Reversibility of Frail Phenotype in Patients with Inflammatory Bowel Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Statistical Analysis
3. Results
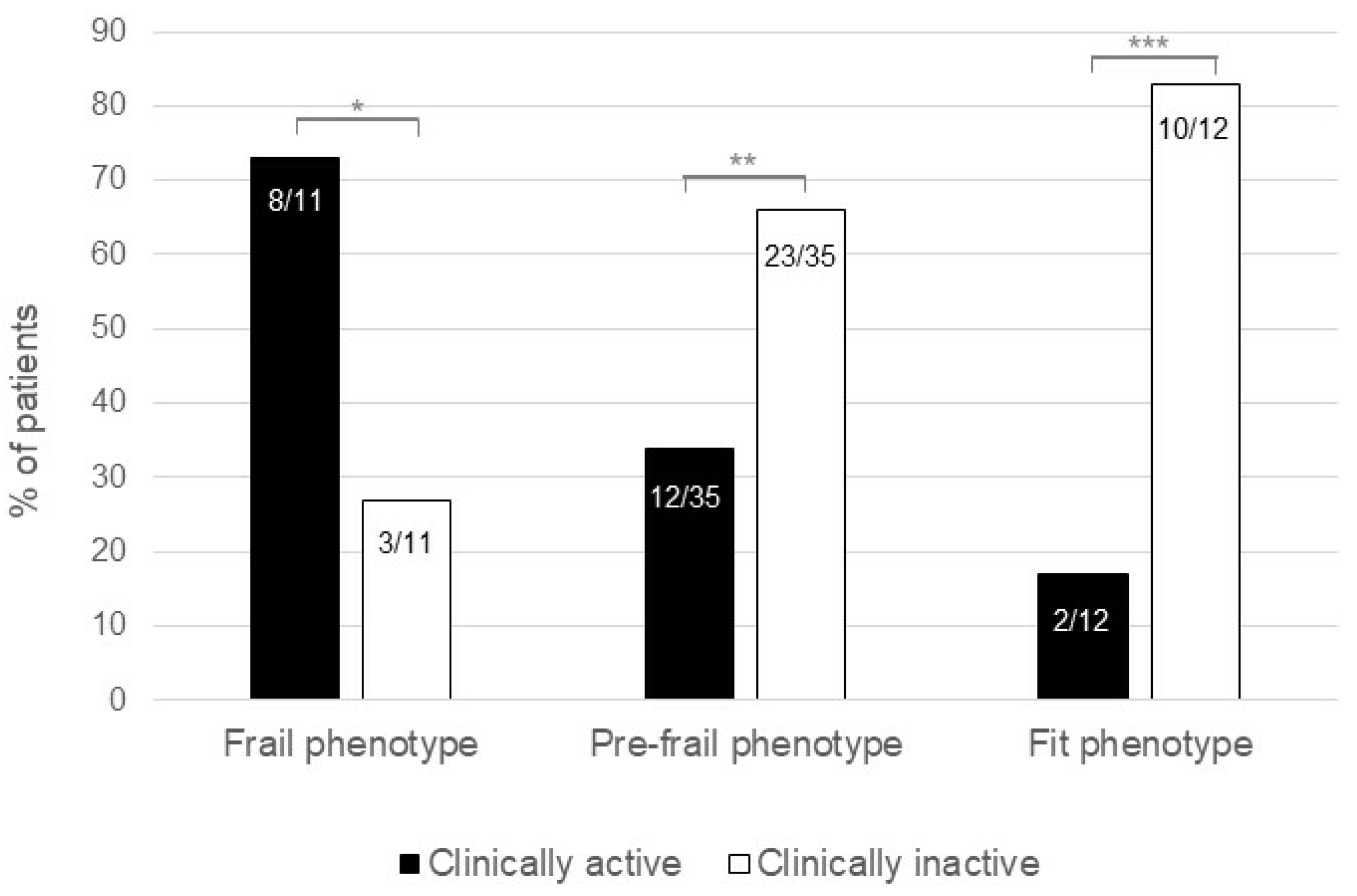
3.1. Study Population and Changes in Frail Phenotype
3.2. Reversibility of Frail Phenotype Occurs More Frequently following Induction of Clinical Remission
3.3. Predictive Factors for Phenotype Improvement
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kochar, B.; Jylhävä, J.; Söderling, J.; Ritchie, C.S.; SWIBREG Study Group; Ludvigssonm, J.F.; Khalili, H.; Olén, O. Prevalence and Implications of Frailty in Older Adults with Incident Inflammatory Bowel Diseases: A Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, 2358–2365.e11. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in Elderly. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Qian, A.S.; Nguyen, N.H.; Elia, J.; Ohno-Machado, L.; Sandborn, W.J.; Singh, S. Frailty Is Independently Associated with Mortality and Readmission in Hospitalized Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 2054–2063.e14. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.H.; Hassab, T.; D’Adamo, C.R.; Svoboda, S.; Demos, J.; Ahuja, V.; Katlic, M. Frailty is a stronger predictor than age for postoperative morbidity in Crohn’s disease. Surgery 2021, 170, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Telemi, E.; Trofymenko, O.; Venkat, R.; Pandit, V.; Pandian, T.; Nfonsam, V.N. Frailty Predicts Morbidity after Colectomy for Ulcerative Colitis. Am. Surg. 2018, 84, 225–229. [Google Scholar] [CrossRef]
- Singh, S.; Heien, H.C.; Sangaralingham, L.; Shah, N.D.; Lai, J.C.; Sandborn, W.J.; Moore, A.A. Frailty and Risk of Serious Infections in Biologic-treated Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, 1626–1633. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Xue, Q.-L.; Bandeen-Roche, K.; Varadhan, R.; Zhou, J.; Fried, L.P. Initial Manifestations of Frailty Criteria and the Development of Frailty Phenotype in the Women’s Health and Aging Study II. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 984–990. [Google Scholar] [CrossRef]
- Fried, L.P.; Xue, Q.-L.; Cappola, A.R.; Ferrucci, L.; Chaves, P.; Varadhan, R.; Guralnik, J.M.; Leng, S.X.; Semba, R.D.; Walston, J.D.; et al. Nonlinear Multisystem Physiological Dysregulation Associated with Frailty in Older Women: Implications for Etiology and Treatment. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64A, 1049–1057. [Google Scholar] [CrossRef]
- Szanton, S.L.; Allen, J.K.; Seplaki, C.; Bandeen-Roche, K.; Fried, L.P. Allostatic Load and Frailty in the Women’s Health and Aging Studies. Biol. Res. Nurs. 2008, 10, 248–256. [Google Scholar] [CrossRef]
- Salvatori, S.; Marafini, I.; Venuto, C.; Laudisi, F.; Neri, B.; Lavigna, D.; Franchin, M.; De Cristofaro, E.; Biancone, L.; Calabrese, E.; et al. Frail Phenotype in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kochar, B.D.; Cai, W.; Ananthakrishnan, A.N. Inflammatory Bowel Disease Patients Who Respond to Treatment with Anti-tumor Necrosis Factor Agents Demonstrate Improvement in Pre-treatment Frailty. Dig. Dis. Sci. 2022, 67, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the mayo score to assess clinical response in Ulcerative Colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.F.; Bradshaw, J.M. Index of Crohn’s disease activity. Lancet 1980, 315, 711. [Google Scholar] [CrossRef]
- Tada, M.; Yamada, Y.; Mandai, K.; Hidaka, N. Correlation between frailty and disease activity in patients with rheumatoid arthritis: Data from the CHIKARA study. Geriatr. Gerontol. Int. 2019, 19, 1220–1225. [Google Scholar] [CrossRef]
- Haider, S.; Grabovac, I.; Berner, C.; Lamprecht, T.; Fenzl, K.-H.; Erlacher, L.; Quittan, M.; Dorner, T.E. Frailty in seropositive rheumatoid arthritis patients of working age: A cross-sectional study. Clin. Exp. Rheum. 2018, 37, 585–592. [Google Scholar]
- Kojima, M.; Kojima, T.; Waguri-Nagaya, Y.; Takahashi, N.; Asai, S.; Sobue, Y.; Nishiume, T.; Suzuki, M.; Mitsui, H.; Kawaguchi, Y.; et al. Depression, physical function, and disease activity associated with frailty in patients with rheumatoid arthritis. Mod. Rheumatol. 2021, 31, 979–986. [Google Scholar] [CrossRef]
- Yao, X.; Li, H.; Leng, S.X. Inflammation and Immune System Alterations in Frailty. Clin. Geriatr. Med. 2011, 27, 79–87. [Google Scholar] [CrossRef]
- Westbrook, R.; Chung, T.; Lovett, J.; Ward, C.; Joca, H.; Yang, H.; Khadeer, M.; Tian, J.; Xue, Q.-L.; Le, A.; et al. Kynurenines link chronic inflammation to functional decline and physical frailty. J. Clin. Investig. 2020, 5, e136091. [Google Scholar] [CrossRef]
- Van Epps, P.; Oswald, D.; Higgins, P.A.; Hornick, T.R.; Aung, H.; Banks, R.E.; Wilson, B.M.; Burant, C.; Gravenstein, S.; Canaday, D.H. Frailty has a stronger association with inflammation than age in older veterans. Immun. Ageing 2016, 13, 27. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, E.; Ito, S.; Kurosawa, Y.; Kobayashi, D.; Otani, H.; Abe, A.; Nakazono, K.; Murasawa, A.; Narita, I.; Ishikawa, H. The Efficacy of Biological Disease-modifying Antirheumatic Drugs on Sarcopenia in Patients with Rheumatoid Arthritis. Intern. Med. 2023, 62, 373–379. [Google Scholar] [CrossRef] [PubMed]


| Characteristics (N = 58) | T0 | T1 | p-Value |
|---|---|---|---|
| Age (years), median [range] | 54 [18–78] | - | - |
| Female gender, n (%) | 36 (62) | - | - |
| CD, n (%) | 31 (53) | - | - |
| CD localization | |||
| L1, n (%) | 19/31 (61) | - | - |
| L2, n (%) | 4/31 (13) | - | - |
| L3, n (%) | 8/31 (26) | - | - |
| L4, n (%) | 0 | - | - |
| CD behaviour | |||
| B1, n (%) | 11/31 (35) | - | - |
| B2, n (%) | 16/31 (52) | - | - |
| B3, n (%) | 4/31 (13) | - | - |
| UC, n (%) | 27 (47) | - | - |
| UC extension | |||
| E1, n (%) | 2/27 (7) | - | - |
| E2, n (%) | 10/27 (37) | - | - |
| E3, n (%) | 15/27 (56) | - | - |
| Age at diagnosis | |||
| A1, n (%) | 5 (9) | - | - |
| A2, n (%) | 34 (58) | - | - |
| A3, n (%) | 19 (33) | - | - |
| Perianal Disease, n (%) | 11 (19) | ||
| BMI (kg/m2), median [range] | 25 [16–42] | 24 [15–41] | 0.09 |
| History of EIMs, n (%) | 19 (33) | - | - |
| History of steroid dependance/resistance, n (%) | 26 (45) | - | - |
| Clinically active disease, n (%) | 45 (78) | 22 (38) | <0.0001 |
| Current therapy with steroids, n (%) | 10 (17) | 4 (7) | 0.109 |
| Current therapy with biologic agents, n (%) | 36 (62) | 38 (66) | 0.77 |
| Current therapy with ISS, n (%) | 2 (3) | 2 (3) | >0.99 |
| Current therapy with mesalamine, n (%) | 37 (64) | 38 (66) | >0.99 |
| Charlson comorbidity index, median [range] | 1 [0–5] | 1 [0–5] | 0.25 |
| Psychiatric diseases, n (%) | 10 (17) | 12 (21) | 0.69 |
| Osteoarticular diseases, n (%) | 13 (22) | 15 (26) | 0.69 |
| Heart failure, n (%) | 1 (2) | 1 (2) | >0.99 |
| Pneumological diseases, n (%) | 5 (9) | 5 (9) | >0.99 |
| Neurodegenerative diseases, n (%) | 1 (2) | 1 (2) | >0.99 |
| Post-COVID fatigue, n (%) | 2 (3) | 2 (3) | >0.99 |
| Characteristics at T1 | Frail Phenotype (N = 11) | Fit Phenotype (N = 12) | p-Value |
|---|---|---|---|
| Age (years), median [range] | 59 [27–78] | 57.5 [24–71] | 0.67 |
| Female gender, n (%) | 8 (73) | 8 (67) | >0.99 |
| CD, n (%) | 6 (55) | 5 (42) | 0.68 |
| UC, n (%) | 5 (45) | 7 (58) | 0.67 |
| Duration of disease (months), median [range] | 180 [24–492] | 168 [36–432] | 0.88 |
| BMI (kg/m2), median [range] | 25 [18–31] | 24 [16–33] | 0.78 |
| History of EIMs, n (%) | 7 (64) | 3 (25) | 0.10 |
| History of steroid dependence/resistance, n (%) | 4 (36) | 6 (50) | 0.68 |
| Clinically active disease, n (%) | 8 (73) | 2 (17) | 0.012 |
| Current therapy with steroids, n (%) | 0 (0) | 1 (8) | >0.99 |
| Current therapy with biologic agents, n (%) | 3 (27) | 9 (75) | 0.039 |
| Current therapy with ISS, n (%) | 0 | 0 | - |
| Current therapy with mesalamine, n (%) | 7 (64) | 11 (92) | 0.15 |
| Charlson comorbidity index, median [range] | 3 [0–5] | 1.5 [0–4] | 0.31 |
| Psychiatric diseases, n (%) | 2 (18) | 1 (8) | 0.59 |
| Heart failure, n (%) | 0 | 0 | - |
| Pneumological diseases, n (%) | 1 (9) | 0 | 0.48 |
| Neurodegenerative diseases, n (%) | 0 | 1 (8) | >0.99 |
| Post-COVID fatigue, n (%) | 1 (9) | 1 (8) | >0.99 |
| Characteristics at T1 | Improved Frail Phenotype (N = 47) | Persistence of Frail Phenotype (N = 11) | p-Value |
|---|---|---|---|
| Age (years), median [range] | 54 [18–71] | 59 [27–78] | 0.27 |
| Female gender, n (%) | 28 (60) | 8 (73) | 0.07 |
| CD, n (%) | 25 (53) | 6 (55) | 0.88 |
| UC, n (%) | 22 (47) | 5 (45) | 0.88 |
| Duration of disease (months), median [range] | 156 [10–624] | 180 [24–492] | 0.99 |
| BMI (kg/m2), median [range] | 24 [15–41] | 23.5 [20–33] | 0.83 |
| History of EIMs, n (%) | 12 (26) | 7 (64) | 0.03 |
| History of steroid dependence/resistance, n (%) | 22 (47) | 4 (36) | 0.74 |
| Clinically active disease, n (%) | 14 (30) | 8 (73) | <0.0001 |
| Current therapy with steroids, n (%) | 4 (9) | 0 | 0.001 |
| Current therapy with biologic agents, n (%) | 35 (74) | 3 (27) | <0.0001 |
| Current therapy with ISS, n (%) | 2 (4) | 0 | 0.048 |
| Current therapy with mesalamine, n (%) | 31 (66) | 7 (64) | 0.88 |
| Charlson Comorbidity Index, median [range] | 1 [0–5] | 3 [0–5] | 0.08 |
| Psychiatric diseases, n (%) | 9 (19) | 2 (18) | 0.90 |
| Heart failure, n (%) | 1 (2) | 0 | 0.50 |
| Pneumological diseases, n (%) | 5 (11) | 1 (9) | 0.81 |
| Neurodegenerative diseases, n (%) | 1 (2) | 0 | 0.50 |
| Post-COVID fatigue, n (%) | 1 (2) | 1 (9) | 0.06 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Risk Factors | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age (years) | 0.97 (0.9–1.0) | 0.26 | - | - |
| Female gender | 0.6 (0.1–2.2) | 0.41 | - | - |
| CD | 0.95 (0.2 3–6) | 0.94 | - | - |
| UC | 1.1 (0.3–4.1) | 0.95 | - | - |
| Duration of disease (months) | 1.0 (0.9–1.0) | 0.96 | - | - |
| BMI (kg/m2) | 1.0 (0.9–1.2) | 0.93 | - | - |
| History of EIMs | 0.2 (0.04–0.7) | 0.02 | 0.1 (0.02–0.8) | 0.04 |
| History of steroid dependence/resistance | 1.5 (0.4–6.5) | 0.52 | - | - |
| Clinically active disease | 0.2 (0.03–0.6) | 0.01 | 0.1 (0.01–0.6) | 0.02 |
| Current therapy with steroids | 1.2 (0.2–24.1) | 0.88 | - | - |
| Current therapy with biologic agents | 7.8 (1.9–40.2) | 0.007 | 21.7 (3.4–263) | 0.004 |
| Current therapy with ISS | 0.7 (0.08–14.6) | 0.75 | - | - |
| Current therapy with mesalamine | 1.1 (0.3–4.3) | 0.88 | - | - |
| Charlson comorbidity index | 0.7 (0.4–1.1) | 0.10 | - | - |
| Psychiatric diseases | 1.1 (0.2–7.8) | 0.94 | - | - |
| Heart failure | 0.4 (0.04–10.1) | 0.52 | - | - |
| Pneumological diseases | 1.2 (0.2–24.1) | 0.88 | - | - |
| Neurodegenerative diseases | 0.4 (0.04–10.1) | 0.52 | - | - |
| Post-COVID fatigue | 0.2 (0.02–1.8) | 0.13 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatori, S.; Marafini, I.; Franchin, M.; Lavigna, D.; Brigida, M.; Venuto, C.; Biancone, L.; Calabrese, E.; Giannarelli, D.; Monteleone, G. Reversibility of Frail Phenotype in Patients with Inflammatory Bowel Diseases. J. Clin. Med. 2023, 12, 2658. https://doi.org/10.3390/jcm12072658
Salvatori S, Marafini I, Franchin M, Lavigna D, Brigida M, Venuto C, Biancone L, Calabrese E, Giannarelli D, Monteleone G. Reversibility of Frail Phenotype in Patients with Inflammatory Bowel Diseases. Journal of Clinical Medicine. 2023; 12(7):2658. https://doi.org/10.3390/jcm12072658
Chicago/Turabian StyleSalvatori, Silvia, Irene Marafini, Martina Franchin, Diletta Lavigna, Mattia Brigida, Chiara Venuto, Livia Biancone, Emma Calabrese, Diana Giannarelli, and Giovanni Monteleone. 2023. "Reversibility of Frail Phenotype in Patients with Inflammatory Bowel Diseases" Journal of Clinical Medicine 12, no. 7: 2658. https://doi.org/10.3390/jcm12072658
APA StyleSalvatori, S., Marafini, I., Franchin, M., Lavigna, D., Brigida, M., Venuto, C., Biancone, L., Calabrese, E., Giannarelli, D., & Monteleone, G. (2023). Reversibility of Frail Phenotype in Patients with Inflammatory Bowel Diseases. Journal of Clinical Medicine, 12(7), 2658. https://doi.org/10.3390/jcm12072658







