976, 57, 371–375. [Google Scholar] [CrossRef]
- Gamallat, Y.; Meyiah, A.; Kuugbee, E.D.; Hago, A.M.; Chiwala, G.; Awadasseid, A.; Bamba, D.; Zhang, X.; Shang, X.; Luo, F.; et al. Lactobacillus Rhamnosus Induced Epithelial Cell Apoptosis, Ameliorates Inflammation and Prevents Colon Cancer Development in an Animal Model. Biomed. Pharmacother. 2016, 83, 536–541. [Google Scholar] [CrossRef]
- Verma, A.; Shukla, G. Probiotics Lactobacillus Rhamnosus GG, Lactobacillus Acidophilus Suppresses DMH-Induced Procarcinogenic Fecal Enzymes and Preneoplastic Aberrant Crypt Foci in Early Colon Carcinogenesis in Sprague Dawley Rats. Nutr. Cancer 2013, 65, 84–91. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Walker, J.W.; Diaz, H.; Madsen, K.L. Bioproduction of Conjugated Linoleic Acid by Probiotic Bacteria Occurs In Vitro and In Vivo in Mice. J. Nutr. 2006, 136, 1483–1487. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Yang, Z.; Wang, Y.; et al. Randomised Clinical Trial: The Effects of Perioperative Probiotic Treatment on Barrier Function and Post-Operative Infectious Complications in Colorectal Cancer Surgery—A Double-Blind Study. Aliment. Pharmacol. Ther. 2011, 33, 50–63. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J. The Association of Diet, Gut Microbiota and Colorectal Cancer: What We Eat May Imply What We Get. Protein Cell 2018, 9, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T. Environmental Factors, Gut Microbiota, and Colorectal Cancer Prevention. Clin. Gastroenterol. Hepatol. 2019, 17, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Fung, T.T.; Hamada, T.; Masugi, Y.; da Silva, A.; et al. Dietary Patterns and Risk of Colorectal Cancer: Analysis by Tumor Location and Molecular Subtypes. Gastroenterology 2017, 152, 1944–1953.e1. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. Interplay between Diet, Gut Microbiota, Epigenetic Events, and Colorectal Cancer. Mol. Nutr. Food Res. 2017, 61, 1500902. [Google Scholar] [CrossRef]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients 2017, 9, 1043. [Google Scholar] [CrossRef]
- Fan, Y.; Jin, X.; Man, C.; Gao, Z.; Wang, X. Meta-Analysis of the Association between the Inflammatory Potential of Diet and Colorectal Cancer Risk. Oncotarget 2017, 8, 59592–59600. [Google Scholar] [CrossRef] [PubMed]
- Norat, T.; Bingham, S.; Ferrari, P.; Slimani, N.; Jenab, M.; Mazuir, M.; Overvad, K.; Olsen, A.; Tjønneland, A.; Clavel, F.; et al. Meat, Fish, and Colorectal Cancer Risk: The European Prospective Investigation into Cancer and Nutrition. J. Natl. Cancer Inst. 2005, 97, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.L.; Hjartåker, A.; Lund, E.; Veierød, M.B. Meat Intake, Cooking Methods and Risk of Proximal Colon, Distal Colon and Rectal Cancer: The Norwegian Women and Cancer (NOWAC) Cohort Study. Int. J. Cancer 2013, 133, 1153–1163. [Google Scholar] [CrossRef]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, Foods, and Colorectal Cancer Prevention. Gastroenterology 2015, 148, 1244–1260.e16. [Google Scholar] [CrossRef]
- Wang, Y.; Nguyen, L.H.; Mehta, R.S.; Song, M.; Huttenhower, C.; Chan, A.T. Association Between the Sulfur Microbial Diet and Risk of Colorectal Cancer. JAMA Netw. Open 2021, 4, e2134308. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Ma, W.; Wang, D.D.; Cao, Y.; Mallick, H.; Gerbaba, T.K.; Lloyd-Price, J.; Abu-Ali, G.; Hall, A.B.; Sikavi, D.; et al. Association Between Sulfur-Metabolizing Bacterial Communities in Stool and Risk of Distal Colorectal Cancer in Men. Gastroenterology 2020, 158, 1313–1325. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Myung, S.-K.; Lee, J.-H. Light Alcohol Drinking and Risk of Cancer: A Meta-Analysis of Cohort Studies. Cancer Res. Treat. 2018, 50, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Li, Y.; Ding, Y.; Chen, K.; Jin, M. Alcohol Drinking and the Risk of Colorectal Cancer Death: A Meta-Analysis. Eur. J. Cancer Prev. 2014, 23, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, A.I.; Haif, S.K.; Kamal, A.; Al-ataby, I.A.; Talib, W.H. Chemoprevention Effect of the Mediterranean Diet on Colorectal Cancer: Current Studies and Future Prospects. Front. Nutr. 2022, 9, 924192. [Google Scholar] [CrossRef]
- Castelló, A.; Rodríguez-Barranco, M.; Fernández de Larrea, N.; Jakszyn, P.; Dorronsoro, A.; Amiano, P.; Chirlaque, M.-D.; Colorado-Yohar, S.; Guevara, M.; Moreno-Iribas, C.; et al. Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Colorectal Cancer Risk: Findings from the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Nutrients 2022, 14, 3085. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-León, D.; Gómez-Abril, S.Á.; Monzó-Beltrán, L.; Estañ-Capell, N.; Arroyo-Montañés, R.; Bañuls, C.; Salas-Salvadó, J.; Sáez, G. Adherence to the Mediterranean Diet Has a Protective Role against Metabolic and DNA Damage Markers in Colorectal Cancer Patients. Antioxidants 2022, 11, 499. [Google Scholar] [CrossRef]
- Agnoli, C.; Grioni, S.; Sieri, S.; Palli, D.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Giurdanella, M.C.; Pala, V.; et al. Italian Mediterranean Index and Risk of Colorectal Cancer in the Italian Section of the EPIC Cohort. Int. J. Cancer 2013, 132, 1404–1411. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and Beverages and Colorectal Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies, an Update of the Evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef]
- Flood, A.; Rastogi, T.; Wirfält, E.; Mitrou, P.N.; Reedy, J.; Subar, A.F.; Kipnis, V.; Mouw, T.; Hollenbeck, A.R.; Leitzmann, M.; et al. Dietary Patterns as Identified by Factor Analysis and Colorectal Cancer among Middle-Aged Americans. Am. J. Clin. Nutr. 2008, 88, 176–184. [Google Scholar] [CrossRef]
- Sain, A.; Sahu, S.; Naskar, D. Potential of Olive Oil and Its Phenolic Compounds as Therapeutic Intervention against Colorectal Cancer: A Comprehensive Review. Br J. Nutr. 2021, 128, 1–17. [Google Scholar] [CrossRef]
- Itsiopoulos, C.; Mayr, H.L.; Thomas, C.J. The Anti-Inflammatory Effects of a Mediterranean Diet: A Review. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.-D.; Agudo, A.; Sánchez, M.-J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhou, R.-L.; Ou, Q.-J.; Chen, Y.-M.; Fang, Y.-J.; Zhang, C.-X. Association of Plant-Based Dietary Patterns with the Risk of Colorectal Cancer: A Large-Scale Case-Control Study. Food Funct. 2022, 13, 10790–10801. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.J.; Mazidi, M.; Lip, G.Y.H.; Kengne, A.P.; Banach, M.; Davies, I.G. The Role of Adiposity, Diet and Inflammation on the Discordance between LDL-C and Apolipoprotein B. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Milesi, G.; Rangan, A.; Grafenauer, S. Whole Grain Consumption and Inflammatory Markers: A Systematic Literature Review of Randomized Control Trials. Nutrients 2022, 14, 374. [Google Scholar] [CrossRef] [PubMed]
- Harland, J.I.; Garton, L.E. Whole-Grain Intake as a Marker of Healthy Body Weight and Adiposity. Public Health Nutr. 2008, 11, 554–563. [Google Scholar] [CrossRef]
- Illikoud, N.; Mantel, M.; Rolli-Derkinderen, M.; Gagnaire, V.; Jan, G. Dairy Starters and Fermented Dairy Products Modulate Gut Mucosal Immunity. Immunol. Lett. 2022, 251–252, 91–102. [Google Scholar] [CrossRef]
- Khorraminezhad, L.; Rudkowska, I. Modulation of Gene Expression Profile Following Consumption of High-Dairy Products in Subjects with Hyperinsulinemia. Nutr. Metab. Cardiovasc. Dis. 2022, 33, 219–226. [Google Scholar] [CrossRef]
- Kim, S.H.; Moon, J.Y.; Lim, Y.J. Dietary Intervention for Preventing Colorectal Cancer: A Practical Guide for Physicians. J. Cancer Prev. 2022, 27, 139–146. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Y.; Dai, H.; Deng, Z. Effects of Bariatric Surgery on Cancer Risk: Evidence from Meta-Analysis. Obes. Surg. 2020, 30, 1265–1272. [Google Scholar] [CrossRef]
- Roslan, N.H.; Makpol, S.; Yusof, Y.A.M. A Review on Dietary Intervention in Obesity Associated Colon Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 1309–1319. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, Fiber and Cancer Risk in African Americans and Rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef]
- Orange, S.T.; Jordan, A.R.; Odell, A.; Kavanagh, O.; Hicks, K.M.; Eaglen, T.; Todryk, S.; Saxton, J.M. Acute Aerobic Exercise-Conditioned Serum Reduces Colon Cancer Cell Proliferation in Vitro through Interleukin-6-Induced Regulation of DNA Damage. Int. J. Cancer 2022, 151, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, M.H. Physical Activity and Nutrition in Primary and Tertiary Prevention of Colorectal Cancer. Visc. Med. 2016, 32, 199–204. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A. Mechanisms Linking Physical Activity with Cancer. Nat. Rev. Cancer 2008, 8, 205–211. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical Activity, Obesity and Sedentary Behavior in Cancer Etiology: Epidemiologic Evidence and Biologic Mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Powell, K.E.; Jakicic, J.M.; Troiano, R.P.; Piercy, K.; Tennant, B. Sedentary Behavior and Health: Update from the 2018 Physical Activity Guidelines Advisory Committee. Med. Sci. Sports Exerc. 2019, 51, 1227–1241. [Google Scholar] [CrossRef]

| Population (n, Total) | Years | CRC Cases, n | Individuals with Obesity and CRC *, % | Individuals with Obesity *, n | CRC Risk OR/RR/HZ (CI) | Ref. |
|---|---|---|---|---|---|---|
| Australia, men (16,556) | 1990–1994 | 153 | 33.33 | 51 (BMI > 29.2) | CRC risk RR = 2.1 (1.3–3.7) 4th vs. 1st quartiles of WHR | [56] |
| United Kingdom (40,467) | 2006–2010 | 1918 | 28.94 | 555 | n/a | [59] |
| Germany (14,552) | 2003–2020 | 747 | 16.2 | 121 (10 years before diagnosis) | CRC risk OR = 2.17 (1.54–3.07) | [60] |
| Singapur (51,251) | 1993–1998 | 980 | 11.63 | 114 (BMI > 27.5) | CRC risk HR = 1.25 (1.01–1.55) Colon cancer HR = 1.48 (1.13–1.92) Rectal cancer HR = 0.93 (0.64–1.36) | [61] |
| Japan, men Cohort I (16,765) Cohort II (28,945) | 1990–2001 | - | - | 420 616 | CRC risk RR = 1.5 (0.7–3.0) RR = 1.5 (0.6–3.03) | [62] |
| Japan, women Cohort I (21,725) Cohort II (32,066) | 1990–2001 | - | - | 700 1009 | CRC risk RR = 0.7 (0.3–2.0) RR = 0.8 (0.3–2.0) | [62] |
| Japan, women (15,054) | 1984–1992 | 115 | 7.8% | 9 | CRC risk RR = 2.06 (1.03–4.13) | [63] |
| The USA (517,144) | 1995–2000 | 3343 | 25.04 | 837 | Colon cancer risk M/W BMI 30–32.5 M, RR = 1.53 (1.23–1.9) W, RR =1.28 (0.97–1.69) BMI ≥ 40 M, RR = 2.39 (1.59–3.58)/ W, RR = 1.49 (0.98 = 2.25) | [64] |
| The USA (36,941) | 1986–2005 | 1464 | 27.46 | 402 | CRC risk RR = 1.56 (1.10–2.22) | [65] |
| Foods and Dietary Patterns Increasing the Risk of Colorectal Cancer | Foods and Dietary Patterns Decreasing the Risk of Colorectal Cancer |
|---|---|
| Western diet [118] Sulfur microbial diet [125,126] Proinflammatory diet [120,121] Red and processed meat [122,123] Refined grains [147] Alcohol [127] | Mediterranean diet [130,137] Fruits and vegetables [132,134] Fiber [41,132] Phenolic of olive oils [135] Fish [133] Vitamin D [137] Whole grains [41]. Dairy products [41,133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rychter, A.M.; Łykowska-Szuber, L.; Zawada, A.; Szymczak-Tomczak, A.; Ratajczak, A.E.; Skoracka, K.; Kolan, M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Why Does Obesity as an Inflammatory Condition Predispose to Colorectal Cancer? J. Clin. Med. 2023, 12, 2451. https://doi.org/10.3390/jcm12072451
Rychter AM, Łykowska-Szuber L, Zawada A, Szymczak-Tomczak A, Ratajczak AE, Skoracka K, Kolan M, Dobrowolska A, Krela-Kaźmierczak I. Why Does Obesity as an Inflammatory Condition Predispose to Colorectal Cancer? Journal of Clinical Medicine. 2023; 12(7):2451. https://doi.org/10.3390/jcm12072451
Chicago/Turabian StyleRychter, Anna Maria, Liliana Łykowska-Szuber, Agnieszka Zawada, Aleksandra Szymczak-Tomczak, Alicja Ewa Ratajczak, Kinga Skoracka, Michalina Kolan, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2023. "Why Does Obesity as an Inflammatory Condition Predispose to Colorectal Cancer?" Journal of Clinical Medicine 12, no. 7: 2451. https://doi.org/10.3390/jcm12072451
APA StyleRychter, A. M., Łykowska-Szuber, L., Zawada, A., Szymczak-Tomczak, A., Ratajczak, A. E., Skoracka, K., Kolan, M., Dobrowolska, A., & Krela-Kaźmierczak, I. (2023). Why Does Obesity as an Inflammatory Condition Predispose to Colorectal Cancer? Journal of Clinical Medicine, 12(7), 2451. https://doi.org/10.3390/jcm12072451





